Introduction
Breast cancer is the most common malignancy among
women worldwide. Among breast cancer subtypes, invasive ductal
carcinoma is the one most commonly associated with metastasis
(1). Invasive lobular cancer is the
second most common invasive type of breast cancer, and is
associated with a heterogeneous group of histologically different
types of metastatic spread and potentially uncommon patterns of
metastatic site involvement (1,2). The
most common sites for breast cancer metastases are the bone, liver,
lung and brain, while gastrointestinal metastasis from breast
cancer is rare. However, acknowledging this entity is important for
accurate and timely diagnosis and treatment (2,3).
We herein report the case of a patient with a
metastatic gastric tumor arising from invasive lobular carcinoma of
the breast, complicated by gastric outlet obstruction (GOO), which
was managed by metallic stent placement.
Case report
A 68-year-old female patient presented to the Kochi
Medical School Hospital (Nankoku, Japan) in March 2018 with nausea
and appetite loss. The patient's medical history included right
mastectomy with sentinel lymph node biopsy for right breast cancer
5 years earlier. The pathological diagnosis was invasive lobular
carcinoma, 6.2 cm in greatest diameter, without lymphovascular
invasion. Immunohistochemical examination of the tumor revealed 40%
estrogen receptor positivity, 1% progesterone receptor positivity
and negative staining for human epidermal growth factor receptor 2
(HER2), with a Ki-67 index of 15%. Two years after the surgery, the
patient developed brain metastasis and underwent metastasectomy to
control neurological symptoms such as unsteadiness and asthenia.
Postoperatively, the patient received systemic chemotherapy using
S-1, followed by bevacizumab plus paclitaxel, although the
treatment was subsequently changed to eribulin due to
bevacizumab-related cardiotoxicity.
The laboratory findings on admission were as
follows: Decreased red blood cell count
(296×104/mm3; normal range,
386–492×104/mm3), decreased white blood cell
count (1.0×103/mm3; normal range,
3.3–8.6×103/mm3) and increased C-reactive
protein levels (34.21 mg/dl; normal values <0.14 mg/dl). On
esophagogastroduodenoscopy, an elevated lesion was identified
occupying the entire circumference of the antrum and causing a
narrowing of the gastric outlet (Fig.
1). Biopsy of the tumor followed by histological examination
revealed infiltration of the wall of the antrum by undifferentiated
neoplastic cells with poor adhesion, resembling invasive lobular
carcinoma (Fig. 2A);
immunohistochemical staining revealed positivity for estrogen
receptor (Fig. 2B), mammaglobin
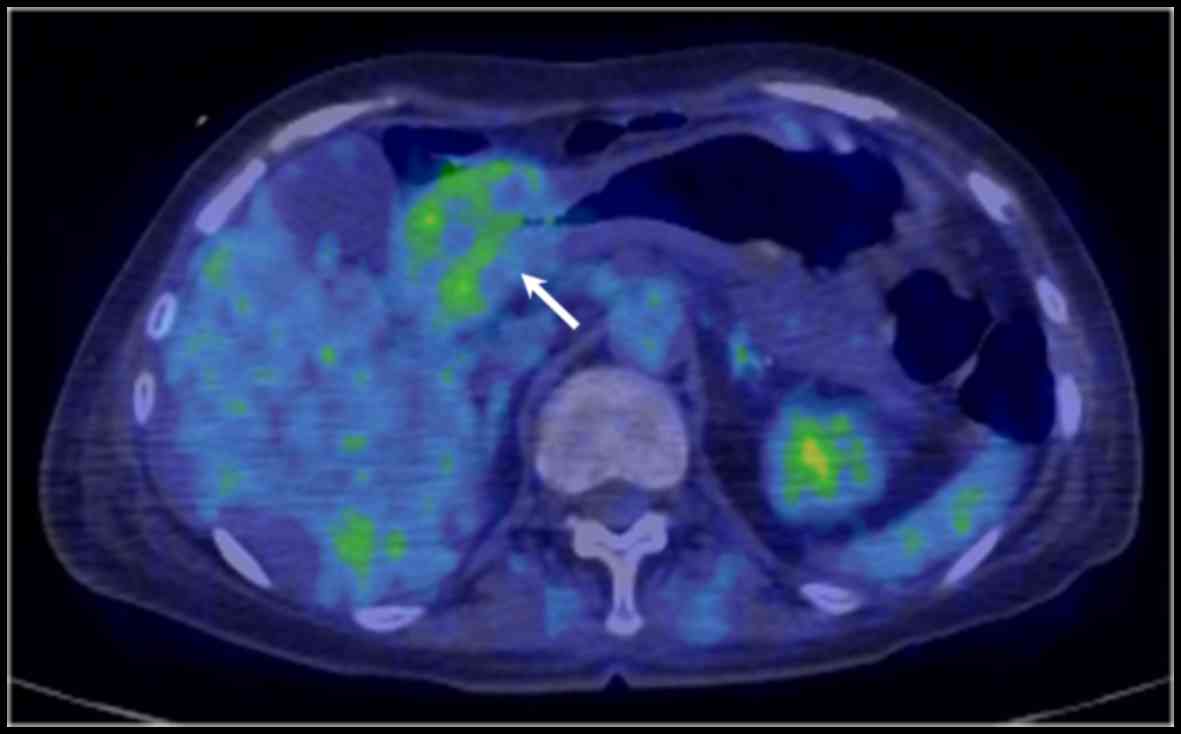
(Fig. 2C) and GATA3 (Fig. 2D). Imaging by
18F-2-deoxy-2-fluoro-D-glucose (FDG) positron emission
tomography combined with computed tomography revealed FDG uptake
across the full thickness of the antral wall (Fig. 3). These findings indicated a clinical
diagnosis of gastric metastasis from the primary breast cancer.
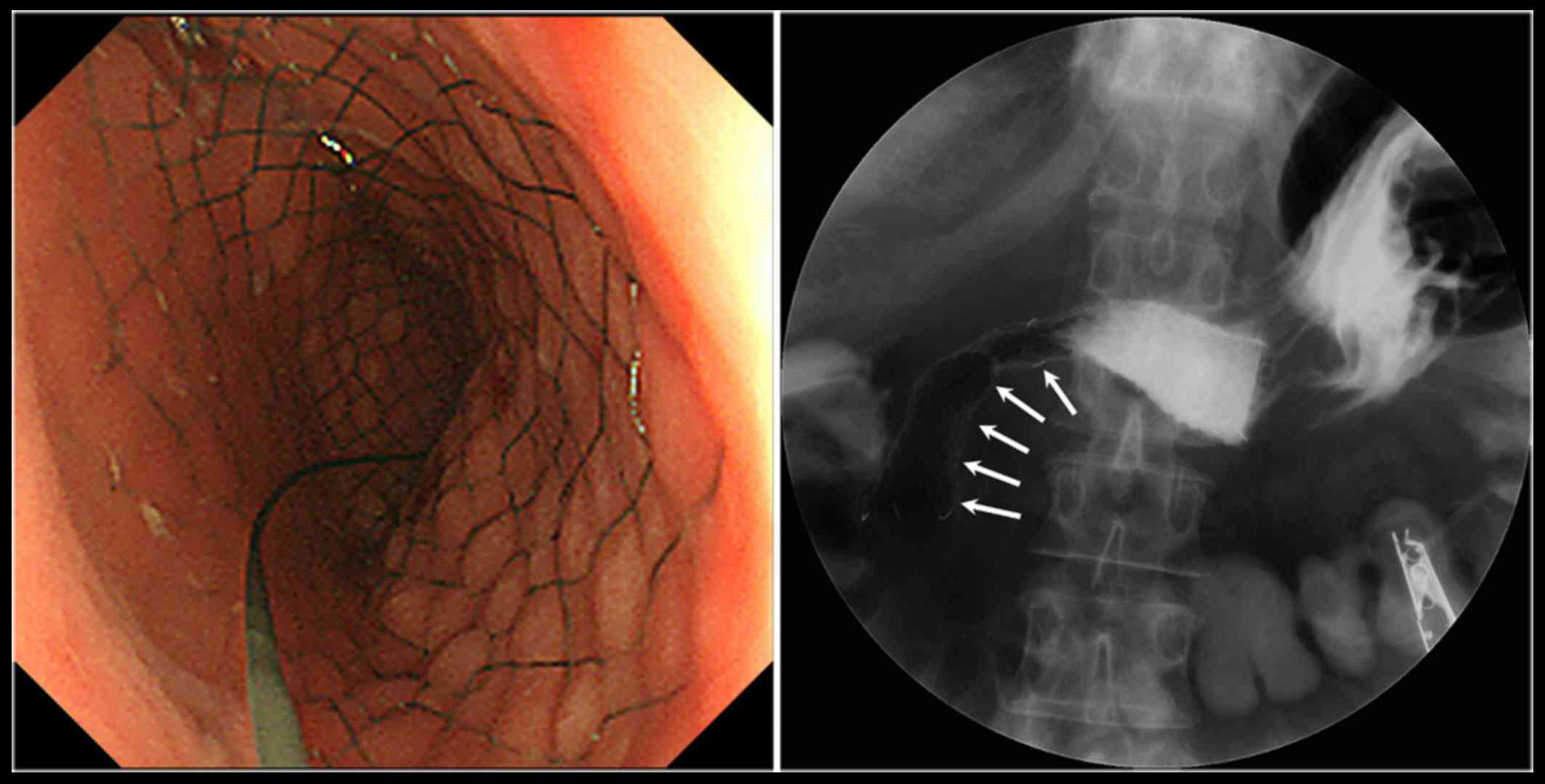
The patient eventually developed GOO that markedly
worsened her quality of life; thus, placement of an endoscopic
self-expandable metallic stent (SEMS) was performed to resolve the
obstruction-induced clinical symptoms (Fig. 4). There were no procedure-related
adverse events. The date of last contact was August 2018, and the
patient remained alive under best supportive care 5 months after
the procedure.
Discussion
We herein present a case of GOO caused by a
metastatic tumor of the stomach originating from an invasive
lobular carcinoma of the breast that was resected 5 years earlier.
SEMS placement was used to relieve the obstruction, as it is an
effective and safe procedure for maintaining the quality of life of
patients under best supportive care. To the best of our knowledge,
this is the first reported case of GOO caused by metastatic gastric
tumor managed by SEMS placement.
Gastric involvement by metastatic breast cancer is a
rare clinical diagnosis. Furthermore, invasive lobular breast
carcinoma is less likely to involve the gastrointestinal tract
compared with invasive ductal carcinoma, with the most frequent
metastatic sites being the bone, gynecological organs, peritoneum
and retroperitoneum (3–5). In this respect, the present case
highlights the importance of considering metastatic tumor of the
stomach secondary to invasive lobular carcinoma of the breast, and
the importance of immunohistochemical analysis, such as staining
for estrogen and progesterone receptors (2,3,5,6).
GATA3 is a multifunctional transcription factor that
is important for the development and function of ductal epithelial
cells, including those of breast, urothelia, epidermis and skin
adnexa, wherein specific nuclear proteins recognize G-A-T-A
nucleotide sequences in target gene promoters (7). As the majority of primary and
metastatic mammary tumors express GATA3 (positive rate of 80–90%),
it is a potentially useful addition to hormonal markers, such as
estrogen and progesterone receptors, for identifying metastatic
cells of mammary origin (8). In the
present case, positive immunohistochemical staining of these three
markers was observed.
The endoscopic and radiological appearance resemble
linitis plastica due to the diffuse infiltration of the submucosa
and muscularis propria, with circumferential thickening and
narrowing of the lumen, as metastatic lobular carcinoma infiltrates
within the serosal, muscular and submucosal layers, with cord-like
projections of small cells (6). The
treatment generally recommended for gastric metastases from breast
cancer is systemic chemotherapy and/or hormonal therapy (3,4,6). When an unusual lesion is detected in a
patient with invasive lobular carcinoma, metastatic disease should
be considered in the differential diagnosis, and
immunohistochemical analysis is recommended for accurate
diagnosis.
Patients with malignant GOO tend to develop
undesirable clinical symptoms that are detrimental to the quality
of life of the patients, such as nausea, vomiting, abdominal pain
and difficulty eating (9).
Fluoroscopic or/and endoscopic SEMS placement as palliative
treatment for malignant GOO is generally safe, easily performed and
effective, and is associated with higher clinical success rates and
lower morbidity and mortality rates compared with palliative
surgery (9,10). By contrast, Jang et al
(11) reported that palliative
gastrojejunostomy was significantly associated with longer overall
survival and lower risk of re-intervention compared with SEMS
placement in patients with malignant GOO caused by unresectable
gastric cancer using a propensity score matching analysis. Overall,
the choice of systemic treatment, such as chemotherapy and/or
hormonal therapy, for breast cancer metastasis is based upon
presenting symptoms, age and general performance status, and
surgical palliation should be considered only under emergency
conditions to bypass the obstruction (3). The bone marrow of the patient in the
present case was exhausted due to long-term systemic chemotherapy;
thus, we selected SEMS placement to avoid unfavorable complications
associated with the bypass procedure.
In conclusion, this case indicates that SEMS
placement may be a promising approach to the management of patients
with GOO caused by unresectable advanced gastric cancer as well as
metastatic gastric tumors, and may contribute to improved quality
of life for these patients. Further investigations with a larger
accumulation of cases and/or prospective studies are required to
establish the optimal treatment for GOO caused by metastatic tumors
to the stomach originating from other primary malignancies.
Acknowledgements
Not applicable.
Funding
No funding was received
Availability of data and materials
Not applicable.
Authors' contributions
MO and TN contributed to the writing of the
manuscript. MK and KH supervised the study. MO, TN, TO, JI, HM, TT,
TY, HK, KD and TS served as the attending physicians for the
presented patient. All the authors have read and approved that
final version of this manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient has given consent for the publication of
the case details and associated images.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Arpino G, Bardou VJ, Clark GM and Elledge
RM: Infiltrating lobular carcinoma of the breast: Tumor
characteristics and clinical outcome. Breast Cancer Res.
6:R149–R156. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Namikawa T, Kobayashi M and Hanazaki K: An
unusual giant duodenal mass lesion. Gastroenterology. 148:e5–e6.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Namikawa T and Hanazaki K:
Clinicopathological features and treatment outcomes of metastatic
tumors in the stomach. Surg Today. 44:1392–1399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Borst MJ and Ingold JA: Metastatic
patterns of invasive lobular versus invasive ductal carcinoma of
the breast. Surgery. 114:637–641; discussion 641–642.
1993.PubMed/NCBI
|
|
5
|
Namikawa T, Munekage E, Ogawa M, Oki T,
Munekage M, Maeda H, Kitagawa H, Sugimoto T, Kobayashi M and
Hanazaki K: Clinical presentation and treatment of gastric
metastasis from other malignancies of solid organs. Biomed Rep.
7:159–162. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taal BG, Peterse H and Boot H: Clinical
presentation, endoscopic features, and treatment of gastric
metastases from breast carcinoma. Cancer. 89:2214–2221. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miettinen M, McCue PA, Sarlomo-Rikala M,
Rys J, Czapiewski P, Wazny K, Langfort R, Waloszczyk P, Biernat W,
Lasota J, et al: GATA3: a multispecific but potentially useful
marker in surgical pathology: a systematic analysis of 2500
epithelial and nonepithelial tumors. Am J Surg Pathol. 38:13–22.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Shi J, Wilkerson ML and Lin F:
Immunohistochemical evaluation of GATA3 expression in tumors and
normal tissues: A useful immunomarker for breast and urothelial
carcinomas. Am J Clin Pathol. 138:57–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yukimoto T, Morisaki T, Komukai S, Yoshida
H, Yamaguchi D, Tsuruoka N, Miyahara K, Sakata Y, Shibasaki S,
Tsunada S, et al: The palliative effect of endoscopic uncovered
self-expandable metallic stent placement versus gastrojejunostomy
on malignant gastric outlet obstruction: A pilot study with a
retrospective chart review in Saga, Japan. Intern Med.
57:1517–1521. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi D, Bao YS and Liu YP:
Individualization of metal stents for management of gastric outlet
obstruction caused by distal stomach cancer: A prospective study.
Gastrointest Endosc. 78:277–284. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jang SH, Lee H, Min BH, Kim SM, Kim HS,
Carriere KC, Min YW, Lee JH and Kim JJ: Palliative
gastrojejunostomy versus endoscopic stent placement for gastric
outlet obstruction in patients with unresectable gastric cancer: A
propensity score-matched analysis. Surg Endosc. 31:4217–4223. 2017.
View Article : Google Scholar : PubMed/NCBI
|