Introduction
Anaplastic thyroid cancer (ATC) is a rare and lethal
type of thyroid cancer. The survival rate of patients with ATC has
not changed markedly over the past 20 years (1). Although clinical trials (randomized and
non-randomized) have been conducted (2-4),
including one with a large cohort of 205 patients, the considerable
improvement in the understanding of the pathogenesis and genetics
of ATC has not notably improved patient outcome (5). According to the American Thyroid
Association guidelines, once a patient is diagnosed with stage IVC
ATC, either systemic treatment and/or palliative treatment,
including best supportive care (BSC), should be immediately
implemented (6). Systemic therapy
trials are time-consuming, and disease progression is quicker than
the trial entry process prior to treatment initiation; therefore,
the patient may not have sufficient time for treatment (7). In 2015, the Japanese Ministry of
Health, Labor and Welfare approved the clinical use of lenvatinib
for the treatment of patients with ATC and differentiated thyroid
cancer (DTC). Therefore, lenvatinib may be used for the prompt
systemic treatment of stage IVC ATC (8). Conversely, when patients treated with
weekly paclitaxel (PTX), palliative radiation, or tracheostomy for
local control manifest disease progression, BSC is administered to
prolong survival. To the best of our knowledge, no studies to date
have compared such cases and their prognoses, or have reported
basic data of such cases. Although ATC is hypothesized to evolve
from DTC, no report has yet proven that ATC originates from DTC
(9,10). The aim of the present study was to
examine whether the clinical course of ATC was different from that
determined using the presence and the shape of calcifications
observed on computed tomography (CT) scans at the time of
diagnosis.
Materials and methods
Patients
A total of 32 patients with pathologically confirmed
stage IVC ATC who were treated at the Kanagawa Cancer Center
(Yokohama, China) between January 2011 and April 2019 were included
in the present study. A total of 16 patients each were included in
the lenvatinib (L) and palliative (P) groups. In the P group, 7
patients were treated with 80 mg/m2 PTX weekly, 2
underwent palliative irradiation, and 2 were subjected to
tracheostomy for local control. Patients treated with PTX were
included in the P group due to insufficient PTX dose for the
evaluation of therapeutic efficacy. Patients in the L group were
treated with lenvatinib alone. The background characteristics of
patients in both groups are shown in Table I. Histopathological diagnosis was
confirmed using core needle biopsy or open biopsy. Post-treatment
disease progression and tumor diameter were assessed using CT scans
every 4 weeks. Blood tests were conducted to determine thyroid
function and the presence of adverse events (AEs). In the L group,
the initial dose of lenvatinib was 24 mg for 12 patients, 14 mg for
3 patients, and 10 mg for 1 patient. A total of 4 patients were
administered a reduced dose due to poor performance status,
malnutrition, and old age (>80 years). The survival curves of
both groups were analyzed using the log-rank test. Tumor
characteristics were classified into three subgroups according to
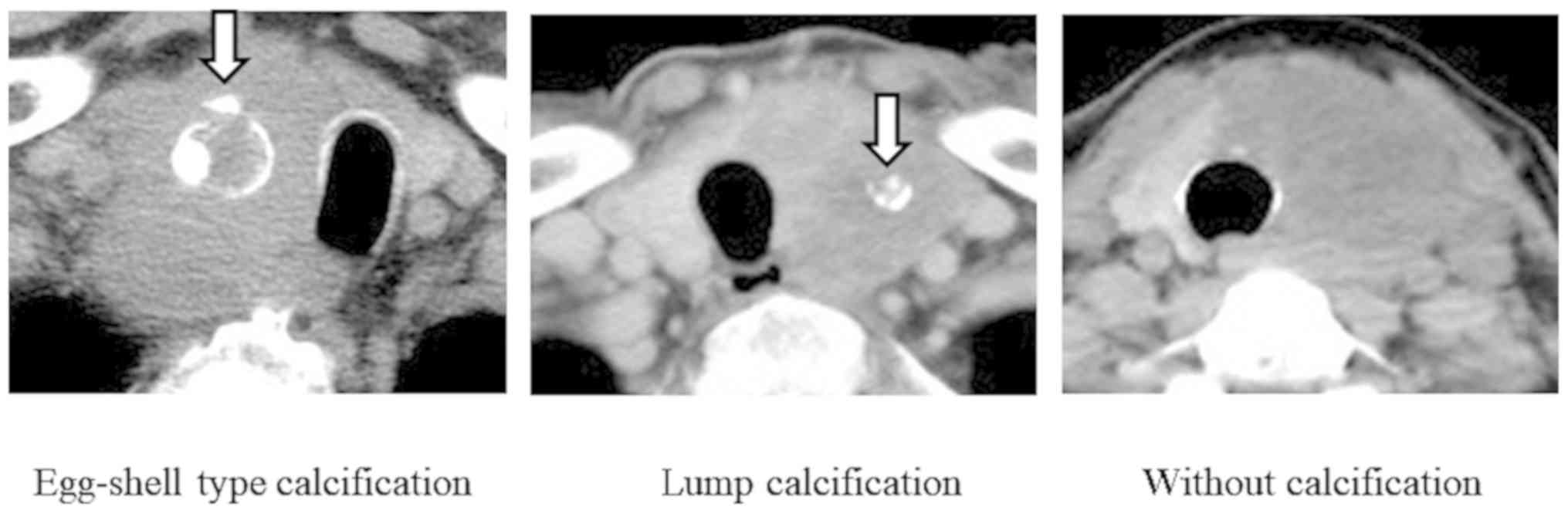
the calcification patterns at the time of diagnosis as follows:
Eggshell calcification, lump calcification, and no calcification
(Fig. 1). Based on this
classification, the results of lenvatinib treatment in cases with
and without calcification were compared.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variables | L group | P group | P-value |
|---|
| N | 16 | 16 | |
| Age, years [mean
(range)] | 73.00
(47.00-89.00) | 72.50
(61.00-86.00) | 0.624 |
| Sex, n (%) | | | 0.722 |
|
Female | 8 (50.0) | 10 (62.5) | |
|
Male | 8 (50.0) | 6 (37.5) | |
| Body weight, kg [mean
(range)] | 57.85
(41.70-88.00) | 45.80
(36.30-76.00) | 0.136 |
| Lung metastasis, n
(%) | 16 (100.0) | 14 (87.5) | 0.484 |
| Other metastasis, n
(%) | 4 (25.0) | 4 (25.0) | 1.000 |
| Calcification, n
(%) | 10 (71.4) | 8 (57.1) | 0.695 |
| Maximum diameter, mm
[mean (range)] | 47.00
(24.00-65.00) | 59.25
(30.00-91.50) | 0.138 |
| FT3, pg/ml [mean
(range)] | 2.03 (1.00-3.19) | 2.22 (1.31-3.06) | 0.57 |
| FT4, ng/dl [mean
(range)] | 1.18 (0.64-1.80) | 1.16 (0.68-1.49) | 0.593 |
| TSH, mIU/l [mean
(range)] | 1.64
(0.01-31.99) | 2.96
(0.09-33.03) | 0.593 |
|
Hypothyroidismb, n (%) | 14 (87.5) | 4 (25.0) | 0.001a |
The Cancer Board of the Kanagawa Cancer Center
approved lenvatinib treatment for ATC. The study protocol was
approved by the Institutional Review Board of the Kanagawa Cancer
Center (no. 49,2016).
Statistical analysis
The overall survival (OS) was calculated with the
Kaplan-Meier method using SPSS software, v.24 (IBM Corp.). The
Kaplan-Meier estimator on the SPSS software was used to calculate
OS and apply the log-rank test. P<0.05 was considered to
indicate statistically significant differences. The OS of the L and
P groups was validated using the log-rank test. The median values
between the two groups were compared using Man-Whitney U test with
statistical significance set at P<0.05. The statistical analyses
were performed using EZR (Saitama Medical Center, Jichi Medical
University, Saitama, Japan), a graphical user interface for R (The
R Foundation for Statistical Computing).
Results
Treatment results
In L group, the median duration of lenvatinib
administration was 107.0 days (range, 30-837 days) (Fig. 2). There were 7 cases (43.8%) of
fistula formation (patients 1-5, 9 and 16), 2 cases (12.5%) of
bleeding (patients 4 and 16), and 1 case (6.3%) of mediastinitis
(patient 5). There were 3 treatment-related deaths: Patient 16
succumbed to hemorrhage from a fistula, patient 4 developed
aspiration pneumonia from a fistula, and patient 5 developed
mediastinitis from a fistula. The remaining 13 deaths were
considered as disease-specific, and some of the patients had other
complications: 2 had brain metastases, 2 had aspiration pneumonia,
and 1 developed suffocation. Patient 15 discontinued lenvatinib
treatment due to grade 4 digestive tract bleeding. Other grade ≥3
or AEs required dose reduction or discontinuation. None of the
patients in the present study had undergone total thyroidectomy for
volume reduction. All patients were diagnosed with stage IVC ATC
and had no history of thyroid surgery. Necrosis of the tumor as
well as of the normal thyroid gland by lenvatinib in the L group
may have caused hypothyroidism.
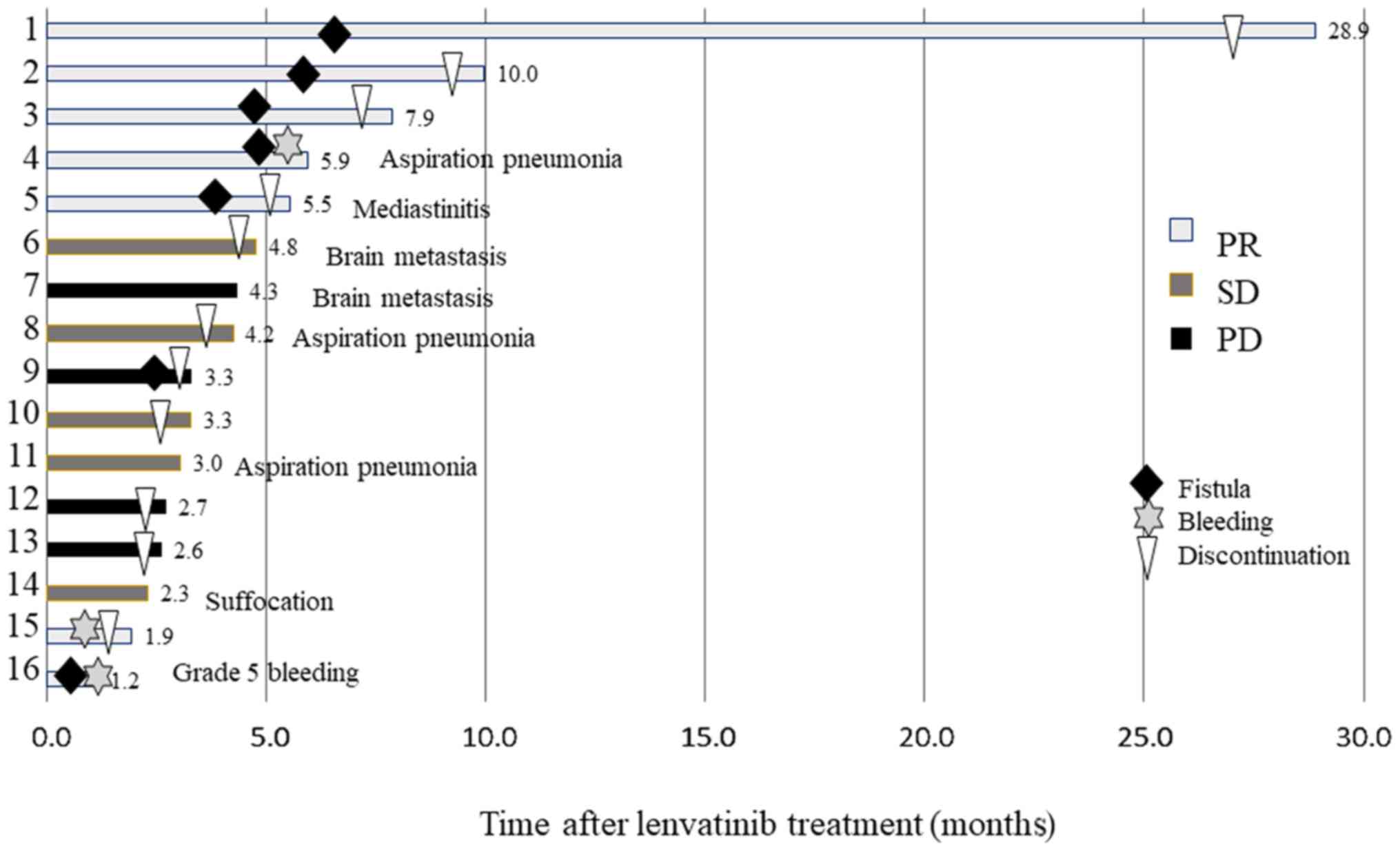 | Figure 2.Treatment progression in 16 patients
with ATC treated only with lenvatinib. Gray box, PR; dark gray box,
SD; bracket box, PD; black diamonds, skin fistula; gray stars,
massive bleeding; triangles, treatment discontinuation. The number
to the right of the box indicates patient survival in months, and
the non-disease related cause of death appears next to it. ATC,
anaplastic thyroid cancer; PR, partial response; SD, stable
disease; PD, progressive disease. |
In the P group, 7 patients (43.8%) were treated with
weekly PTX (80 mg/m2) as previously reported (11), but PTX was only administered 1-7
times. All patients in the P group had progressive disease and the
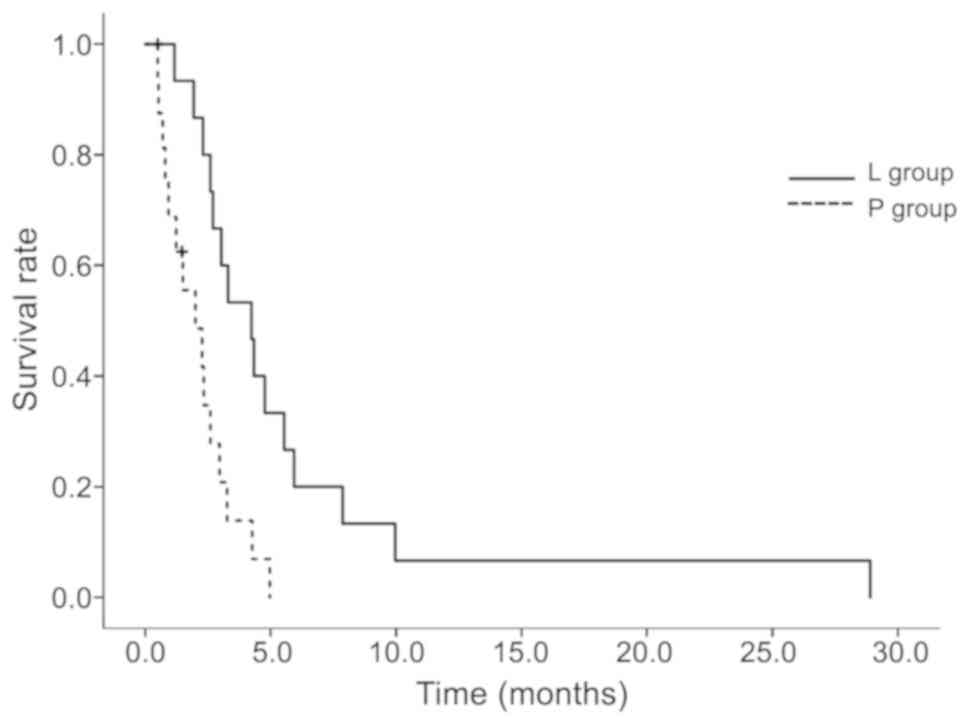
treatment was discontinued. As shown in Table II and Fig. 3, a significantly different median
survival benefit was observed between the L and P groups at 4.2 and
2 months (P=0.00298), respectively.
 | Table IIComparison of survival data between
the L and P groups. |
Table II
Comparison of survival data between
the L and P groups.
| Groups | N | 3-month survival rate
(95% CI) | Median survival
[months (95% CI)] | P-value |
|---|
| L | 16 | 0.600
(0.318-0.797) | 4.2 (2.3-5.5) | 0.00298a |
| P | 16 | 0.211
(0.053-0.440) | 2.0 (0.8-3.0) | |
Antitumor effect
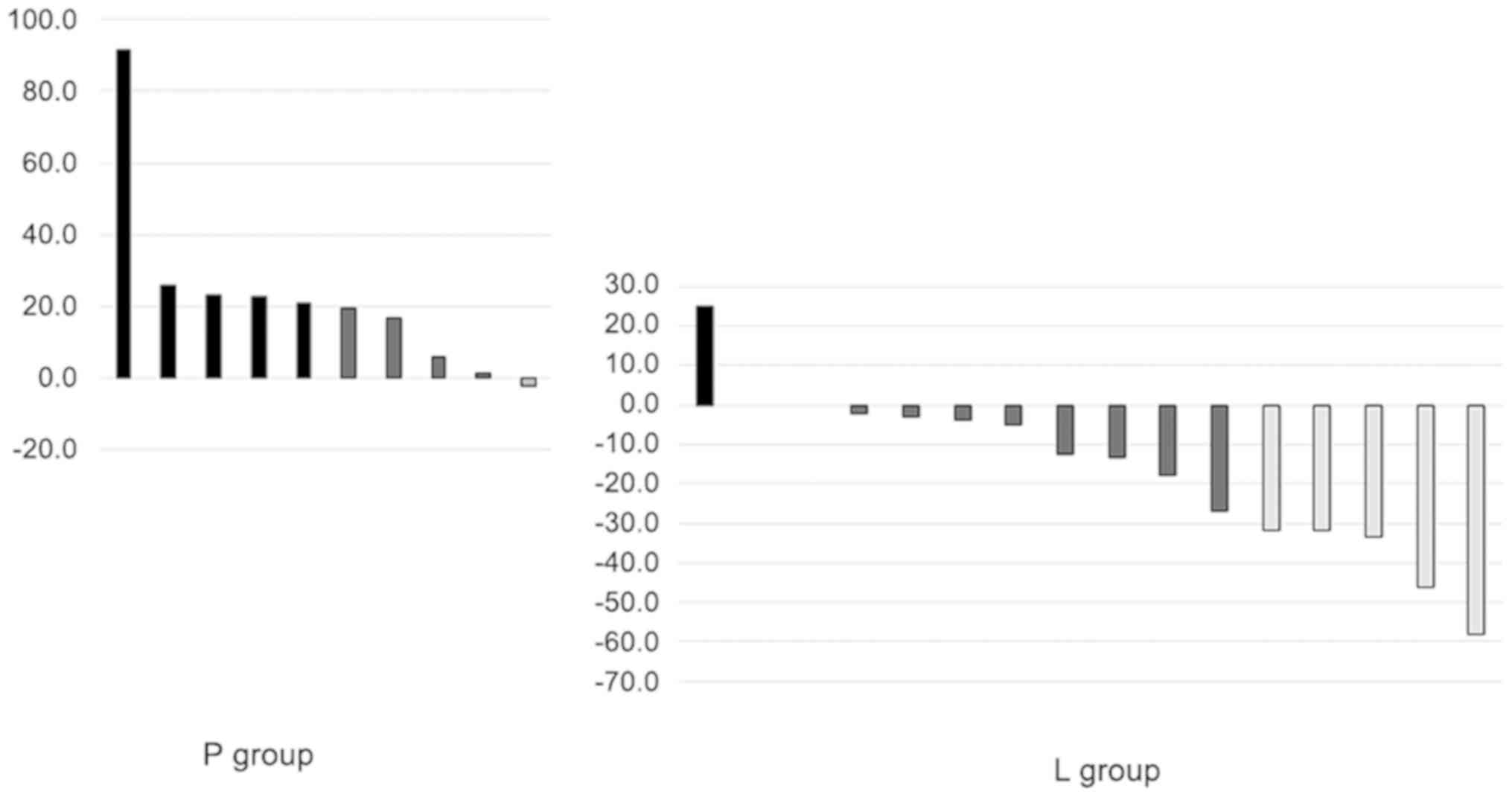
Changes in tumor diameter after first 4 weeks of
treatment are shown in Fig. 4. A
reduction in tumor diameter by ≥30% [clinical partial response
(cPR)] was observed in 5 patients (31.3%) in the L group and none
in the P group. A total of 6 patients in the P group did not
undergo a CT scan 1 month after the treatment and died of BSC
policy. In the L group, the tumor reduction effect of lenvatinib
was observed at either the full or low dose. In addition,
treatment-related hypothyroidism occurred during the course of
lenvatinib and PTX treatment; as a result, 14 patients (87.5%) in
the L group and 4 patients (25.0%) in the P group required thyroid
hormone replacement therapy. When the treatment period exceeded 3
months, particularly in the L group, the thyroid function in
patients was severely compromised; thus, these patients required
thyroid hormone replacement therapy. Although none of the patients
in the present study had undergone total thyroidectomy for volume
reduction, tumor necrosis by lenvatinib, also affecting normal
thyroid gland tissue, caused hypothyroidism.
Difference by calcification
pattern
Regarding tumor characteristics, 11 patients had
tumors displaying an eggshell calcification pattern, 8 had lumpy
calcified tumors, and 9 had non-calcified tumors. In the remaining
4 patients, calcification was found out of the tumor and its
association with the tumor was unknown; therefore, it was excluded
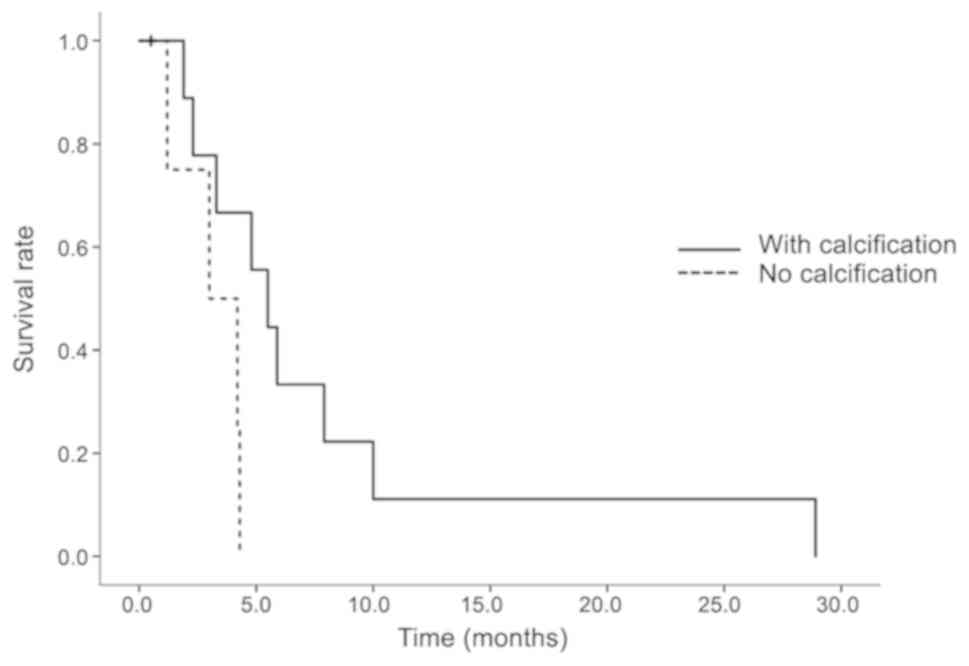
from this analysis. Comparing the treatment results of lenvatinib
with and without calcification, the median OS was 5.5 months for
patients with calcification and 3.0 months for those without
calcification. The difference in the OS curves was statistically
significant (P=0.0473; Fig. 5).
Discussion
The genetic analysis of ATC (12) demonstrated that some ATCs evolve from
PTCs, FTCs and aggressive DTCs; the remaining cases are de
novo carcinomas. An interesting previous study demonstrated
that PTC coexisted with ATC and harbored the same BRAF mutation
(13). Moreover, previous reports
hypothesized that DTC is a pre-existing condition in the majority
of ATC cases (9,10). In fact, it is not uncommon to observe
the coexistence of DTC in ATC pathological tissues (14,15). In
the present study, a co-existing PTC in the biopsied specimen was
detected in 3 patients, and their CT images displayed
calcification. The co-existence of DTCs in ATC surgical specimens
is occasionally observed, but it is difficult to prove pre-existing
DTC. It is desirable to determine the presence of DTC prior to the
development of ATC. In the present study, CT examination at the
time of diagnosis revealed calcification in the primary lesion,
which may have resulted from a thyroid disease, such as DTC or
multinodular goiter. Few studies have reported image findings for
ATC. Takashima et al reported that 58% of ATC patients had
dense calcifications (16). Although
this incidence was lower compared to the 67.9% in the present
study, over half ATCs displayed calcifications. Thus, it may be
hypothesized that pre-existing diseases may result in calcification
in ATC. It was suggested that differentiation may affect lenvatinib
efficacy, and the treatment results of lenvatinib were superior in
patients who had calcifications in the primary lesion. In order to
avoid treatment-related deaths due to fistula formation in all
patients, discontinuation and dose reduction must be considered
before the condition becomes life-threatening.
In a phase II study, the use of lenvatinib in 17
patients with ATC was reported (5,6). The
median progression-free survival was 7.4 months and the median OS
was 10.6 months. The majority of the patients had been pretreated
prior to lenvatinib treatment: 14 patients underwent surgery, 7
patients underwent chemotherapy, and 9 patients received external
irradiation. The present study only compared patients receiving
lenvatinib with those receiving palliative treatment for stage IVC
ATC. Following lenvatinib treatment, 13 patients exhibited a
reduction in tumor size, of whom 5 (31.3%) achieved a cPR; 1
patient displayed an increase in tumor size after 1 month and 2 did
not display any changes. Although this treatment response does not
appear to be a bad result, we believe that extensive tumor
necrosis, AE-related mortality and treatment discontinuation caused
by formation of tumor-skin fistula may severely compromise
treatment outcomes. It is crucial that a median 2.2-month survival
benefit was obtained with lenvatinib compared with palliative
therapy. In addition, it was reported that an initial low dose of
lenvatinib (10, 14 and 20 mg) did not alter the results of DTC
(17). Recently, ATC has been
treated with low-dose lenvatinib, depending on the patient's
condition. In ATC, as the tumor is close to the skin, treatment
should be initiated as soon as possible after confirmation of
diagnosis using core needle biopsy, as a fistula may form where the
tumor is exposed at the biopsy site. As ATC progresses rapidly,
prompt initiation of treatment is required; moreover, it is
necessary to arrest tumor progression and administer a proper dose
to avoid deterioration of the fistula. This is also to avoid
treatment-related deaths due to profuse bleeding from large vessels
located close to the fistula. In the present study, co-existing PTC
was detected in 3 patients, who also displayed calcifications on
the CT scan. It has been reported that DTC coexisted in 35% of
patients with ATC (2) and that ATC
coexists not only with DTC, but also with thyroid diseases, such as
nodular goiter (3). The
calcification patterns identified in our study displayed the
typical characteristics of PTC, and 3 patients had coexisting PTC,
which was detected by core needle biopsy; therefore, transformation
of PTC to ATC was suggested.
In addition to than lenvatinib, BRAF inhibitors
serve as effective therapeutic agents for type I ATC with BRAF
mutation (18). Programmed death-1
checkpoint blockers may be also efficacious in patients with
aggressive forms of thyroid cancer (19). However, these agents are not approved
for use in Japan. The combination of PLX4720 and dasatinib induced
apoptosis, increased immune cell infiltration and reduced tumor
volume in a preclinical model of ATC, and may be expected to be
approved as treatment for patients with ATC in the future (20). The results of the present study may
serve as a basis for progress in ATC treatment, including
combination treatment with lenvatinib.
In conclusion, the L group demonstrated a median
survival benefit of 2.2 months compared with the P group in stage
IVC ATC. Although cPR was confirmed in 5 patients who received
lenvatinib, 2 patients died. These results suggest that an
appropriate lenvatinib dose reduction is necessary.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
HI and ST designed the study. NS and DM performed
analysis and data interpretation, particularly statistical
analysis. HN and KM contributed to data acquisition. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The Chemotherapy Committee of Kanagawa Cancer Center
(Yokohama, Japan), approved this regimen of lenvatinib for use in
patients with ATC. The cancer board of our Kanagawa Cancer Center
also approved lenvatinib treatment, including surgery, for patients
with ATC. All patients provided a comprehensive consent form
stating that personal data may be used for academic presentation or
paper presentation, while ensuring complete anonymity, prior to
receiving the treatment.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
financial interests. An abstract (submission ID #34) covering this
article was presented at the WCTC meeting held in Rome, Italy, on
June 20-22, 2019.
Authors' information
The first author HI is an endocrine surgeon working
at the Kanagawa Cancer Center and has extensive experience on
several surgeries for advanced thyroid cancer, as well as ATC
treatment.
References
|
1
|
Oh EM, Lee KE, Kwon H, Kim EY, Bae DS and
Youn YK: Analysis of patients with anaplastic thyroid cancer
expected to have curative surgery. J Korean Surg Soc. 83:123–129.
2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shimaoka K, Schoenfeld DA, DeWys WD,
Creech RH and DeConti R: A randomized trial of doxorubicin versus
doxorubicin plus cisplatin in patients with advanced thyroid
carcinoma. Cancer. 56:2155–2160. 1985.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Mooney CJ, Nagaiah G, Fu P, Wasman JK,
Cooney MM, Savvides PS, Bokar JA, Dowlati A, Wang D, Agarwala SS,
et al: A phase II trial of fosbretabulin in advanced anaplastic
thyroid carcinoma and correlation of baseline serum-soluble
intracellular adhesion molecule-1 with outcome. Thyroid.
19:233–240. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sosa JA, Elisei R, Jarzab B, Balkissoon J,
Lu SP, Bal C, Marur S, Gramza A, Yosef RB, Gitlitz B, et al:
Randomized safety and efficacy study of fosbretabulin with
paclitaxel/carboplatin against anaplastic thyroid carcinoma.
Thyroid. 24:232–240. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bisof V, Rakusic Z and Despot M: Treatment
of patients with anaplastic thyroid cancer during the last 20
years: Whether any progress has been made? Eur Arch
Otorhinolaryngol. 272:1553–1567. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Smallridge RC, Ain KB, Asa SL, Bible KC,
Brierley JD, Burman KD, Kebebew E, Lee NY, Nikiforov YE, Rosenthal
MS, et al: American thyroid association guidelines for management
of patients with anaplastic thyroid cancer. Thyroid. 22:1104–1139.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwasaki H, Yamazaki H, Suganuma N,
Nakayama H, Toda S and Masudo K: Role of tyrosine kinase inhibitor
thyrapy in anaplastic thyroid cancer. Int J Recent Adv
Multidisciplinary Res. 05:4270–4274. 2018.
|
|
8
|
Iwasaki H, Yamazaki H, Takasaki H,
Suganuma N, Nakayama H, Toda S and Masudo K: Lenvatinib as a novel
treatment for anaplastic thyroid cancer: A retrospective study.
Oncol Lett. 16:7271–7277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Venkatesh YS, Ordonez NG, Schultz PN,
Hickey RC, Goepfert H and Samaan NA: Anaplastic carcinoma of the
thyroid. A clinicopathologic study of 121 cases. Cancer.
66:321–330. 1990.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kebebew E, Greenspan FS, Clark OH, Woeber
KA and McMillan A: Anaplastic thyroid carcinoma. Treatment outcome
and prognostic factors. Cancer. 103:1330–1335. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Onoda N, Sugino K, Higashiyama T, Kammori
M, Toda K, Ito K, Yoshida A, Suganuma N, Nakashima N, Suzuki S, et
al: The safety and efficacy of weekly paclitaxel administration for
anaplastic thyroid cancer patients: A nationwide prospective study.
Thyroid. 26:1293–1299. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Pozdeyev N, Gay LM, Sokol ES, Hartmaier R,
Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan AC, et
al: Genetic analysis of 779 advanced differentiated and anaplastic
thyroid cancers. Clin Cancer Res. 24:3059–3068. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Begum S, Rosenbaum E, Henrique R, Cohen Y,
Sidransky D and Westra WH: BRAF mutations in anaplastic thyroid
carcinoma: Implications for tumor origin, diagnosis and treatment.
Mod Pathol. 17:1359–1363. 2004.PubMed/NCBI
|
|
14
|
McIver B, Hay ID, Giuffrida DF, Dvorak CE,
Grant CS, Thompson GB, van Heerden JA and Goellner JR: Anaplastic
thyroid carcinoma: A 50-year experience at a single institution.
Surgery. 130:1028–1034. 2001.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pacheco-Ojeda LA, Martinez AL and Alvarez
M: Anaplastic thyroid carcinoma in ecuador: Analysis of prognostic
factors. Int Surg. 86:117–121. 2001.PubMed/NCBI
|
|
16
|
Takashima S, Morimoto S, Ikezoe J, Takai
S, Kobayashi T, Koyama H, Nishiyama K and Kozuka T: CT evaluation
of anaplastic thyroid carcinoma. AJR Am J Roentgenol.
154:1079–1085. 1990.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yamazaki H, Iwasaki H, Takasaki H,
Suganuma N, Sakai R, Masudo K, Nakayama H, Rino Y and Masuda M:
Efficacy and tolerability of initial low-dose lenvatinib to treat
differentiated thyroid cancer. Medicine (Baltimore).
98(e14774)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Subbiah V, Kreitman RJ, Wainberg ZA, Cho
JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME,
Urbanowitz G, et al: Dabrafenib and trametinib treatment in
patients with locally advanced or metastatic BRAF V600-mutant
anaplastic thyroid cancer. J Clin Oncol. 36:7–13. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bastman JJ, Serracino HS, Zhu Y, Koenig
MR, Mateescu V, Sams SB, Davies KD, Raeburn CD, McIntyre RC Jr,
Haugen BR, et al: Tumor-Infiltrating T cells and the PD-1
checkpoint pathway in advanced differentiated and anaplastic
thyroid cancer. J Clin Endocrinol Metab. 101:2863–2873.
2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vanden Borre P, Gunda V, McFadden DG,
Sadow PM, Varmeh S, Bernasconi M, Parangi S, et al: Combined
BRAF(V600E)-and SRC-inhibition induces apoptosis, evokes an immune
response and reduces tumor growth in an immunocompetent orthotopic
mouse model of anaplastic thyroid cancer. Oncotarget. 5:3996–4010.
2014.PubMed/NCBI View Article : Google Scholar
|