Introduction
For colorectal cancer (CRC), 5-fluorouracil (5-FU)
has been a major medicinal treatment. However, gastrointestinal
adverse events and myelosuppression, are reportedly associated with
the conventional 5-FU bolus schedule (1).
Capecitabine is an oral fluoropyrimidine that may
replace 5-FU as a single-agent therapy for CRC due to its good
efficacy, favorable tolerability and convenience. To compare
capecitabine with bolus 5-FU/leucovorin (LV) therapy (Mayo Clinic
regimen) as an adjuvant chemotherapy in stage III colon cancer, a
phase III clinical study (X-ACT trial) has been performed (2-4).
Capecitabine treatment was at least equivalent to 5-FU/LV therapy
(Mayo Clinic regimen) regarding the primary endpoint of three-year
disease-free survival (DFS) (5).
Capecitabine also achieved improved relapse-free survival over 5-FU
and was associated with significantly fewer adverse events (AEs)
(5,6).
However, AEs including hand-foot syndrome (HFS)
still prevent patients from completing the adjuvant chemotherapy
course. Twelves et al (5)
reported that the grade (G)3/4 toxicity was 20% for
hyperbilirubinemia, 17% for HFS and 11% for diarrhea (5). According to Emi et al (7), 24 out of 97 patients (24.7%)
discontinued the treatment due to side effects. The most common
reason was HFS in 7 patients (7.2%), followed by 5 patients with
hematological toxicities (5.2%) and 5 patients with liver
dysfunction (5.2%) (7). These
adverse events prevent patients from completing the adjuvant
chemotherapy courses. Overall DFS and quality of life (QOL) may be
improved by a higher capecitabine completion rate, achieved by
reduction of side effects.
Previous clinical studies have adopted the
conventional oral capecitabine regimen, wherein the drug was taken
for 14 consecutive days every 21 days. An alternative
5-days-on/2-days-off schedule of capecitabine has been proposed as
a way to reduce side effects in patients with advanced solid
cancers (8). According to this
schedule, patients are treated with capecitabine (2,500
mg/m2/day), which is taken for five days, followed by an
interval of two days. One course lasts for three weeks, and eight
courses (24 weeks) are administered. Modulation of the methods of
capecitabine administration may change the profile of the side
effects of capecitabine and lead to an increased total dose and
improved QOL.
The present study aimed to assess the potency of the
5-days-on/2-days-off schedule for patients with CRC compared with
the conventional schedule for the capecitabine regimen.
Patients and methods
Inclusion criteria
The patients were enrolled between March 2014 and
October 2014. The follow-up period was one year from the last case
registration. Patients were enrolled at the Wakayama Medical
University Hospital (Wakayama, Japan) and its associated teaching
hospitals, National Hospital Organization Minami Wakayama Medical
Center (Wakayama, Japan), Wakayama Rosai Hospital (Wakayama, Japan)
and Izumiotsu Municipal Hospital (Osaka, Japan). Patients on the
5-days-on/2-days-off regimen who met all of the following criteria
were eligible for the present study, regardless of sex:
Histologically or cytologically diagnosed with CRC; capecitabine
used as adjuvant chemotherapy; age between 20 and 80 years; Eastern
Cooperative Oncology Group (ECOG) performance status (PS) of 0 or
1; principal organ functions sufficiently maintained (see criteria
below); first-line treatment; written informed consent to
participate in the study.
Furthermore, patients were required to be CRC stage
III or high-risk stage II and undergoing curative operation
followed by adjuvant chemotherapy (staging criteria in accordance
with the TNM Classification of Malignant Tumors, 7th edition)
(9). According to the American
Society of Clinical Oncology guidelines (10) and the European Society for Medical
Oncology guidelines (11), high-risk
stage II CRC has <12 lymph nodes, T4 lesions, perforation,
poorly differentiated histology, vascular or lymphatic or
perineural invasion, and clinical presentation with intestinal
occlusion or perforation.
The study was performed in accordance with the
Declaration of Helsinki and the Ethical Guidelines for Clinical
Research and has been approved by the Ethics Committee of Wakayama
Medical University (Wakayama, Japan). Written informed consent was
obtained from all patients prior to enrollment in this study.
Regarding the laboratory test results, the following
criteria were also required to be satisfied: White cell count
>1,500/mm3; neutrophil count ≥1,500/mm3;
platelet count ≥100,000/mm3; hemoglobin ≥9.0 g/dl; total
bilirubin ≤1.5 mg/dl; aspartate transaminase, alanine
aminotransferase ≤100 IU/l; serum creatinine ≤1.5 mg/dl.
Exclusion criteria. Patients who met any of
the following criteria were excluded from participation in the
study: Pregnant or lactating; likely to, or planning to, become
pregnant; history of malignancy; uncontrollable congestive heart
failure, hypertension, angina pectoris, arrhythmia; severe drug
hypersensitivity; side effects (or possible side effects) of
fluoropyrimidine-type drugs with suspicion that dihydropyrimidine
dehydrogenase deficiency may have developed; uncontrollable
infection; oral ingestion difficulty; otherwise inappropriate for
enrollment based on the researcher's judgment. Protocol treatment
was not started in cases of rapid exacerbation after registration,
protocol violation or discovery of ineligibility after
registration.
Toxicities
Toxicities were graded according to the common
terminology criteria for adverse events version 4.0(12). Patients visited the hospital on the
first day of admission and were checked for adverse events by blood
examination and clinical examination. If a G3 adverse event was
identified, the patient visited the clinic weekly until the adverse
events were alleviated. If there were no G3 adverse events, the
patient visited the clinic every three weeks. Adverse effects of
capecitabine were checked by YM, SY, MO, YK and HY. If any
discrepancies arose, all members discussed them in order to reach a
consensus.
Treatment and assessment
The patients enrolled were treated with capecitabine
(2,500 mg/m2/day), which was taken on five consecutive
days, followed by an interval of two days (5-days-on/2-days-off
schedule). This trial was single-arm study and comparison was
performed with a retrospectively enrolled conventional treatment
group. The retrospective conventional treatment group was composed
of 21 consecutive patients who received conventional treatment.
They underwent surgery between January 2011 and March 2016 and
received capecitabine treatment (2,500 mg/m2/day) taken
on 14 consecutive days every 21 days according to the conventional
schedule, prior to March 2014. One course lasted for three weeks,
and eight courses (24 weeks) were administered in total. To assess
the feasibility of the treatment schedule, sex, age, agreed date of
acquisition of adverse events, ECOG PS, body height, body weight,
body surface area, location of CRC, surgical procedure, lymph node
dissection range, tumor diameter, histopathological classification,
patient history, and comorbidities within one year were recorded.
To examine hematologic side effects of the protocol treatment,
baseline peripheral blood counts were measured and biochemical
examination of blood was performed within 14 days prior to the
start of drug administration.
General characteristics, clinical findings
(subjective symptoms and objective signs), peripheral blood counts,
blood chemistry values, tumor markers and incidence of HFS were
typically recorded between weeks three and five during treatment.
Imaging evaluation was performed at three months by computed
tomography or magnetic resonance imaging. QOL evaluation (EORTC
QLQ-C30) (13,14) was performed prior to chemotherapy and
after two, four, six and eight courses of treatment.
Treatment completion was defined as the completion
of the eight three-week cycles on the 5-days-on/2-days-off schedule
according to protocol in patients who were registered and began
treatment within eight weeks post-operatively. None of the patients
received neo-adjuvant chemotherapy.
Protocol discontinuation criteria
Discontinuation meant that the intake of the drug
was completely terminated. Treatment was stopped for any of the
following reasons: i) Decision made by the clinician to terminate
protocol treatment due to AEs; ii) patient decided to discontinue
treatment according to the protocol for reasons associated with
AEs; iii) patient decided to stop treatment according to the
protocol due to reasons not associated with AEs; iv) mortality
during treatment.
Statistical analysis
Statistical analysis regarding patient
characteristics was performed using non-parametric methods,
including the Chi-squared test and Fisher's exact probability test.
Statistical analysis regarding the treatment course, completion
rate and relative dose intensity was performed using Fisher's exact
probability test. For the time to treatment failure (TTF),
evaluation was performed using Kaplan-Meier analysis and the
log-rank test was used to identify statistically significant
differences between the groups. The Cox proportional hazard ratios
for the risk of treatment failure were calculated with a 95%
confidence interval (CI). P<0.05 was considered to indicate
statistical significance.
Results
Patient characteristics
A total of 27 patients [18 males and 9 females;
median age, 67 years (range 47-80 years)] were enrolled. There was
no significant difference in the age and sex ratio of patients
between the 5-days-on/2-days-off regimen group and the
retrospectively included conventional regimen group. The follow-up
period was one year from the last case registration. These patients
underwent curative surgery and were diagnosed either as
pathological stage III or high-risk stage II CRC. A total of 6
patients were excluded: One was changed to a different
chemotherapy, one declined to participate in the trial, two
patients started treatment from a dose lower than that prescribed
in the study protocol, one was excluded due to bowel-diverting
stoma operation and one lost the medicine after registration. A
total of 21 patients were therefore eligible and treated with
capecitabine (2,500 mg/m2/day), which was taken for five
days followed by an interval of two days (5-days-on/2-days-off
schedule). One course lasted for three weeks, and eight courses (24
weeks) were administered. The median age of patients on the
5-days-on/2-days-off schedule was 67 years (range, 38-80 years).
The patients had a PS of 0 or 1. Rectal cancer patients accounted
for 23.8% (Table I). To compare the
age, sex, PS, lymph node dissection, location of cancer, stage,
invasion, lymphatic invasion, venous invasion and histological
classification between the 5-days-on/2-days-off regimen group and
conventional regimen group, two-sided P-values were calculated and
P<0.05 was considered significant. A significant difference
between the two groups was only identified in the stage (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | 5-days-on/2-days-off
(n=21) | Conventional
(n=21) | P-value |
|---|
| Age (years) | 67 (38-80) | 68 (46-75) | 0.9354 |
| Sex | | | 0.0614 |
|
Male | 6 (28.6) | 12 (57.1) | |
|
Female | 15 (71.4) | 9 (42.9) | |
| PS | | | 1.0000 |
|
0 | 21(100) | 21(100) | |
|
1 | 0 (0) | 0 (0) | |
| Lymph node
dissection | | | 0.2931 |
|
D0 | 0 (0) | 0 (0) | |
|
D1 | 0 (0) | 0 (0) | |
|
D2 | 2 (9.5) | 1 (54.8) | |
|
D3 | 19 (90.5) | 20 (95.2) | |
| Location of
cancer | | | 0.9588 |
|
C | 1 (4.8) | 2 (9.5) | |
|
A | 4 (19.0) | 4 (19.0) | |
|
T | 2 (9.5) | 3 (14.3) | |
|
D | 1 (4.8) | 1 (4.8) | |
|
S | 8 (38.1) | 6 (28.6) | |
|
RS | 2 (9.5) | 2 (9.5) | |
|
Ra | 3 (14.3) | 2 (9.5) | |
|
Rb | 0 (0) | 1 (4.8) | |
| Stagea | | | 0.0397 |
|
IIA | 5 (23.8) | 0 (0) | |
|
IIB | 1 (4.8) | 0 (0) | |
|
IIIA | 3 (14.3) | 9 (42.9) | |
|
IIIB | 12 (57.1) | 11 (52.4) | |
|
IIIC | 0 (0) | 1 (4.8) | |
| Tumor depth of
invasion | | | 0.0727 |
|
T1 | 2 (9.5) | 4 (19.0) | |
|
T2 | 1 (4.8) | 5 (23.8) | |
|
T3 | 17 (81.0) | 8 (38.1) | |
|
T4 | 1 (4.8) | 4 (19.0) | |
| Lymphatic
invasion | | | 0.6200 |
|
Ly0 | 11 (52.4) | 10 (47.6) | |
|
Ly1 | 6 (28.6) | 6 (28.6) | |
|
Ly2 | 3 (14.3) | 5 (23.8) | |
|
Ly3 | 1 (4.8) | 0 (0) | |
| Venous
invasion | | | 0.1885 |
|
V0 | 13 (64.9) | 9 (42.9) | |
|
V1 | 6 (28.6) | 7 (33.3) | |
|
V2 | 1 (4.8) | 3 (14.3) | |
|
V3 | 1 (4.8) | 0 (0.0) | |
|
Unknown | 0 (0.0) | 2 (9.5) | |
| Histological
classification | | | 0.4283 |
|
Pap | 1 (4.8) | 0 (0.0) | |
|
Wel | 7 (33.3) | 10 (47.6) | |
|
Mod | 13 (61.9) | 11 (52.4) | |
Toxicities
The most common treatment-associated AEs were HFS
(13 patients, 61.9%), anemia (7 patients, 33.3%), pigmentation (6
patients, 28.5%), hyperbilirubinemia (6 patients, 28.5%) and
aspartate aminotransferase increase (6 patients, 28.5%; Table II). The major G3/4 AE was HFS only
(9.5%). Although AEs in the conventional group were only
investigated retrospectively, most common AEs were HFS (5 patients,
23.9%) and hyperbilirubinemia (2 patients, 9.5%). The AEs in the
conventional group are provided in Table III. When comparing the AEs between
the two groups, it appeared that HFS was more common in the
5-days-on/2-days-off regimen but G3/4 AEs were less common.
 | Table IIAdverse events in the
5-days-on/2-days-off regimen. |
Table II
Adverse events in the
5-days-on/2-days-off regimen.
| Adverse event | All grades | Grade 1 | Grade 2 | Grade 3/4 |
|---|
| Diarrhea | 2 (9.5) | 2 (9.5) | 0 (0.0) | 0 (0.0) |
| Hand-foot
syndrome | 13 (61.9) | 7 (33.3) | 4 (19.0) | 2 (9.5) |
| Nausea and
vomiting | 1 (4.7) | 1 (4.7) | 0 (0.0) | 0 (0.0) |
| Anorexia | 3 (14.3) | 3 (14.3) | 0 (0.0) | 0 (0.0) |
| Dysgeusia | 3 (14.3) | 2 (9.5) | 1 (4.7) | 0 (0.0) |
| Pigmentation | 6 (28.5) | 6 (28.5) | 0 (0.0) | 0 (0.0) |
| Stomatitis | 2 (9.5) | 2 (9.5) | 0 (0.0) | 0 (0.0) |
| Fatigue | 3 (14.3) | 2 (9.5) | 1 (4.7) | 0 (0.0) |
| Neutropenia | 4 (19.0) | 2 (9.5) | 2 (9.5) | 0 (0.0) |
| Anemia | 7 (33.3) | 4 (19.0) | 3 (14.3) | 0 (0.0) |
|
Thrombocytopenia | 1 (4.7) | 1 (4.7) | 0 (0.0) | 0 (0.0) |
|
Hyperbilirubinemia | 6 (28.5) | 5 (23.8) | 1 (4.7) | 0 (0.0) |
| Alanine
aminotransferase increase | 3 (14.3) | 3 (14.3) | 0 (0.0) | 0 (0.0) |
| Aspartate
aminotransferase increase | 6 (28.5) | 6 (28.5) | 0 (0.0) | 0 (0.0) |
 | Table IIIAdverse events in the conventional
regimen group. |
Table III
Adverse events in the conventional
regimen group.
| Adverse events | All grades | Grade 1 | Grade 2 | Grade 3/4 |
|---|
| Diarrhea | 1 (4.7) | 0 (0.0) | 0 (0.0) | 1 (4.7) |
| Hand-foot
syndrome | 5 (23.9) | 1 (4.7) | 3 (14.3) | 1 (4.7) |
| Anorexia | 1 (4.7) | 0 (0.0) | 0 (0.0) | 1 (4.7) |
| Fatigue | 1 (4.7) | 1 (4.7) | 0 (0.0) | 0 (0.0) |
|
Hyperbilirubinemia | 2 (9.5) | 0 (0.0) | 2 (9.5) | 0 (0.0) |
| Allergic
reaction | 1 (4.7) | 0 (0.0) | 1 (4.7) | 0 (0.0) |
Continuity of 5-days-on/2-days-off
regimen as adjuvant chemotherapy for colorectal cancer
To address whether the 5-days-on/2-days-off regimen
allows patients to continue adjuvant chemotherapy of stage III or
high-risk stage II CRC, the median number and range of treatment
courses in the 5-days-on/2-days-off regimen were compared with
those of the conventional regimen. The median number of treatment
courses in the 5-days-on/2-days-off regimen group was significantly
higher than that in the conventional regimen group (P=0.0438). In
terms of completion rate, 20 out of 21 patients (95.2%) received
the complete scheduled treatment according to the
5-days-on/2-days-off regimen. On the other hand, of the 21 patients
following a conventional schedule, only 15 patients (71.4%)
completed the entire scheduled treatment (Table IV). Although the result was not
significantly different (P=0.0931), the 5-days-on/2-days-off
treatment tended to have better feasibility than the conventional
regimen, and regarding the completion of the new vs. conventional
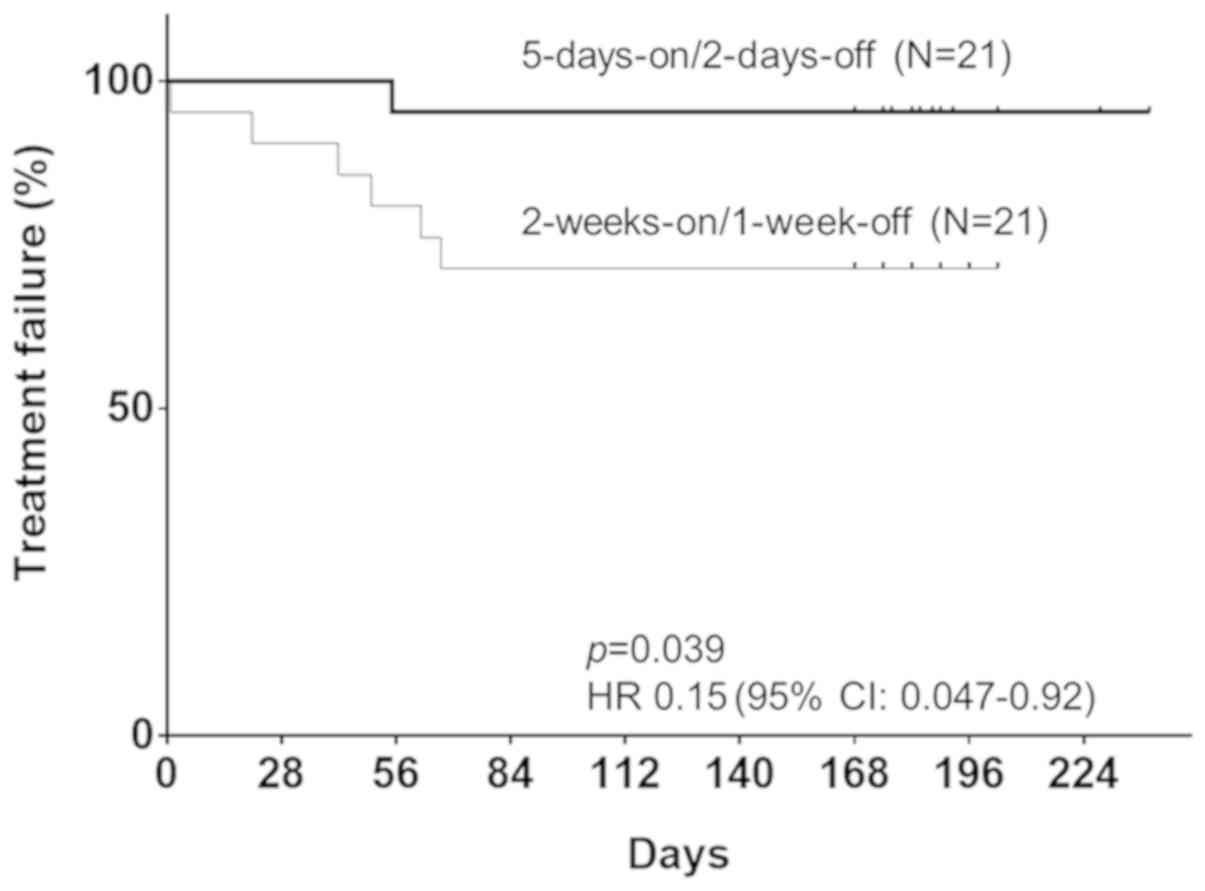
regimen, the odds ratio was 8.0 (95% CI: 0.87-73.7). When the TTF
of the 5-days-on/2-days-off group was compared with that of the
conventional group, the 5-days-on/2-days-off regimen group had a
significantly lower risk of failure of the scheduled treatment
(P=0.0389; HR=0.15; 95% CI: 0.047-0.92; Fig. 1).
 | Table IVContinuity in the
5-days-on/2-days-off regimen compared with the conventional
regimen. |
Table IV
Continuity in the
5-days-on/2-days-off regimen compared with the conventional
regimen.
| Item |
5-days-on/2-days-off (n=21) | Conventional
(n=21) | P-value |
|---|
| Treatment
courses | 8.0 (1-8) | 8.0 (2-8) | 0.0438 |
| Completion
ratea | | | 0.0931 |
| Complete
predetermined dose | 20 (95.2) | 15 (71.4) | |
| Incomplete
predetermined dose | 1 (4.8) | 6 (28.6) | |
| Relative dose
intensity | 1 (0.15-1) | 1 (0.125-1) | 0.158 |
The median relative dose intensity was 1.0 (range,
0.15-1) in the 5-days-on/2-days-off group and 1 (range, 0.125-1) in
the conventional group, respectively. Table V presents the various reasons for
discontinuation and dose reduction in the conventional group. A
dose reduction was required for seven patients (33.3%) in the
5-days-on/2-days-off group due to AEs. One of these seven patients
discontinued the treatment. In the conventional group, four
patients (19%) reduced the capecitabine dose but completed all
eight courses. The other six patients (28.6%) stopped the regimen
without dose reduction.
 | Table VReasons for protocol dose reduction
or incompletion. |
Table V
Reasons for protocol dose reduction
or incompletion.
| Item |
5-days-on/2-days-off | Conventional |
|---|
| Reason for dose
reduction |
|
HFS | 7 | 3 |
|
Fatigue | 0 | 1 |
| Reason for
incompletion |
|
HFS | 1 | 1 |
|
Total
bilirubin increase | 0 | 2 |
|
Anorexia | 0 | 1 |
|
Rash | 0 | 1 |
|
Diarrhea | 0 | 1 |
QOL of patients under the
5-days-on/2-days-off regimen
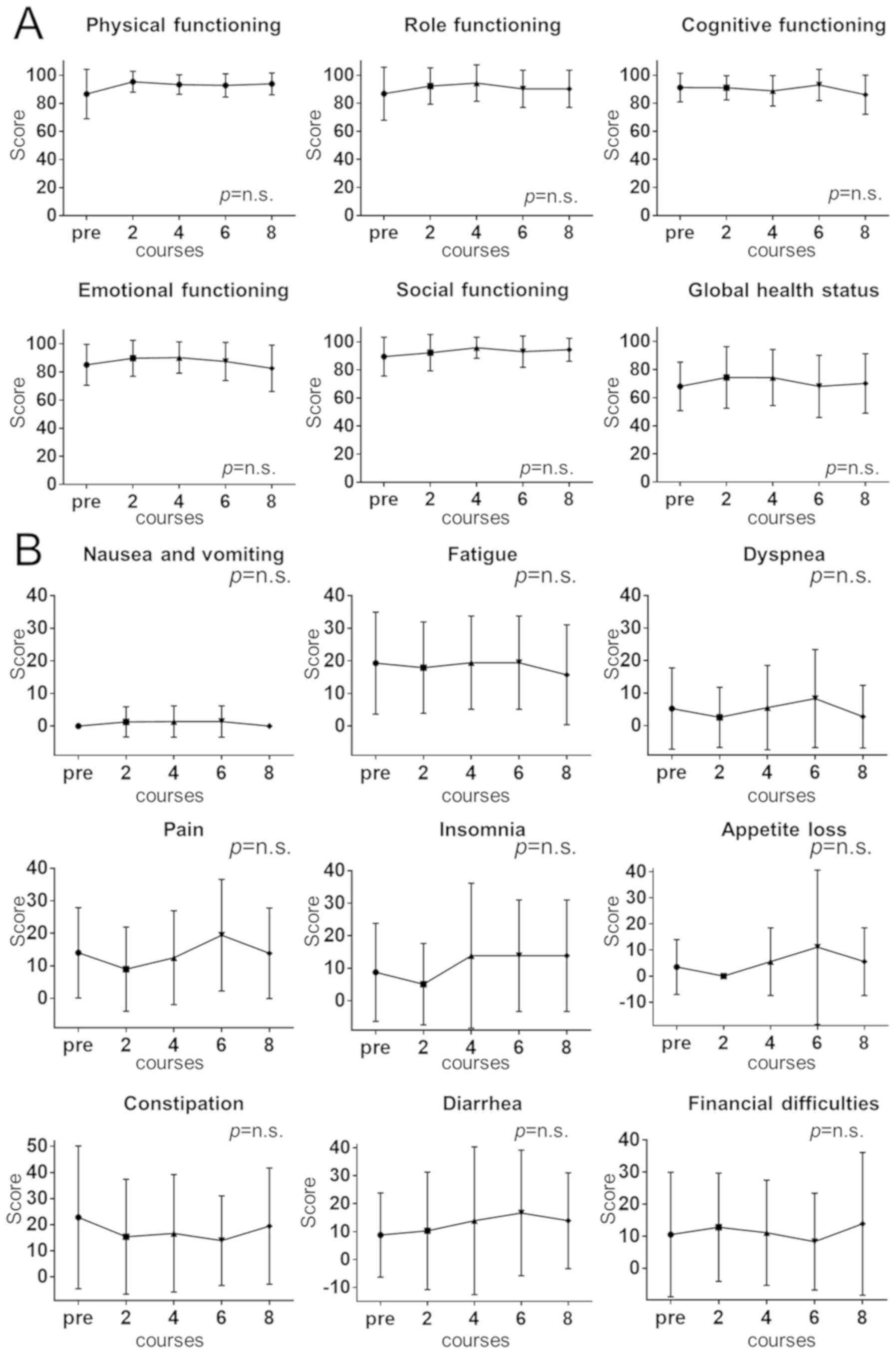
The results obtained with the EORTC QLQ-C30 are
presented in Fig. 2. The QLQ-C30
questionnaire consists of two scales: A functional scale (Fig. 2A) and a symptom scale (Fig. 2B), each scoring from 0 to 100. Higher
scores are considered to indicate a better result on the functional
scale and lower scores are better on the symptom scale. On the two
scales, there was no significant change in any of the QOL functions
and symptoms in the 5-days-on/2-days-off group over the course of
the treatment (P>0.05).
Comparison of toxicities in the
5-days-on/2-days-off regimen with that in other studies
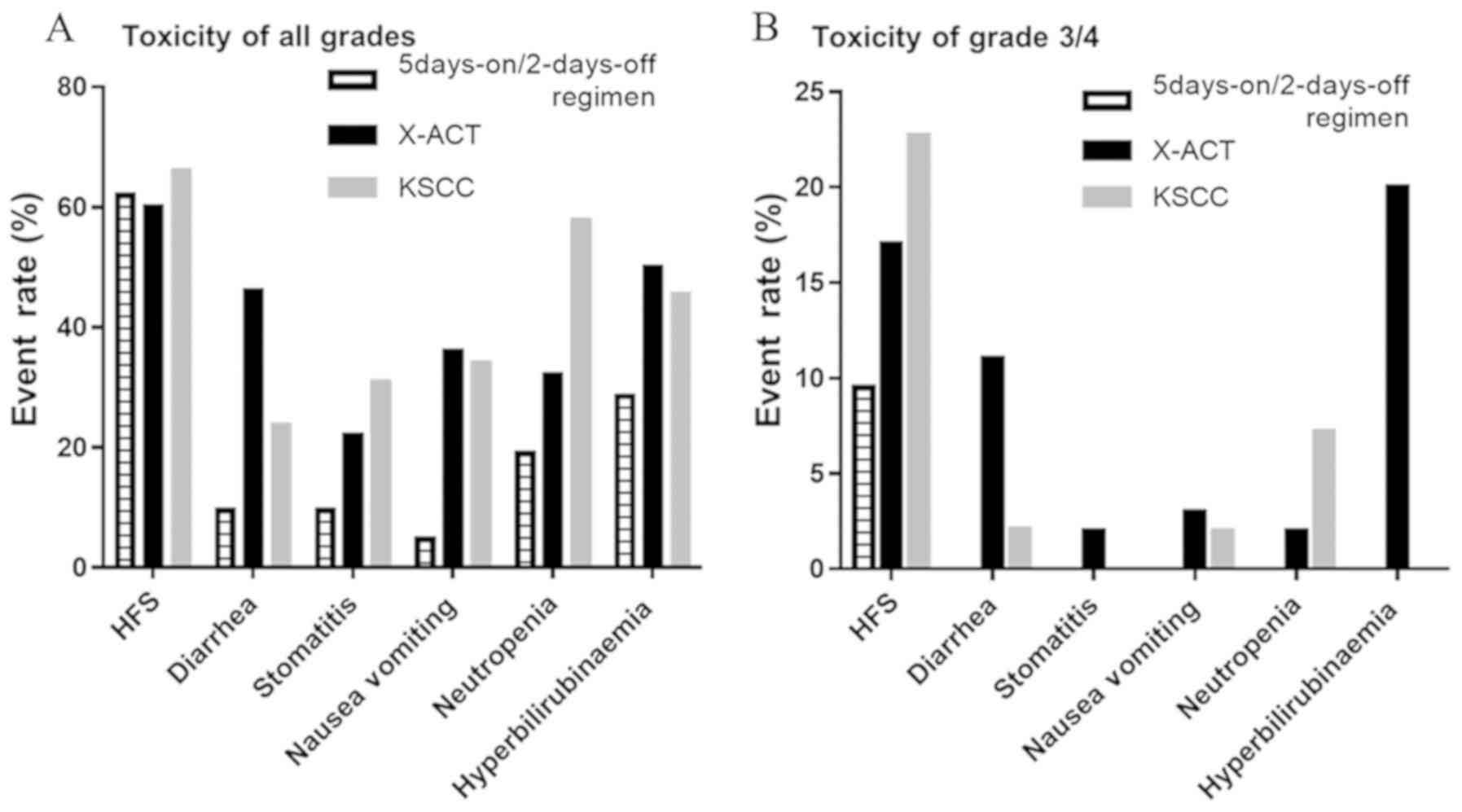
To address the degree of toxicity occurring under
the 5-days-on/2-days-off regimen, the toxicities of the
5-days-on/2-days-off regimen were compared with those of other
larger studies, including the Xeloda in Adjuvant Colon Cancer
Therapy (X-ACT) (5) and Kyushu Study
group of Clinical Cancer (KSCC)0803(7) (Fig. 3).
The X-ACT and KSCC0803 trials were performed using the
2-weeks-on/1-week-off regimen. Toxicities of all grades (Fig. 3A) and G3/4 toxicities (Fig. 3B) were compared among the three
studies. The AEs in the present study, except for HFS, had a lower
frequency and severity than those in the X-ACT and KSCC0803 trials.
In the present study, low-grade HFS occurred at the same frequency
but fewer cases of high-grade HFS were noted as compared with those
in the X-ACT and KSCC0803 trials.
Discussion
The conventional capecitabine regimen for adjuvant
chemotherapy for stage III CRC patients, according to which the
medicine is taken for 14 consecutive days every 21 days, has been
reported to be equivalent to a bolus 5-FU/LV regimen in a previous
study (5). Capecitabine has been
accepted as a standard adjuvant chemotherapy after radical surgery
for patients with stage III CRC, but has considerable side effects.
Twelves et al (5) reported an
occurrence rate of all grades of HFS of 60%, diarrhea of 46%,
nausea and vomiting of 36%, stomatitis of 22%, hyperbilirubinemia
of 50% and neutropenia of 32%. In a Japanese study on adjuvant
capecitabine therapy for CRC, 66% of patients had symptoms of HFS,
23.7% of diarrhea, 34% of nausea and vomiting, 30.9% of stomatitis,
45.4% of hyperbilirubinemia and 57.7% of neutropenia. If these side
effects are suppressed in patients undergoing adjuvant
chemotherapy, their QOL may be improved and the regimen may be
continued, leading to higher efficacy of adjuvant therapy.
In the present cohort under the 5-days-on/2-days-off
regimen, diarrhea occurred in only 9.5% of patients and there were
no G3 or G4 toxicities. The frequency of other side effects also
tended to be lower. One reason for the 5-days-on/2-days-off regimen
being more favorable than the 2-weeks-on/1-week-off regimen was
provided by Pentheroudakis et al (8): Under the 5-days-on/2-days-off regimen,
the time the drug prevails in the blood, presented by the maximum
serum concentration Cmax or area under the curve for
5-FU, is extended as long as possible. Furthermore, it is desirable
as an administration method, as no accumulation of the metabolite
of 5-FU occurs (8). The emergence of
AEs was then compared between the X-ACT study, the KSCC0803 study
and the present 5-days-on/2-days-off regimen. All-grade toxicity of
HFS was unmodulated by the change in administration method to the
5-days-on/2-days-off regimen. However, G3/4 toxicity of HFS and
other toxicities were reduced under the 5-days-on/2-days-off
regimen. It appears that the HFS was more common with the
5-days-on/2-days-off regimen but G3/4 AEs were less common. The
median of the course during which HFS first occurred under the
5-day-on/2-day-off regimen was the third course, while it was the
second course under the conventional regimen. There was no
statistically significant difference regarding the median course of
occurrence of HFS. (P<0.8411). Of note, only the patients in the
experimental group (under the 5-day-on/2-day-off regimen) were
enrolled in the present trial, and their behavior may have been
influenced to continue the treatment, or the behavior of the
doctors may have encouraged them to continue it. However, the
patients in the retrospectively included conventional group did not
participate in any clinical trial and no such bias existed for
them. Therefore, the reason for the 5-day-on/2-day-off regimen
having a better completion rate remains elusive, and a randomized
controlled study is required to address this issue.
A limitation of the present study is that it is a
single-arm preliminary study. The data of the group under the
5-days-on/2-days-off regimen were compared with those from external
groups or the retrospective conventional 2-weeks-on/1-week-off
regimen group. The present analysis included 21 patients in each
cohort, and therefore, the sample size is not sufficiently large
for detection of differences in efficacy between the two groups.
Furthermore, although dose reduction under the 5-days-on/2-days-off
regimen was precisely defined, there was no exact definition of
dose reduction in the retrospectively included conventional regimen
group. The comparison of regimen completion between the two groups
is therefore limited. A randomized study is required to further
assess the feasibility and safety of the 5-days-on/2-days-off
regimen of capecitabine for adjuvant chemotherapy for CRC.
In spite of these limitations, the present study
suggests that toxicities in the 5-days-on/2-days-off regimen were
lower than in the conventional regimen. TTF was also favorable in
the new regimen with a tendency toward good feasibility. The
5-days-on/2-days-off regimen of capecitabine may facilitate longer
continuity of adjuvant chemotherapy for CRC patients. Although 5-FU
monotherapy is the standard regimen for adjuvant chemotherapy, the
combination of 5-FU with oxaliplatin is also an important modality
of adjuvant chemotherapy for colon cancer. If a phase III
randomized trial was to further demonstrate that a modified
5-days-on/2-days-off regimen improves the continuity of adjuvant
chemotherapy, combination therapy of 5-FU with oxaliplatin should
be evaluated in the next step.
Acknowledgements
The authors acknowledge the proofreading of the
manuscript by Mr. Benjamin Phillis at the Clinical Study Support
Center, Wakayama Medical University (Wakayama, Japan).
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YM and SY were responsible for the design and
implementation of the study. YM performed the statistical analysis
and drafted the manuscript. HY made substantial contributions to
the conception, framework and design of the study. KM, KT, YM, HI,
YN, DM, MO and YK made substantial contributions to clinical data
collection and clinical management. All authors were involved in
drafting, reading and approving the manuscript, and all agree to be
accountable for the results. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was performed in accordance with the
Declaration of Helsinki and the Ethical Guidelines for Clinical
Research and has been approved by the Ethics Committee of Wakayama
Medical University (Wakayama, Japan). Written informed consent was
obtained from all patients prior to enrollment in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cassidy J, Twelves C, Van Cutsem E, Hoff
P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, et
al: First-line oral capecitabine therapy in metastatic colorectal
cancer: A favorable safety profile compared with intravenous
5-fluorouracil/leucovorin. Ann Oncol. 13:566–575. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Reddy GK: Efficacy of adjuvant
capecitabine compared with bolus 5-fluorouracil/leucovorin regimen
in Dukes C colon cancer: Results from the X-ACT trial. Clin
Colorectal Cancer. 4:87–88. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cassidy J, Douillard JY, Twelves C,
McKendrick JJ, Scheithauer W, Bustova I, Johnston PG,
Lesniewski-Kmak K, Jelic S, Fountzilas G, et al: Pharmacoeconomic
analysis of adjuvant oral capecitabine vs intravenous 5-FU/LV in
Dukes' C colon cancer: The X-ACT trial. Br J Cancer. 94:1122–1129.
2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Twelves C, Scheithauer W, McKendrick J,
Seitz JF, Van Hazel G, Wong A, Diaz-Rubio E, Gilberg F and Cassidy
J: Capecitabine versus 5-fluorouracil/folinic acid as adjuvant
therapy for stage III colon cancer: Final results from the X-ACT
trial with analysis by age and preliminary evidence of a
pharmacodynamic marker of efficacy. Ann Oncol. 23:1190–1197.
2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Twelves C, Wong A, Nowacki MP, Abt M,
Burris H III, Carrato A, Cassidy J, Cervantes A, Fagerberg J,
Georgoulias V, et al: Capecitabine as adjuvant treatment for stage
III colon cancer. N Engl J Med. 352:2696–2704. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Twelves CJ: Xeloda in Adjuvant Colon
Cancer Therapy (X-ACT) trial: Overview of efficacy, safety, and
cost-effectiveness. Clin Colorectal Cancer. 6:278–287.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Emi Y, Kakeji Y, Oki E, Saeki H, Ando K,
Kitazono M, Sakaguchi Y, Morita M, Samura H, Ogata Y, et al:
Initial report of KSCC0803: Feasibility study of capecitabine as
adjuvant chemotherapy for stage III colon cancer in Japanese
patients. Int J Clin Oncol. 18:254–259. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pentheroudakis G, Pappas P, Golfinopoulos
V, Fountzilas G, Nikolaidou M, Boumba VA, Vougiouklakis T,
Nikiforidis L, Tzamakou E, Siarabi O, et al: Weekday on-weekend off
oral capecitabine: A phase I study of a continuous schedule better
simulating protracted fluoropyrimidine therapy. Cancer Chemother
Pharmacol. 60:733–739. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. 7th Edition.
Wiley-Blackwell, Chichester. 2009.
|
|
10
|
Benson AB III, Schrag D, Somerfield MR,
Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J,
McAllister P, Van Cutsem E, et al: American Society of Clinical
Oncology recommendations on adjuvant chemotherapy for stage II
colon cancer. J Clin Oncol. 22:3408–3419. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
National Cancer Institute (NCI): Common
Terminology Criteria for Adverse Events (CTCAE). Version 4.0.
http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm.
|
|
13
|
Aaronson NK, Ahmedzai S, Bergman B,
Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman
SB and de Haes JC: The European Organization for Research and
Treatment of Cancer QLQ-C30: A quality-of-life instrument for use
in international clinical trials in oncology. J Natl Cancer Inst.
85:365–376. 1993.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kobayashi K, Takeda F, Teramukai S, Gotoh
I, Sakai H, Yoneda S, Noguchi Y, Ogasawara H and Yoshida K: A
cross-validation of the European Organization for Research and
Treatment of Cancer QLQ-C30. Eur J Cancer. 34:810–815.
1998.PubMed/NCBI View Article : Google Scholar
|