Several tumor markers and biomarkers, both tissue-
and serum-based, are currently used in the management of patients
with breast cancer (1-4),
among which, carbohydrate antigen 15-3 (CA 15-3) is considered a
specific tumor marker for breast cancer. At present, the main
utility of CA 15-3 is monitoring therapy in patients with advanced
breast cancer, especially in women with non-evaluable disease. Most
expert panels advise against the routine use of CA 15-3 in the
surveillance of asymptomatic patients who have undergone surgery
for breast cancer (5).
Systemic sclerosis (SSc) is a multi-system
autoimmune disorder characterized by autoantibody production,
endothelial damage with obliterative microvascular disease,
inflammation, and fibrosis affecting the skin and internal organs
(6,7). Cardiopulmonary involvement is a
common manifestation in SSc, which presents as either interstitial
lung disease (ILD) or pulmonary arterial hypertension (8), and is currently the leading cause of
disease-related morbidity and mortality in patients with
scleroderma (9). The most studied
and characterized biomarker for ILD is Krebs von den Lungen 6
(KL-6) (10,11). CA 15-3 is the shed or soluble form
of MUC-1 protein. MUC1 is strongly expressed by atypical and/or
regenerating type II pneumocytes in tissue sections obtained from
patients with ILDs (12-14).
Although CA 15-3 has been investigated as a biomarker in SSc-ILD,
its role remains unclear (15-17).
Herein, we report a case of coexisting SSc and recurrent breast
cancer who showed improvement in high CA 15-3 levels with
amelioration of ILD without any systemic cancer treatment.
A 60-year-old woman underwent mastectomy with
axillary lymph node dissection at JA Hiroshima General Hospital
(Hatsukaichi, Japan) in October 2014 after preoperative
chemotherapy (four cycles of docetaxel and trastuzumab, followed by
four cycles of cyclophosphamide, epirubicin, and fluorouracil) for
estrogen receptor-negative, HER2-positive right breast invasive
ductal cancer, T2N1M0 stage IIB (18). Postoperative radiation therapy with
50 Gy in 25 fractions to the supraclavicular lymph nodes and chest
wall was performed, followed by 14 cycles of 3-weekly
trastuzumab.
At 63 years old, contrast-enhanced computed
tomography (CT) performed as postoperative follow-up indicated
brain metastasis in the right occipital lobe without liver, lung,
or bone metastasis. She underwent γ-knife radiosurgery (20 Gy)
followed by the administration of perutuzumab, trastuzumab, and
weekly paclitaxel. After four cycles of perutuzumab, trastuzumab,
and weekly paclitaxel, she experienced shortness of breath with
minimal exertion, so the fifth course was canceled. CT revealed
ground-glass opacities and linear shadows in the peripheral lower
lobes of both lungs (Fig. 1).
Although the development of lung involvement associated with breast
cancer such as carcinomatous lymphangitis was initially suspected,
because of the increase in CA 15-3, we investigated other possible
causes of ILD (Fig. 2). From only
CT image, the possibility of interstitial lung disease due to
trastuzumab or pertuzumab cannot be ruled out (19). Bilateral sclerodactyly and facial
skin thickness were found on clinical examination without a history
of Raynaud's phenomenon and the finding of nail fold bleeding. A
test for anti-nuclear antibodies with a nucleolar pattern was
positive, at a titer of 1:320. Anti-double stranded DNA antibody,
specific antibodies against centromere, SSA/SSB, Scl-70, RNP, and
RNA polymerase III were negative. Pulmonary function tests showed a
severely reduced %VC of 50.8%, indicating restrictive ventilatory
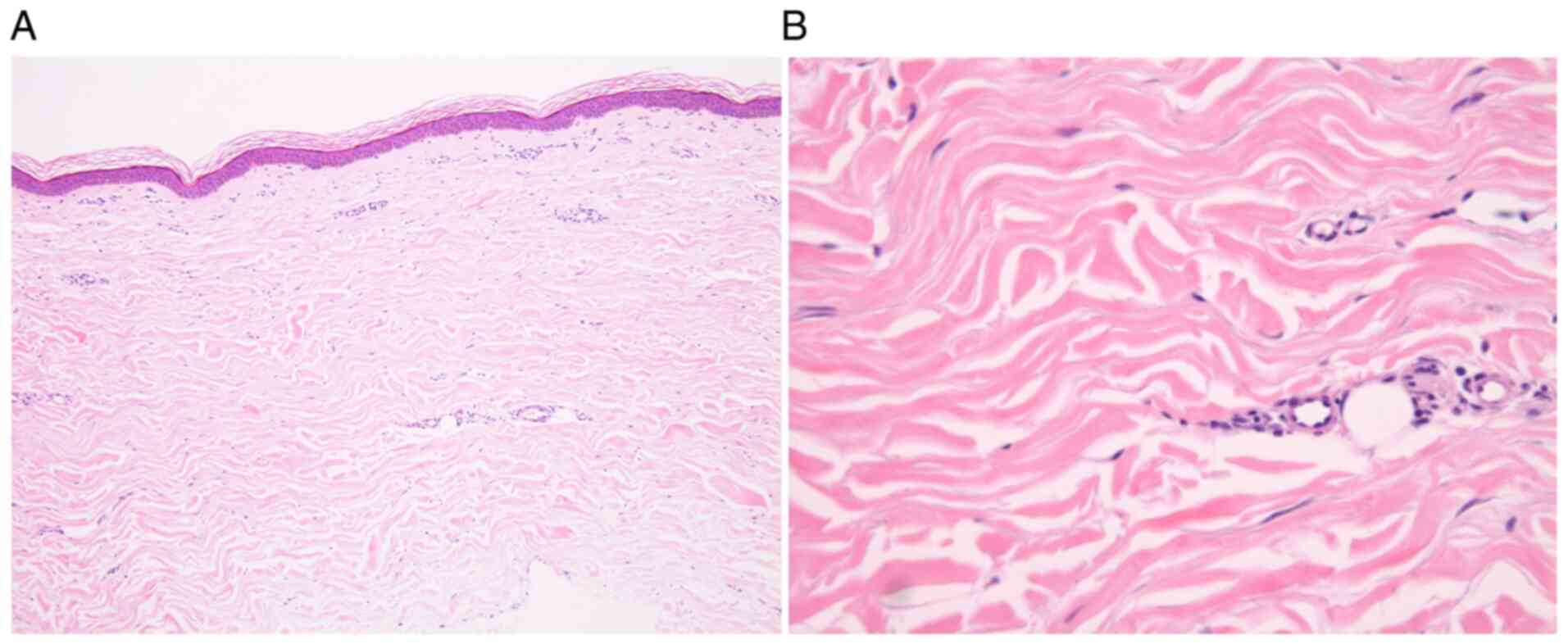
impairment. Skin biopsies taken from the left index finger base and
extension side of the left elbow demonstrated increased thickness
of the dermis composed of broad and sclerotic collagen bundles
extending to the underlying subcutis without inflammatory cell
infiltration in hematoxylin and eosin stained-samples. These
findings were consistent with the late stage of scleroderma
(Fig. 3). From these findings, the
diagnosis of SSc-ILD was made according to the diagnostic criteria
for SSc proposed by the Ministry of Health, Labour and Welfare of
Japan. The treatment for recurrent breast cancer was discontinued,
and combination prednisone (PSL) (15 mg/day) and intravenous
cyclophosphamide (IVCY) (500 mg/4 weeks) therapy was administered
for induction treatment of SSc-ILD. PSL was tapered and
discontinued at 1 year and IVCY was given five times in total. At 6
months after the start of treatment, her symptoms, including cough
and dyspnea, had improved. CA 15-3 and KL-6 levels decreased
simultaneously, reflecting the therapeutic effect (Fig. 2), and CT showed improvement in the
ground-glass opacities in the peripheral lower lobes of both lungs
as compared with those before treatment (Fig. 4). This patient is receiving
treatment for SSc-ILD. The patient's SSc-ILD has not worsening and
her breast cancer has not recurred despite not receiving treatment
for four years.
SSc is a devastating disease of unknown etiology
that is characterized by systemic, immunological, vascular, and
fibrotic abnormalities and a heterogeneous clinical course.
Fibrosis, the hallmark of the disease, can affect the skin and
internal organs, including lung (20). As is well known, pulmonary
involvement is one of the most important features of SSc and often
the leading cause of exitus. SSc-ILD is one of the most severe
complications and is the main cause of SSc-related deaths (9,21);
however, review of contemporary literature suggests improved
survival among patients with SSc-ILD due to more aggressive
monitoring and treatment (22,23).
In clinical trials of SSc-ILD, change in forced vital capacity
(FVC) is commonly used as a primary outcome measure, as low FVC
predicts morbidity and mortality (24). Two landmark clinical trials, SLS-I
(25) and SLS-II (26), established cyclophosphamide and
mycophenolate mofetil as disease modifying therapies for SSc
patients with active ILD.
Taxanes, as well as other antineoplastic agents,
have many toxic effects. Therefore, whether the etiology of the
present case is drug-induced remains unclear. Taxane-induced
scleroderma-like skin changes were first reported in 1995, and
clinical characteristics include preceding edema, absence of
Raynaud's phenomenon, and negative scleroderma-specific
autoantibodies (46-49).
The clinical course is refractory to treatment and commonly
progressive even after discontinuation of the trigger drugs
(50). However, unlike the present
study, previous reports showed mainly skin disorders without ILD.
In addition, the positivity of anti-nuclear antibodies with a
nucleolar pattern in this case, which suggested the existence of
SSc-specific antibodies against RNA polymerase III, Th/To, U3-RNP,
and PM-Scl, helped us determine the diagnosis of SSc regardless of
taxane exposure (51).
CA 15-3 is the shed or soluble form of MUC-1
protein. MUC1 is a transmembrane mucin with marked overexpression
in human breast cancers as compared with that in normal ductal
breast epithelial cells (52). On
the other hand, MUC1 is strongly expressed by atypical and/or
regenerating type II pneumocytes in tissue sections obtained from
patients with ILDs (12-14).
Serum levels of KL-6, an N-terminal subunit of MUC-1 protein,
increases in the acute exacerbation of idiopathic pulmonary
fibrosis (IPF). Serum levels of KL-6 correlate with IPF severity
and prognosis (53). As both KL-6
and CA 15-3 exist in different positions of MUC1(54), CA 15-3 may retain significant
potential as an alternative biomarker for KL-6 in fibrotic lung
diseases (16-18,55).
In primary breast cancer, various studies have demonstrated that
elevated serum CA15-3 values at diagnosis are associated with
higher breast cancer stage, tumor size, positive axillary lymph
nodes, and worse overall survival and disease-free survival
(56-60).
In metastatic breast cancer, CA15-3 was measured serially in a
number of studies assessing their applications in early detection
of disease progression and monitoring therapy response (61-65).
On the other hand, CA15-3 was previously shown to be elevated in
serum of SSc-ILD patients and was associated with severe ILD
measured by fibrosis on HRCT, decreased FVC and DLCO and the
presence of dyspnoea (16,66). In a study performed by Celeste
et al on 221 SSc patients, among which 168 with ILD, CA15-3
serum levels were found to correlate with the extent of fibrosis
detected on HRCT as well as to be predictive for progression-free
survival, with progression being defined by a decline in either FVC
or DLCO (17). The present study
suggested that oncologists treating the patients with breast cancer
should know CA15-3 is also associated with the condition of ILD.
The concentration of some tumor-associated antigens (TAA) such as
CA19-9 and CA125 were reported to be elevated in the sera of
patients with SSc or systemic lupus erythematosus in comparison to
healthy subjects (67). Because of
public insurance coverage, the number of TAA for patients with
breast cancer that could be measured at one time is limited. CEA
could be measured for patients with breast cancer at the same time.
In this patient, CEA did not show outlier or abnormal changes.
Meanwhile, KL-6 has been reported as a tumor marker
in not only lung cancer, but also gastrointestinal, hepatic,
pancreatic, and breast cancers (68,69).
Kohno, the developer of KL-6 monoclonal antibody, noted that the
serum levels of KL-6 mucin were elevated in patients with
pulmonary, breast, and pancreatic adenocarcinomas (12). Elevation of KL-6 mucin in serum is
significantly associated with the behavior of breast cancer
(70) or lung cancer (68). Immunohistochemical analyses have
clarified KL-6 mucin's clinicopathological significance in
digestive organ cancer tissues. As the expression profile and
clinicopathological significance of KL-6 mucin differ among each
organ or disease, the biological role of KL-6 mucin might have a
different importance in each location and state (68). Thus, the nature of KL-6 mucin
remains unclear, and further investigations of KL-6 mucin in cancer
are expected in the future.
In conclusion, serum levels of CA 15-3 correlated
with the condition of SSc-ILD in a patient with recurrent breast
cancer. This case suggests the importance of considering a
differential diagnosis including ILD concurrently while screening
for the progression of recurrent breast cancer when encountering
patients with breast cancer and elevated levels of CA 15-3.
The authors would like to thank Mrs. Takamoto and
Mrs. Nishimoto (Nursing Department, Hiroshima General Hospital) for
their special help with breast cancer nursing care.
Funding: No funding was received.
All data generated or analyzed during this study are
included in this published article.
MO wrote the draft and critically revised the
manuscript for important intellectual content. MO performed
surgical and post-operative treatment. MO, YK, TS and KK
contributed to the conception of the work, and interpreted and
revised the results of the CT included in this report. YY treated
the patient for SSc-ILD. YD diagnosed the disease pathologically.
YY, SM, AT, AO, IN, MS, KI, MW, and YD collected and analyzed both
the clinical laboratory and histopathological data. MO and YY
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Not applicable.
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
The authors declare that they have no competing
interests.
|
1
|
Sturgeon CM, Duffy MJ, Stenman UH, Lilja
H, Brünner N, Chan DW, Babaian R, Bast RC Jr, Dowell B, Esteva FJ,
et al: National academy of clinical biochemistry laboratory
medicine practice guidelines for use of tumor markers in
testicular, prostate, colorectal, breast, and ovarian cancers. Clin
Chem. 54:e11–e79. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Duffy MJ: Biochemical markers in breast
cancer: Which ones are clinically useful? Clin Biochem. 34:347–352.
2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Duffy MJ: Predictive markers in breast and
other cancers: A review. Clin Chem. 51:494–503. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Duffy MJ, Walsh S, McDermott EW and Crown
J: Biomarkers in breast cancer: Where Are we and where are we
going? Adv Clin Chem. 71:1–23. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Duffy MJ, Evoy D and McDermott EW: CA
15-3: Uses and limitation as a biomarker for breast cancer. Clin
Chim Acta. 411:1869–1874. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Silver RM: Clinical aspects of systemic
sclerosis (scleroderma). Ann Rheum Dis. 50 (Suppl 4):S854–S861.
1991.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Varga J and Abraham D: Systemic sclerosis:
A prototypic multisystem fibrotic disorder. J Clin Invest.
117:557–567. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Wells AU, Steen V and Valentini G:
Pulmonary complications: One of the most challenging complications
of systemic sclerosis. Rheumatology (Oxford). 48 (Suppl
3):iii40–iii44. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Steen VD and Medsger TA: Changes in causes
of death in systemic sclerosis, 1972-2002. Ann Rheum Dis.
66:940–944. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Prasse A and Müller-Quernheim J:
Non-invasive biomarkers in pulmonary fibrosis. Respirology.
14:788–795. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ishikawa N, Hattori N, Yokoyama A and
Kohno N: Utility of KL-6/MUC1 in the clinical management of
interstitial lung diseases. Respir Investig. 50:3–13.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kohno N, Akiyama M, Kyoizumi S, Hakoda M,
Kobuke K and Yamakido M: Detection of soluble tumor-associated
antigens in sera and effusions using novel monoclonal antibodies,
KL-3 and KL-6, against lung adenocarcinoma. Jpn J Clin Oncol.
18:203–216. 1988.PubMed/NCBI
|
|
13
|
Tanaka S, Hattori N, Ishikawa N, Shoda H,
Takano A, Nishino R, Okada M, Arihiro K, Inai K, Hamada H, et al:
Krebs von den Lungen-6 (KL-6) is a prognostic biomarker in patients
with surgically resected nonsmall cell lung cancer. Int J Cancer.
130:377–387. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yamasaki H, Ikeda S, Okajima M, Miura Y,
Asahara T, Kohno N and Shimamoto F: Expression and localization of
MUC1, MUC2, MUC5AC and small intestinal mucin antigen in pancreatic
tumors. Int J Oncol. 24:107–113. 2004.PubMed/NCBI
|
|
15
|
Ricci A, Mariotta S, Bronzetti E, Bruno P,
Vismara L, De Dominicis C, Laganà B, Paone G, Mura M, Rogliani P,
et al: Serum CA 15-3 is increased in pulmonary fibrosis.
Sarcoidosis Vasc Diffuse Lung Dis. 26:54–63. 2009.PubMed/NCBI
|
|
16
|
Valerio Marzano A, Morabito A, Berti E and
Caputo R: Elevated circulating CA 15.3 levels in a subset of
systemic sclerosis with severe lung involvement. Arch Dermatol.
134(645)1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Celeste S, Santaniello A, Caronni M,
Franchi J, Severino A, Scorza R and Beretta L: Carbohydrate antigen
15.3 as a serum biomarker of interstitial lung disease in systemic
sclerosis patients. Eur J Intern Med. 24:671–676. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Greene FL: Breast tumours. In: TNM
classification of malignant tumours. Sobin LH, Gospodarowicz MK and
Wittekind C (eds). 7th edition. Wiley-Blackwell, Oxford, pp181-193,
2009.
|
|
19
|
Hackshaw MD, Danysh HE, Singh J, Ritchey
ME, Ladner A, Taitt C, Camidge DR, Iwata H and Powell CA: Incidence
of pneumonitis/interstitial lung disease induced by HER2-targeting
therapy for HER2-positive metastatic breast cancer. Breast Cancer
Res Treat. 183:23–39. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Varga J: Systemic sclerosis: An update.
Bull NYU Hosp Jt Dis. 66:198–202. 2008.PubMed/NCBI
|
|
21
|
Tyndall AJ, Bannert B, Vonk M, Airò P,
Cozzi F, Carreira PE, Bancel DF, Allanore Y, Müller-Ladner U,
Distler O, et al: Causes and risk factors for death in systemic
sclerosis: A study from the EULAR scleroderma trials and research
(EUSTAR) database. Ann Rheum Dis. 69:1809–1815. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Volkmann ER and Fischer A: Update on
morbidity and mortality in systemic sclerosis-related interstitial
lung disease. J Scleroderma Relat Disord. 6:11–20. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rubio-Rivas M, Royo C, Simeón CP, Corbella
X and Fonollosa V: Mortality and survival in systemic sclerosis:
Systematic review and meta-analysis. Semin Arthritis Rheum.
44:208–219. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Steen VD, Conte C, Owens GR and Medsger TA
Jr: Severe restrictive lung disease in systemic sclerosis.
Arthritis Rheum. 37:1283–1289. 1994.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tashkin DP, Elashoff R, Clements PJ,
Goldin J, Roth MD, Furst DE, Arriola E, Silver R, Strange C,
Bolster M, et al: Cyclophosphamide versus placebo in scleroderma
lung disease. N Engl J Med. 354:2655–2666. 2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tashkin DP, Roth MD, Clements PJ, Furst
DE, Khanna D, Kleerup EC, Goldin J, Arriola E, Volkmann ER, Kafaja
S, et al: Mycophenolate mofetil versus oral cyclophosphamide in
scleroderma-related interstitial lung disease (SLS II): A
randomised controlled, double-blind, parallel group trial. Lancet
Respir Med. 4:708–719. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Colaci M, Giuggioli D, Vacchi C, Lumetti
F, Iachetta F, Marcheselli L, Federico M and Ferri C: Breast cancer
in systemic sclerosis: Results of a cross-linkage of an Italian
rheumatologic center and a population-based cancer registry and
review of the literature. Autoimmun Rev. 13:132–137.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abu-Shakra M, Guillemin F and Lee P:
Cancer in systemic sclerosis. Arthritis Rheum. 36:460–464.
1993.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Szekanecz É, Szamosi S, Horváth Á, Németh
Á, Juhász B, Szántó J, Szücs G and Szekanecz Z: Malignancies
associated with systemic sclerosis. Autoimmun Rev. 11:852–855.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Scope A, Sadetzki S, Sidi Y, Barzilai A,
Trau H, Kaufman B, Catane R and Ehrenfeld M: Breast cancer and
scleroderma. Skinmed. 5:18–24. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hill CL, Nguyen AM, Roder D and
Roberts-Thomson P: Risk of cancer in patients with scleroderma: A
population based cohort study. Ann Rheum Dis. 62:728–731.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Maria ATJ, Partouche L, Goulabchand R,
Rivière S, Rozier P, Bourgier C, Le Quellec A, Morel J, Noël D and
Guilpain P: Intriguing relationships between cancer and systemic
sclerosis: Role of the immune system and other contributors. Front
Immunol. 9(3112)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Straub RH, Zeuner M, Lock G, Schölmerich J
and Lang B: High prolactin and low dehydroepiandrosterone sulphate
serum levels in patients with severe systemic sclerosis. Br J
Rheumatol. 36:426–432. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang M, Wu X, Chai F, Zhang Y and Jiang J:
Plasma prolactin and breast cancer risk: A meta-analysis. Sci Rep.
6(25998)2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li CI, Daling JR, Tang MT, Haugen KL,
Porter PL and Malone KE: Use of antihypertensive medications and
breast cancer risk among women aged 55 to 74 years. JAMA Intern
Med. 173:1629–1637. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Gómez-Acebo I, Dierssen-Sotos T,
Palazuelos C, Pérez-Gómez B, Lope V, Tusquets I, Alonso MH, Moreno
V, Amiano P, Molina de la Torre AJ, et al: The use of
antihypertensive medication and the risk of breast cancer in a
case-control study in a spanish population: The MCC-spain study.
PLoS One. 11(e0159672)2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bernal-Bello D, García de Tena J,
Simeón-Aznar C and Fonollosa-Pla V: Systemic sclerosis, breast
cancer and calcium channel blockers: A new player on the scene?
Autoimmun Rev. 13:880–881. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Brasky TM, Krok-Schoen JL, Liu J,
Chlebowski RT, Freudenheim JL, Lavasani S, Margolis KL, Qi L,
Reding KW, Shields PG, et al: Use of calcium channel blockers and
breast cancer risk in the women's health initiative. Cancer
Epidemiol Biomarkers Prev. 26:1345–1348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Baltus JA, Boersma JW, Hartman AP and
Vandenbroucke JP: The occurrence of malignancies in patients with
rheumatoid arthritis treated with cyclophosphamide: A controlled
retrospective follow-up. Ann Rheum Dis. 42:368–373. 1983.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gulamhusein A and Pope JE: Squamous cell
carcinomas in 2 patients with diffuse scleroderma treated with
mycophenolate mofetil. J Rheumatol. 36:460–462. 2009.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Okada K, Endo Y, Miyachi Y, Koike Y,
Kuwatsuka Y and Utani A: Glycosaminoglycan and versican deposits in
taxane-induced sclerosis. Br J Dermatol. 173:1054–1058.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Hung CH, Chan SH, Chu PM and Tsai KL:
Docetaxel facilitates endothelial dysfunction through oxidative
stress via modulation of protein kinase C beta: The protective
effects of sotrastaurin. Toxicol Sci. 145:59–67. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Abu-Shakra M and Lee P: Exaggerated
fibrosis in patients with systemic sclerosis (scleroderma)
following radiation therapy. J Rheumatol. 20:1601–1603.
1993.PubMed/NCBI
|
|
44
|
Darras-Joly C, Wechsler B, Blétry O and
Piette JC: De novo systemic sclerosis after radiotherapy: A report
of 3 cases. J Rheumatol. 26:2265–2267. 1999.PubMed/NCBI
|
|
45
|
Shah DJ, Hirpara R, Poelman CL, Woods A,
Hummers LK, Wigley FM, Wright JL, Parekh A, Steen VD, Domsic RT and
Shah AA: Impact of radiation therapy on scleroderma and cancer
outcomes in scleroderma patients with breast cancer. Arthritis Care
Res (Hoboken). 70:1517–1524. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Verhulst L, Noë E, Morren MA, Verslype C,
Van Cutsem E, Van den Oord JJ and De Haes P: Scleroderma-like
cutaneous lesions during treatment with paclitaxel and gemcitabine
in a patient with pancreatic adenocarcinoma. Review of literature.
Int J Dermatol. 57:1075–1079. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Itoh M, Yanaba K, Kobayashi T and Nakagawa
H: Taxane-induced scleroderma. Br J Dermatol. 156:363–367.
2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sokołowska-Wojdyło M, Kłudkowska J,
Olszewska B, Seredyńska J, Biernat W, Błażewicz I,
Rustowska-Rogowska A and Nowicki RJ: The first case of drug-induced
pseudoscleroderma and eczema craquelé related to nab-paclitaxel
pancreatic adenocarcinoma treatment. Postepy Dermatol Alergol.
35:106–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Shibao K, Okiyama N, Maruyama H, Jun-Ichi
F and Fujimoto M: Scleroderma-like skin changes occurring after the
use of paclitaxel without any chemical solvents: A first case
report. Eur J Dermatol. 26:317–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Battafarano DF, Zimmerman GC, Older SA,
Keeling JH and Burris HA: Docetaxel (Taxotere) associated
scleroderma-like changes of the lower extremities. A report of
three cases. Cancer. 76:110–115. 1995.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Hamaguchi Y: Autoantibody profiles in
systemic sclerosis: Predictive value for clinical evaluation and
prognosis. J Dermatol. 37:42–53. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kufe D, Inghirami G, Abe M, Hayes D,
Justi-Wheeler H and Schlom J: Differential reactivity of a novel
monoclonal antibody (DF3) with human malignant versus benign breast
tumors. Hybridoma. 3:223–232. 1984.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wakamatsu K, Nagata N, Kumazoe H, Oda K,
Ishimoto H, Yoshimi M, Takata S, Hamada M, Koreeda Y, Takakura K,
et al: Prognostic value of serial serum KL-6 measurements in
patients with idiopathic pulmonary fibrosis. Respir Investig.
55:16–23. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Baldus SE, Engelmann K and Hanisch FG:
MUC1 and the MUCs: A family of human mucins with impact in cancer
biology. Crit Rev Clin Lab Sci. 41:189–231. 2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Kruit A, Gerritsen WB, Pot N, Grutters JC,
van den Bosch JM and Ruven HJ: CA 15-3 as an alternative marker for
KL-6 in fibrotic lung diseases. Sarcoidosis Vasc Diffuse Lung Dis.
27:138–146. 2010.PubMed/NCBI
|
|
56
|
Uehara M, Kinoshita T, Hojo T,
Akashi-Tanaka S, Iwamoto E and Fukutomi T: Long-term prognostic
study of carcinoembryonic antigen (CEA) and carbohydrate antigen
15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 13:447–451.
2008.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Shering SG, Sherry F, McDermott EW,
O'Higgins NJ and Duffy MJ: Preoperative CA 15-3 concentrations
predict outcome of patients with breast carcinoma. Cancer.
83:2521–2527. 1998.PubMed/NCBI
|
|
58
|
Shao Y, Sun X, He Y, Liu C and Liu H:
Elevated levels of serum tumor markers CEA and CA15-3 are
prognostic parameters for different molecular subtypes of breast
cancer. PLoS One. 10(e0133830)2015.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fu Y and Li H: Assessing clinical
significance of serum CA15-3 and carcinoembryonic antigen (CEA)
levels in breast cancer patients: A meta-analysis. Med Sci Monit.
22:3154–3162. 2016.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Di Gioia D, Dresse M, Mayr D, Nagel D,
Heinemann V and Stieber P: Serum HER2 in combination with CA 15-3
as a parameter for prognosis in patients with early breast cancer.
Clin Chim Acta. 440:16–22. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Wojtacki J, Kruszewski WJ, Sliwińska M,
Kruszewska E, Hajdukiewicz W, Sliwiński W, Rolka-Stempniewicz G,
Góralczyk M and Leśniewski-Kmak K: Elevation of serum Ca 15-3
antigen: An early indicator of distant metastasis from breast
cancer. Retrospective analysis of 733 cases. Przegl Lek.
58:498–503. 2001.PubMed/NCBI(In Polish).
|
|
62
|
Tampellini M, Berruti A, Bitossi R,
Gorzegno G, Alabiso I, Bottini A, Farris A, Donadio M, Sarobba MG,
Manzin E, et al: Prognostic significance of changes in CA 15-3
serum levels during chemotherapy in metastatic breast cancer
patients. Breast Cancer Res Treat. 98:241–248. 2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chourin S, Veyret C, Chevrier A, Loeb A,
Gray C and Basuyau J: Routine use of serial plasmatic CA 15-3
determinations during the follow-up of patients treated for breast
cancer. Evaluation as factor of early diagnosis of recurrence. Ann
Biol Clin (Paris). 66:385–392. 2008.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
64
|
Yang Y, Zhang H, Zhang M, Meng Q, Cai L
and Zhang Q: Elevation of serum CEA and CA15-3 levels during
antitumor therapy predicts poor therapeutic response in advanced
breast cancer patients. Oncol Lett. 14:7549–7556. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kim HS, Park YH, Park MJ, Chang MH, Jun
HJ, Kim KH, Ahn JS, Kang WK, Park K and Im YH: Clinical
significance of a serum CA15-3 surge and the usefulness of CA15-3
kinetics in monitoring chemotherapy response in patients with
metastatic breast cancer. Breast Cancer Res Treat. 118:89–97.
2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Wong RC, Brown S, Clarke BE, Klingberg S
and Zimmerman PV: Transient elevation of the tumor markers CA 15-3
and CASA as markers of interstitial lung disease rather than
underlying malignancy in dermatomyositis sine myositis. J Clin
Rheumatol. 8:204–207. 2002.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Inagaki Y, Xu H, Nakata M, Seyama Y,
Hasegawa K, Sugawara Y, Tang W and Kokudo N: Clinicopathology of
sialomucin: MUC1, particularly KL-6 mucin, in gastrointestinal,
hepatic and pancreatic cancers. Biosci Trends. 3:220–232.
2009.PubMed/NCBI
|
|
68
|
Szekanecz E, Szucs G, Szekanecz Z, Tarr T,
Antal-Szalmás P, Szamosi S, Szántó J and Kiss E: Tumor-associated
antigens in systemic sclerosis and systemic lupus erythematosus:
Associations with organ manifestations, immunolaboratory markers
and disease activity indices. J Autoimmun. 31:372–376.
2008.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Ogawa Y, Ishikawa T, Ikeda K, Nakata B,
Sawada T, Ogisawa K, Kato Y and Hirakawa K: Evaluation of serum
KL-6, a mucin-like glycoprotein, as a tumor marker for breast
cancer. Clin Cancer Res. 6:4069–4072. 2000.PubMed/NCBI
|
|
70
|
Kohno N: Serum marker KL-6/MUC1 for the
diagnosis and management of interstitial pneumonitis. J Med Invest.
46:151–158. 1999.PubMed/NCBI
|