1. Introduction
Cancer is the second leading cause of mortality
worldwide, with an estimated 19.3 million new cancer cases
(1). Due to the exponentially higher
incidence of cancer in later life, 60% of newly diagnosed
malignancies and 70% of cancer-related deaths occur in patients
aged >65 years (2-3). By 2040, population growth and aging
alone are expected to increase the global burden to 27.5 million
new cancer cases and 16.3 million cancer-related deaths (4). However, the prevalence of
risk-increasing factors, such as smoking, an unhealthy diet and a
lack of physical activity could significantly increase the future
cancer burden (5). Cancer is
primarily a disease of the elderly, with >80% of cancer cases
arising in humans >50 years. As this amount of the population is
likely to increase gradually over several decades, an urgent need
to address this impending health and medical crisis by developing
policies to decrease cancer rates in older adults. Evolving
suitable plans will require recognizing individuals at risk of
developing cancer and providing them with specific risk-reduction
procedures that go above current endorsements for prevention and
screening. Monogenic and polygenic effects play a crucial role in
cancer susceptibility and pathogenesis; however, cancer in the
elderly is less expected to be related to single-germline variant
genes (4). The degree to which
polygenic variants precisely contribute to the risk of cancer
development is the topic of the present review.
The fundamental processes that mediate
susceptibility to cancer, which are common among all cancers are
also likely to mediate vulnerability to other non-cancerous
diseases and conditions. These developments are most likely related
to aging and longevity, due to the appearance of a number of common
age-related chronic diseases. By bringing together genetic
information about multiple cancers, diseases, aging and life
expectancy, it is possible to develop better predictive models
through risk stratification, and to also learn more about the
influences of aging and the genetic causes of cancer. Tumors arise
from the oncogenic transformation changes of only single cells.
Some tumors gain the ability to leave their site of origin and
invade other bodily organs (6). As
with all diagnostic tests, tumor markers help doctors determine
whether it is a new cancer, recurrence, cancer progression or
mortality, and/or specific treatments to reduce risk. They are a
very useful surrogate indicator (7).
The value of tumor markers is to enable the more efficient
application of therapy, thus allowing treatment to be used in
patients most likely to benefit, while reducing toxicity in those
who do not benefit and reducing exposure (8). Despite the fact that data on the
histological scoring of breast tumor subtypes and prostate cancer
have enabled the investigation of long-term associations with risk
factors, a dilution effect could not be completely ruled out.
However, this may have resulted in a non-differential measurement
error (both in cases and non-cases), which most likely would have
understated the strength of the associations that were present.
2. Biological aging and cancer tumor
markers
An area of particular interest in biological aging
mechanisms, which may constitute a shared set of pathways that
increase the likelihood of developing a variety of diseases
normally associated with aging, including cardiovascular disease,
is numerous common forms of cancer and degenerative diseases
(‘geriatric science hypothesis’) (9). Biomarkers of aging can be used to
forecast the overall risk of chronic disease and offer new insight
into the causes of diseases and their underlying biological
pathways (10). Furthermore,
increasing evidence suggests that biological aging mechanisms and
processes can be specifically targeted and moderated by preventive
and therapeutic interventions, including alterations in dietary
intake and energy restriction, and increased physical exercise or
drug therapy (11). The biological
aging process is related to a series of biological variations. At
the cellular level, this includes amplified genomic instability,
epigenetic alterations, mitochondrial dysfunction, higher oxidative
stress, protein imbalance, impaired nutrient perception and
senescence cell chemistry (12). At
a more systemic level, aging is categorized by a loss and
physiological changes in respiratory, cardiovascular, neurological,
metabolic, musculoskeletal, liver and renal functions (13), which may be mirrored in the various
circulating blood biomarkers (14).
One of the main risk factors of cancer is aging. In
a World Health Organization report, the incidence of cancer was
examined across various age groups. The prevalence of other cancers
rises based on diabetes [breast (post-menopausal) and pancreatic
cancers], infections (hepatocellular carcinoma and anal cancer),
weight and metabolic syndrome [esophageal, pancreatic, thyroid,
gallbladder, colon and rectal, breast (post-menopausal),
endometrial and kidney cancers] and sharply with age; unlike
leukemia, which mostly affects younger individuals, a strong link
has been found between cancer and aging, suggesting that cancer is
an aspect of aging (15). Telomere
shortening, an accumulation of genetic mutations, oxidative stress
and the breakdown of cells and organs, are only a few of the
numerous elements that contribute to aging, a complex and universal
physiological process. The capacity of the body to maintain a
stable state steadily declines with age, and the chance of
developing diseases such as cancer, cardiovascular issues and
neurological conditions also increases (16). Nevertheless, due to the limited
genetic backdrop, the human aging processes is not completely
understood. Tumor markers play a crucial role in patient
monitoring. A clinical issue that increases the reliability and
utility of tumor markers is the accuracy of the marker results for
the desired submission. Marker results classify patients into two
or more populations with markedly dissimilar results, and patients
and their caregivers are treated as distinct groups (17). The incidence of tumors in the aging
population is high, and hence older individuals are keen to
participate in health programs (18). However, although cancer and senescent
cells are also basically opposed, the underlying mechanism of both
cancer and senescence is the time-dependent accumulation of
cellular damage (19). Irrespective
of the mechanisms or networks underlying the aging process, or the
criteria used to define patients as ‘old’, aging itself is
considered to be the most significant risk factor for the growth of
unfavorable cancers. When interpreting laboratory data, it is
crucial to take into account the effects of age in order to
correctly evaluate the results; however, whether or not aging alone
is accountable for changes in certain biological pointers and
whether the aberrations are due to pathological causes or
degenerative diseases with a high prevalence in the elderly
population are questionable (20).
3. Tumor markers and the elderly
To demonstrate the potential association between
cancer and aging, a large-scale genome-wide study of DNA
methylation profiles in aging and cancer was previously completed
(21). Applying an age and
cancer-related weighting network produces biologically meaningful
results, representing that the association between age-related
variations in DNA methylation and carcinogenesis is not accidental
(22). Age is a risk factor for
cancer; however, the association between age and cancer is unclear.
Increased or decreased methylation is detected up to the age of 70,
although these phenomena plateau with aging in healthy populations.
Molecular markers are DNA fragments that signify genetic signatures
for detecting changes in gene sequence, expression levels and
protein structure or function (23).
The extensive cancer research offered by genomics helps to
characterize tumors at the molecular level. Similar advancements in
molecular technology have elevated cancer biology. For instance,
the creation of medications that target pertinent molecules has
been influenced by this information. A platform for developing
cancer molecular markers requires a solid understanding of the
genes involved in cancer development, according to recent findings
from a research project by the International Cancer Genome
Consortium (ICGC) and The Cancer Genome Atlas (TCGA) (24). The process of studying these
oncogenes requires both molecular therapeutics and biomarkers,
leading to the growth of drugs against specific cancers (25). The number of molecular markers has
multiplied over the past decade due to the better understanding of
the molecular biology of cancer, as well as the molecular basis of
tumor progression and therapeutic response.
There are basically three types of biomarkers
(prognostic, preventive and diagnostic markers) available. The
tumor biomarkers in cancer are presented in Table I (26-37).
While all subtypes occur in all age categories, an older age is
associated with a slightly lower incidence of high-grade tumors,
fewer triple-negative breast cancer and HER2+ subtypes,
and more luminal tumors than a younger age (38). Age-related differences in the
landscape of tumor mutations include the fact that older patients
with breast cancer have a lower incidence of TP53 mutations than
younger patients (28). There have
been reports of age-dependent alterations in peritumoral and
systemic immunity; nevertheless, additional studies in various
subtypes of breast cancer are necessary. With an advancing age,
distinct histological (more common squamous cell carcinoma) and
molecular (increased tumor mutational burden and distinct EGFR
mutation subtypes) changes appear to manifest that may affect the
management of lung cancer. Age groups do not appear to exhibit
differences as regards the expression of programmed cell death
protein 1 (PD-1) and PD-L1; however, further studies are required
to fully characterize the tumor microenvironment and identify any
differences between older and younger patients (39). A higher Gleason score, a higher
D'Amico risk classification, more often occurring in the luminal B
subtype, more common intraductal carcinoma of prostate
architecture, a higher p53 positivity, and more tumors with a
high-risk Decipher score all indicate that prostate cancer in older
males generally appears to behave more aggressively (35,40). The
interpretation of serum prostate-specific antigen is hampered by
aging, which is why age-based standards and alternative biomarkers
are used. When compared to younger individuals, elderly patients
with colorectal cancer exhibit notable biochemical changes.
Right-sided tumors are more common among older adults, and serrated
polyps, as opposed to the traditional adenoma-carcinoma pathway,
are more frequently linked to the development of cancer. In light
of the increasing use of targeted therapies and immunotherapy,
there is a larger frequency of BRAF mutations, microsatellite
instability phenotype and CpG island methylator phenotype-high
tumors at the molecular level (41).
These findings may have significant therapeutic consequences. The
predictive value of an immunoscore appears to be age-independent
(42).
 | Table ICancer and tumor markers. |
Table I
Cancer and tumor markers.
| Tumor markers | Type of cancer | Age-related
differences in molecular markers | (Refs.) |
|---|
| Luminal a | Breast | 50% ≥40 | (26) |
| Luminal b | Breast | 38% ≥70 | (27) |
| HER2 | Breast | 17% ≥65 | (28) |
| TP53 | Breast | 42% ≥40-65 | (29) |
| GATA3 | Breast | 22% ≥40 | (26) |
| EGFR | Lung | 21% ≥60 | (30) |
| PDL1 | Lung | 55% ≥65 | (31) |
| KRAS | Lung | 47% ≥50 | (32) |
| Gleason score | Prostate | 47% ≥50 | (33) |
| Serum PSA | Prostate | 85% ≥60 | (34) |
| p53 | Prostate | 24% ≥70 | (35) |
| MSI | Colon | 19.5% ≥75 | (36) |
| BRAF | Colon | 20% ≥60 | (37) |
4. Prognostic, predictive and diagnostic
markers
Prognostic markers assess the complete outcomes of
patients following typical therapy and predict disease progression
or the response to therapeutic intervention in subjects with
similar characteristics (43).
Predictive markers quantity the usefulness of a particular clinical
intervention, or different outcomes of two or more interventions,
and specify sensitivity or resistance to a particular therapy.
Diagnostic markers, on the other hand, recognize whether a patient
is suffering from a particular disease by measuring susceptibility
or resistance to a particular treatment. Biomarkers help assess
preventive and therapeutic measures and detect early stages of
malignant transformation of the oral mucosa. Biomarkers reveal
genetic and molecular alterations associated with early,
intermediate and late endpoints of the oral carcinogenesis process.
Circulating biomarkers, such as circulating tumor DNA, exosomes and
circulating microRNAs (miRNAs/miRs) can be used for early
prognosis, diagnosis and treatment. Circulating miRNAs, such as
miR-138, miRa-99a, miR-21, miR-181a, miR-222 and miR-7, have
enhanced the diagnostic ability for head and oral cancer (44). Pharmaceutical research and
development are focused on oncology. The discovery of therapeutic
breakthroughs in oncology accounts for 29% of the total R&D
spending. Given that cancer is a highly heterogeneous disease, not
only in terms of histology and clinical outcomes, but also at the
molecular level, oncology is one of the first fields to use
targeted therapy (45). Tumor
barkers and their specific genetic alterations are summarized in
Table II (46-55).
 | Table IITumor barkers and their specific
genetic alterations. |
Table II
Tumor barkers and their specific
genetic alterations.
| Tumor type | Targeted
therapeutics | FDA approved
specific Genetic Alterations | (Refs.) |
|---|
| Non-small cell lung
cancer | Gefitinib,
crizotinib and erlotinib | • EGFR exon 19
deletions, L858R | (46,47) |
| | | • EGFR exon 20
insertions | |
| | | • EGFR nonresistant
mutations other than exon 19 deletions and L858R | |
| Breast cancer | Lapatinib | • ERBB2
amplification | (48) |
| Metastatic
melanoma | Vemurafenib,
Dabrafenib | • BRAF V600E | (49) |
| Colorectal
cancer | Panitumumab,
cetuximab | • KRAS and/or NRAS
exon 2, 3, and 4 mutations | (50) |
| Fallopian tube,
ovarian, primary peritoneal carcinoma | Bevacizumab,
olaparib | • Deleterious
germline or somatic mutations in BRCA1 and/or BRCA2 | (51) |
| Esophagogastric
cancer | Trastuzumab,
cisplatin, capecitabine or fluorouracil | • ERBB2
amplification | (52) |
| Endometrial
cancer | Dostarlimab | • dMMR and/or
MSI-H | (53) |
| Ovarian cancer | Niraparib | • GIS-positive or
HRD-positive | (51) |
| Bladder cancer | Erdafitinib | • FGFR2
fusions | (54) |
|
Cholangiocarcinoma | Pemigatinib,
infigratinib | • FGFR2
fusions | (55) |
Increased markers in the elderly do not reflect the
presence of tumors, but may have some association with the
carcinogenic process. The starting point may be the increase in
multiple hyperplasia that occurs with aging and rarely leads to
clinical neoplasms (56).
Hyperplasia in the epithelial cells of the gastrointestinal tract
has been widely studied and has been revealed to be closely related
with stomach cancer, colonic metaplasia and age, with a noticeable
increase in premalignant abrasions with age (57). Another possibility is that the
markers merely represent age-related, non-specific organ
degeneration (57). To measure risk
aspects and predict efficacy/toxicity, treatment tolerability and
overall risk/benefit ratio in elderly patients are critical
parameters to consider in the decision-making of patients with
cancer. Additionally, clinically relevant indicators are required
for the delivery of xenobiotics. There currently limited data
available due to the dearth of age-specific effectiveness and
safety data in clinical trials (58). Elevated marker values are not due to
the increased prevalence of chronic disease and not due to the
presence or preclinical stage of occult malignancies, in most
cases. The study by Lopez et al (60) compared the decreased sensitivity of
the markers in subjects without clinical signs of cancer; it was
noted that the increase was not associated with the presence of
antigen-associated tumors. Circulating cytokines may be related to
the increased mortality in aging patients with cancer. Potential
biomarkers require extensive investigation before being validated
for clinical use (59).
Comprehensive geriatric assessment as an interdisciplinary
framework for assessing the impact of age-related physiological
factors, as divergent to chronological age, that may affect health
and disease in older adults was developed (60). Those identified at risk by the
screening test need to be further evaluated with a full CGA, and
the results used may help inform specific healthcare strategies to
improve cancer outcomes in older adults (61). There is also an understanding of the
metabolic and molecular changes associated with cancer. Elderly
patients continue to be underrepresented in clinical trials, making
evidence-based decision-making for older patients difficult. In
particular, race, sex and accounting for age-related inequalities
remain subjects of debate concerning participation in cancer
clinical trials. There is an urgent need to better understand the
clinical, molecular and physiological implications of cancer in the
elderly and the factors that regulate treatment response, toxicity,
and tolerability in older patients with cancer (62).
5. Genomic instability in aging cells
Mutations contribute to tumorigenesis, almost all
human cancers exhibit genomic instability, and mutations occur at a
greater pace than in healthy cells. Preserving genomic stability
appears to be an essential function for preventing cancer
progression and aging processes. Alterations and instability in the
genome can contribute to aging via a variety of mechanisms, from
minor point mutations to significant translocations and deletions.
Alterations in regulatory sequences can lead to a progressive
decline in organ function as a result of variations in the proteome
and homeostasis (63). Indeed, there
is a significant age-related disparity in the expression of genes
among cells in the same tissue. The gene heterogeneity is
considered to cause the stochastic deregulation of gene expression
amongst adjacent cells, which ultimately accelerates aging
(64). It is conceivable that
gradual alterations may occur over time, triggered by somatic
mutations caused by genomic instability. These individual
variations in mutations may have an effect on shared biological
pathways or gene regulatory networks, which eventually result in
age-related functional decline and universally prevalent disease
patterns. Diseases can be caused by errors in genes that maintain
genome stability and repair DNA. Additionally, as individuals age,
somatic mutations spread across tissues, disrupting important
transcriptional programs, as well as other vital biological
functions, leading to a loss of fitness and organismal
deterioration. Overall, it appears that the argument that DNA
damage and genomic instability are key causes of aging and
aging-related disorders, such as cancer, is becoming more and more
compelling (65). Cancer, on the
other hand, is the consequence of advantageous mutations that
provide the neoplastic cell with an advantage in terms of growth
and metastasis. In order to delay disease development and premature
aging, genomic stability must be maintained. Human syndromes of
early aging are caused by mutations in DNA repair enzymes (66), and genomic instability is a key
characteristic of cancer (67).
Numerous studies have linked DNA shape and organization to genomic
instability and aging, highlighting the significance of how DNA is
packed within our cells (68).
Importantly, a number of signaling pathways and molecular
functions, such as insulin signaling, mTOR signaling, cellular
senescence (69), telomere
shortening and sirtuin activity (70), have also been connected to organismal
aging. However, it also signifies the stochastic hazard of
accumulating changes that reassure uncontrolled cell division and
expansion in cancer. Finally, therapeutic interventions that target
both aging as a process and carcinogenesis may be probable with a
deeper understanding of genomic instability (71,72). A
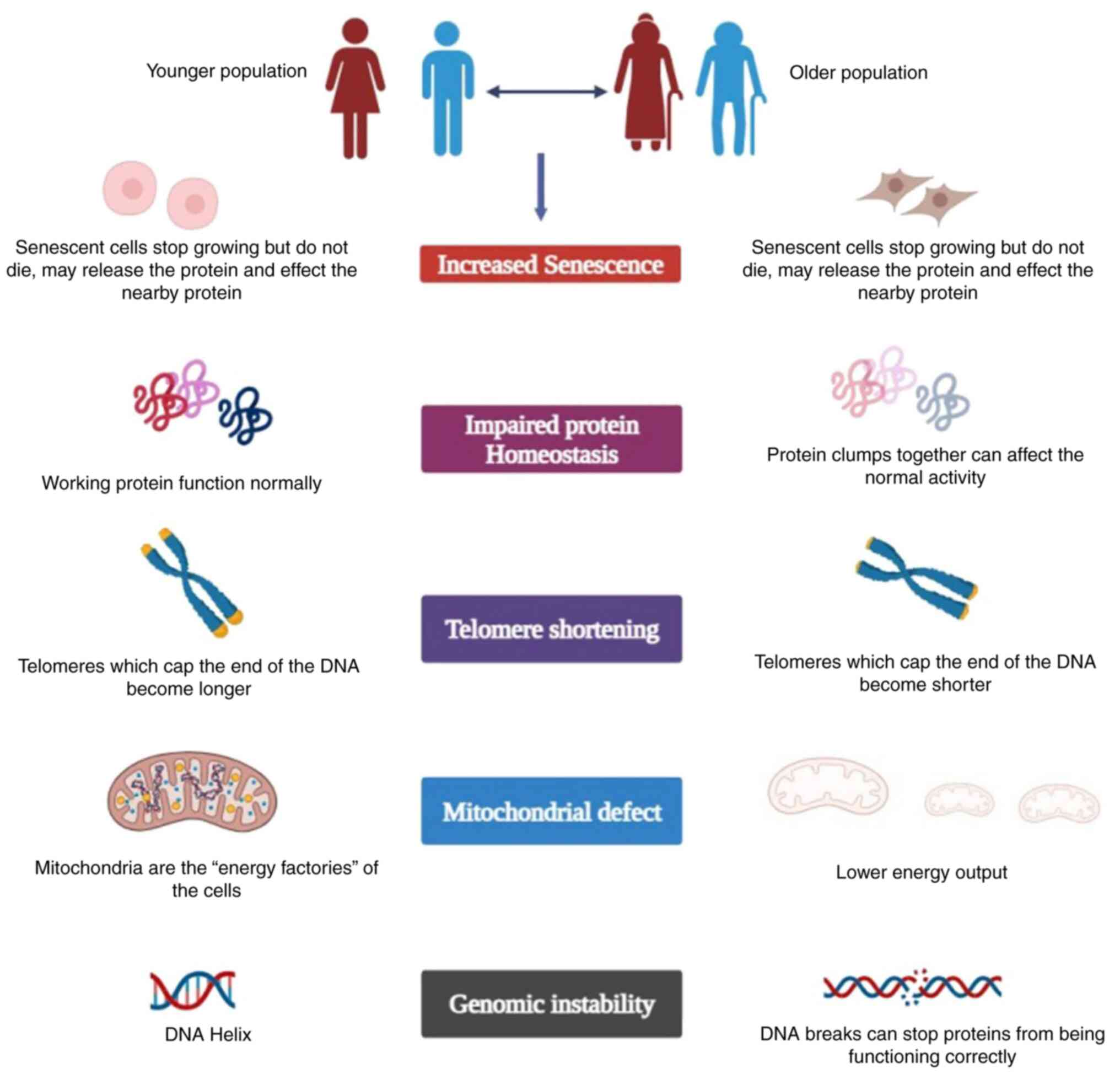
schematic representation of aging and cancer is presented in
Fig. 1.
6. Age-stratified and sex-specific reference
intervals
Specific characteristics of the research
participants, including their age, sex, region and way of life
should be taken into consideration in order to establish a reliable
and well-designed reference interval. These factors may have an
effect on the levels of biomarkers in individuals who will be the
focus of the investigation. When determining a reference interval
that performs well, another key consideration is the volume of the
sample that will be included in the study. Although the Clinical
and Laboratory Standards Institute 24 (CLSI24) needs at least 120
samples to meet the sample volume requirement for founding a
reference interval considering the cost of conducting, a larger
volume sample, will, if the budget permits, provide a better
Poisson distribution and represent a ‘near-true’ population value.
A standardized assessment of tumor markers on a large population
with age-, sex- and geographic location-specific
well-representation of healthy subjects is compulsory to carry out
a reference interval study for a tumor biomarker. It is difficult
to simultaneously establish a reference interval using a
multi-marker lung cancer biomarker panel in numerous hospitals
(73). However, the majority of the
previous studies included older women, a population that is more
likely to be associated with suspicion of cancer. Patients with
dermoid cysts have different clinical features depending on their
age, according to an age-focused study. The likelihood of
developing cancer increased after the age of 40, and tumor size was
higher in younger patients than in older women (74-76).
No association between was found between elevated cancer antigen
125 used for the diagnosis of ovarian, endometrial, peritoneal and
fallopian tube cancers and carbohydrate antigen 19-9 for colon,
stomach and bile duct cancer levels in adolescents and young
adults. On the other hand, in the older age group, an increase in
the levels of carbohydrate antigen 19-9 was associated with larger
tumors (77). In a similar manner,
the majority of studies that have already been published and found
a link between carbohydrate antigen 19-9 levels and tumor size
displayed a higher median age (78,79).
The dynamics of telomeres plays a crucial role in
aging, age-related diseases and cancer. Stress, depression, smoking
and exercise are instances of non-genetic factors that affect
telomere maintenance (80).
It has been well-established that hereditary
telomere disorders are caused by single-gene inactivating mutations
in telomere maintenance components. Typically, these modifications
cause in vivo telomeres to shorten. The classic phenotypes
of accelerated aging include diabetes, cardiovascular disease,
graying of hair, an altered skin colour, loss of immunological
function and susceptibility to specific malignancies. Males have
shorter telomeres than females at any age, according to previous
research, and >50% of telomere length is inherited (14,81). In
addition, telomere length is heritable. It has been found that
age-related telomere attrition is accelerated in patients with
cancer who are diagnosed later in life, although the attrition
reduces 3 to 4 years prior to the diagnosis, resulting in longer
telomeres (82). This could indicate
that telomere shortening plays a role in early carcinogenesis
before cancer hijacks and initiates telomerase activation and other
methods of telomere elongation (83). This may also affect blood leukocytes,
which are critical for the initiation and development of cancer. If
this is validated in further research, it may be used as an early
biomarker for cancer identification. Numerous age-related disorders
include protein aggregation build-up and associated harmful
consequences, and proteostasis is affected. Cancer stops this
process by increasing the activity of the proteasome, lysosome and
chaperone systems. All paths are inhibited in oncological therapy,
and new technologies and drugs are constantly being developed
(83). Cancer and aging are
basically different, as malignant cells avoid senescence by
producing additional mutations, such as the deletion of tumor
suppressors (p16INK4a or p53), an example of antagonistic
pleiotropy, whereas accumulating DNA damage typically will cause an
increase in cell cycle inhibitors leading to senescence or
apoptosis (84). In conclusion,
cancer and aging are connected in time and mechanism, and a number
of the same drugs and strategies can be used to target both. On the
other hand, antagonistic pleiotropy can work, and inhibiting one
can cause the other to be activated.
7. Conclusion and future perspectives
In addition, despite the fact that previous studies
have established strong associations between these markers and the
risk of mortality and chronological age, they cannot be interpreted
as indicators of biological aging. Additionally, there has been no
cross-validation of the multi-marker combination. Despite the fact
that the markers are linked to aging, it is difficult to draw a
clear conclusion about how closely these associations relate to any
one aspect of biological aging. This is due to the fact that these
markers are not only linked to aging, but may also reflect other
causal pathways, such as cellular stress, cardiovascular health,
glucose intolerance, inflammation, or renal dysfunction. While
several of the analyses had a reduced statistical power due to the
limited number of cases needed to identify links in stratified and
tumor subtypes/grades models, the aim of the present review was to
summarize the data obtained to date. In order to more efficiently
determine when and how to treat older patients with cancer, it is
important to discriminate between chronological age, physiological
age, and associated geriatric and comorbidities. Of note,
multidisciplinary research including geriatricians, oncologists and
policymakers, with more data on the elderly population, is
warranted in order to improve clinical decision-making. However, it
appears reasonable to conclude that the relation of tumor markers
with tumor size has limited diagnostic value among the elderly.
Consolidating the research results of clinical data from systematic
reviews and meta-analyses along with a specific focus on the values
of tumor markers in geriatric patients with cancer in further
studies with larger study groups may provide answers to the
currently pending questions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SS was responsible for the conceptualization of the
study and prepared the manuscript. PR was involved in conception
and design of the study. SKL was involved in the drafting of the
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ries LAG, Eisner MP, Kosary CL, Hankey BF,
Miller BA, Clegg LX and Edwards BK: SEER cancer statistics review,
1973-1998. National Institute of Health.
|
|
3
|
National Institute on Aging: Exploring the
role of cancer centers for integrating aging and cancer research
(workshop report). NIA, Bethesda, MD, 2002.
|
|
4
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Piñeros M, Znaor A, Soerjomataram I and Bray F (eds): Cancer
Today (powered by GLOBOCAN 2018). World Health Organization and
International Agency for Research on Cancer, Lyon, 2018.
|
|
5
|
American Cancer Society: Global Cancer
Facts & Figures 4th Edition. Atlanta: American Cancer Society;
2018.
|
|
6
|
Imran A, Qamar HY, Ali Q, Naeem H, Riaz M,
Amin S, Kanwal N, Ali F, Sabar MF and Nasir IA: Role of molecular
biology in cancer treatment: A review article. Iran J Public
Health. 46:1475–1485. 2017.PubMed/NCBI
|
|
7
|
Hayes DF, Bast RC, Desch CE, Fritsche H
Jr, Kemeny NE, Jessup JM, Locker GY, Macdonald JS, Mennel RG,
Norton L, et al: Tumor marker utility grading system: A framework
to evaluate clinical utility of tumor markers. J Natl Cancer Inst.
88:1456–1466. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hayes DF: Prognostic and predictive
factors for breast cancer: Translating technology to oncology. J
Clin Oncol. 23:1596–1597. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Franceschi C, Garagnani P, Morsiani C,
Conte M, Santoro A, Grignolio A, Monti D, Capri M and Salvioli S:
The continuum of aging and age-related diseases: Common mechanisms
but different rates. Front Med (Lausanne). 5(61)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Colloca G, Di Capua B, Bellieni A, Fusco
D, Ciciarello F, Tagliaferri L, Valentini V and Balducci L:
Biological and functional biomarkers of aging: Definition,
characteristics, and how they can impact everyday cancer treatment.
Curr Oncol Rep. 22:1–21. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Guo J, Huang X, Dou L, Yan M, Shen T, Tang
W and Li J: Aging and aging-related diseases: From molecular
mechanisms to interventions and treatments. Signal Transduct Target
Ther. 7(391)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kennedy BK, Berger SL, Brunet A, Campisi
J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI,
Pessin JE, et al: Geroscience: Linking aging to chronic disease.
Cell. 159:709–713. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sharma G and Goodwin J: Effect of aging on
respiratory system physiology and immunology. Clin Interv Aging.
1:253–260. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vaiserman A and Krasnienkov D: Telomere
length as a marker of biological age: State-of-the-art, open
issues, and future perspectives. Front Genet.
11(630186)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
White MC, Holman DM, Boehm JE, Peipins LA,
Grossman M and Henley SJ: Age and cancer risk: A potentially
modifiable relationship. Am J Prev Med. 46:S7–S15. 2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li Z, Zhang Z, Ren Y, Wang Y, Fang Y, Yue
H, Ma S and Guan F: Aging and age-related diseases: From mechanisms
to therapeutic strategies. Biogerontology. 22:165–187.
2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Economopoulou P, de Bree R, Kotsantis I
and Psyrri A: Diagnostic tumor markers in head and neck squamous
cell carcinoma (HNSCC) in the clinical setting. Front Oncol.
9(827)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
National Research Council (US) Panel on
Statistics for an Aging Population Dorothy M. Gilford, editors: The
aging population in the twenty-first century: Statistics for health
policy. Washington (DC): National Academies Press (US); ISBN-10:
0-309-03881-2, 1988.
|
|
19
|
Aunan JR, Cho WC and Søreide K: The
biology of aging and cancer: A brief overview of shared and
divergent molecular hallmarks. Aging Dis. 8:628–642.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cavalieri TA, Chopra A and Bryman PN: When
outside the norm is normal: Interpreting lab data in the aged.
Geriatrics. 47:66–70. 1992.PubMed/NCBI
|
|
21
|
Wilson J, Heinsch M, Betts D, Booth D and
Kay-Lambkin F: Barriers and facilitators to the use of e-health by
older adults: A scoping review. BMC Public Health.
21(1556)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shir D, Mielke MM, Hofrenning EI, Lesnick
TG, Knopman DS, Petersen RC, Jack CR, Algeciras-Schimnich A, Vemuri
P and Graff-Radford J: Associations of neurodegeneration biomarkers
in cerebrospinal fluid with markers of Alzheimer's disease and
vascular pathology. J Alzheimer Dis. 92:887–898. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tran KA, Kondrashova O, Bradley A,
Williams ED, Pearson JV and Waddell N: Deep learning in cancer
diagnosis, prognosis and treatment selection. Genome Med. 13:1–7.
2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kiely M, Tse LA, Koka H, Wang D, Lee P,
Wang F, Wu C, Tsang KH, Chan WC, Law SH, et al: Age-related DNA
methylation in paired normal and tumor breast tissue in Chinese
breast cancer patients. Epigenetics. 16:677–691. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mealey NE, O'Sullivan DE, Pader J, Ruan Y,
Wang E, Quan ML and Brenner DR: Mutational landscape differences
between young-onset and older-onset breast cancer patients. BMC
Cancer. 20(212)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Azim HA Jr, Nguyen B, Brohée S, Zoppoli G
and Sotiriou C: Genomic aberrations in young and elderly breast
cancer patients. BMC Med. 13(266)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
De Kruijf EM, Bastiaannet E, Rubertá F, de
Craen AJ, Kuppen PJ, Smit VT, van de Velde CJ and Liefers GJ:
Comparison of frequencies and prognostic effect of molecular
subtypes between young and elderly breast cancer patients. Mol
Oncol. 8:1014–1025. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang MX, Ren JT, Tang LY and Ren ZF:
Molecular features in young vs elderly breast cancer patients and
the impacts on survival disparities by age at diagnosis. Cancer
Med. 7:3269–3277. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhong W, Zhao J, Huang K, Zhang J and Chen
Z: Comparison of clinicopathological and molecular features between
young and old patients with lung cancer. Int J Clin Exp Pathol.
11:1031–1035. 2018.PubMed/NCBI
|
|
31
|
Lin C, Chen X, Li M, Liu J, Qi X, Yang W,
Zhang H, Cai Z, Dai Y and Ouyang X: Programmed death-ligand 1
expression predicts tyrosine kinase inhibitor response and better
prognosis in a cohort of patients with epidermal growth factor
receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer.
16:e25–e35. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Boldrini L, Giordano M, Lucchi M, Melfi F
and Fontanini G: Expression profiling and microRNA regulation of
the LKB1 pathway in young and aged lung adenocarcinoma patients.
Biomed Rep. 29:198–205. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pettersson A, Robinson D, Garmo H,
Holmberg L and Stattin P: Age at diagnosis and prostate cancer
treatment and prognosis: A population-based cohort study. Ann
Oncol. 29:377–385. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang H, Messing EM, Travis LB, Hyrien O,
Chen R, Milano MT and Chen Y: Age and racial differences among
PSA-detected (AJCC stage T1cN0M0) prostate cancer in the U.S.: A
population-based study of 70,345 men. Front Oncol.
3(312)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Calvocoressi L, Uchio E, Ko J,
Radhakrishnan K, Aslan M and Concato J: Prostate cancer
aggressiveness and age: Impact of p53, BCL-2 and microvessel
density. J Investig Med. 66:1142–1146. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Aparicio T, Schischmanoff O, Poupardin C,
Soufir N, Angelakov C, Barrat C, Levy V, Choudat L, Cucherousset J,
Boubaya M, et al: Deficient mismatch repair phenotype is a
prognostic factor for colorectal cancer in elderly patients. Dig
Liver Dis. 45:245–250. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Phipps AI, Buchanan DD, Makar KW,
Burnett-Hartman AN, Coghill AE, Passarelli MN, Baron JA, Ahnen DJ,
Win AK, Potter JD and Newcomb PA: BRAF mutation status and survival
after colorectal cancer diagnosis according to patient and tumor
characteristics. Cancer Epidemiol Biomarkers Prev. 21:1792–1798.
2012.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lodi M, Scheer L, Reix N, Heitz D, Carin
AJ, Thiébaut N, Neuberger K, Tomasetto C and Mathelin C: Breast
cancer in elderly women and altered clinico-pathological
characteristics: A systematic review. Breast Cancer Res Treat.
166:657–668. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Velcheti V, Schalper KA, Carvajal DE,
Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L
and Rimm DL: Programmed death ligand-1 expression in non-small cell
lung cancer. Lab Invest. 94:107–116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Brody R, Zhang Y, Ballas M, Siddiqui MK,
Gupta P, Barker C, Midha A and Walker J: PD-L1 expression in
advanced NSCLC: Insights into risk stratification and treatment
selection from a systematic literature review. Lung Cancer.
112:200–215. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jędroszka D, Orzechowska M, Hamouz R,
Górniak K and Bednarek AK: Markers of epithelial-to-mesenchymal
transition reflect tumor biology according to patient age and
Gleason score in prostate cancer. PLoS One.
12(e0188842)2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sacher AG, Dahlberg SE, Heng J, Mach S,
Jänne PA and Oxnard GR: Association between younger age and
targetable genomic alterations and prognosis in non-small-cell lung
cancer. JAMA Oncol. 2:313–320. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Van Herck Y, Feyaerts A, Alibhai S,
Papamichael D, Decoster L, Lambrechts Y, Pinchuk M, Bechter O,
Herrera-Caceres J, Bibeau F, et al: Is cancer biology different in
older patients? Lancet Healthy Longev. 2:e663–e677. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hasan N, Choudhary S, Naaz N, Sharma N and
Laskar RA: Recent advancements in molecular marker-assisted
selection and applications in plant breeding programmes. J Genet
Eng Biotechnol. 19(128)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhu L, Zhao L, Wang Q, Zhong S, Guo X, Zhu
Y, Bao J, Xu K and Liu S: Circulating exosomal miRNAs and cancer
early diagnosis. Clin Transl Oncol. 24:393–406. 2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chakravarty D, Johnson A, Sklar J,
Lindeman NI, Moore K, Ganesan S, Lovly CM, Perlmutter J, Gray SW,
Hwang J, et al: Somatic genomic testing in patients with metastatic
or advanced cancer: ASCO provisional clinical opinion. J Clin
Oncol. 40:1231–1258. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Shaw AT, Ou SHI, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Subbiah V, Lassen U, Elez E, Italiano A,
Curigliano G, Javle M, de Braud F, Prager GW, Greil R, Stein A, et
al: Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated
biliary tract cancer (ROAR): A phase 2, open-label, single-arm,
multicentre basket trial. Lancet Oncol. 21:1234–1243.
2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Swetter SM, Thompson JA, Albertini MR,
Barker CA, Baumgartner J, Boland G, Chmielowski B, DiMaio D, Durham
A, Fields RC, et al: NCCN guidelines® insights:
Melanoma: Cutaneous, version 2.2021. J Natl Compr Canc Netw.
19:364–376. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Battaglin F, Naseem M, Puccini A and Lenz
HJ: Molecular biomarkers in gastro-esophageal cancer: Recent
developments, current trends and future directions. Cancer Cell
Int. 18(99)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Olson SH, Atoria CL, Cote ML, Cook LS,
Rastogi R, Soslow RA, Brown CL and Elkin EB: The impact of race and
comorbidity on survival in endometrial cancer. Cancer Epidemiol
Biomarkers Prev. 21:753–760. 2012.PubMed/NCBI View Article : Google Scholar
|
|
54
|
US Food and Drug Administration: FDA
grants accelerated approval to erdafitinib for metastatic
urothelial carcinoma, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-erdafitinib-metastatic-urothelial-carcinoma.
|
|
55
|
Javle MM, Roychowdhury S, Kelley RK,
Sadeghi S, Macarulla T, Waldschmidt DT, Goyal L, Borbath I,
El-Khoueiry AB, Yong WP, et al: Final results from a phase II study
of infigratinib (BGJ398), an FGFR-selective tyrosine kinase
inhibitor, in patients with previously treated advanced
cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement.
J Clin Oncol. 39(265)2021.
|
|
56
|
Martin GM: Proliferative homeostasis and
its age-related aberrations. Mech Ageing Dev. 9:385–391.
1979.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Atillasoy E and Holt PR: Gastrointestinal
proliferation and aging. J Gerontol. 48:B43–B49. 1993.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nagayo T: Gastric cancer preceded by
severe dysplasia. Histol Histopatnol. l:171–80. 1986.PubMed/NCBI
|
|
59
|
You WC, Blot WJ, Li JY, Chang YS, Jin ML,
Kneller R, Zhang L, Han ZX, Zeng XR and Liu WD: Precancerous
gastric lesions in a population at high risk of stomach cancer.
Cancer Res. 53:1317–1321. 1993.PubMed/NCBI
|
|
60
|
Lopez LA, Villar VD, Ulla M, Fernandez F,
Fernandez LA, Santos I, Rabadan L and Gutierrez M: Prevalence of
abnormal levels of serum tumor markers in elderly people. Age
Ageing. 25:45–50. 1996.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cohen HJ, Harris T and Pieper CF:
Coagulation and activation of inflammatory pathways in the
development of functional decline and mortality in the elderly. Am
J Med. 114:180–187. 2003.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Jencks DS, Adam JD, Borum ML, Koh JM,
Stephen S and Doman DB: Overview of current concepts in gastric
intestinal metaplasia and gastric cancer. Gastroenterol Hepatol (N
Y). 14:92–101. 2018.PubMed/NCBI
|
|
63
|
Owusu C and Berger NA: Comprehensive
geriatric assessment in the older cancer patient: Coming of age in
clinical cancer care. Clin Pract (Lond). 11:749–762.
2014.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Balducci L and Extermann M: Management of
cancer in the older person: A practical approach. Oncologist.
5:224–237. 2000.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Sarhadi VK and Armengol G: Molecular
biomarkers in cancer. Biomolecules. 12(1021)2022.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Pal M, Muinao T, Boruah HPD and Mahindroo
N: Current advances in prognostic and diagnostic biomarkers for
solid cancers: Detection techniques and future challenges. Biomed
Pharmacother. 146(112488)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Usman S, Jamal A, The MT and Waseem A:
Major molecular signaling pathways in oral cancer associated with
therapeutic resistance. Front Oral Health. 1(603160)2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Pan MR, Li K, Lin SY and Hung WC:
Connecting the dots: From DNA damage and repair to aging. Int J Mol
Sci. 17(685)2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Weichhart T: mTOR as regulator of
lifespan, aging, and cellular senescence: A mini-review.
Gerontology. 64:127–134. 2018.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lee SH, Lee JH, Lee HY and Min KJ: Sirtuin
signaling in cellular senescence and aging. BMB Rep. 52:24–34.
2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Tomasetti C and Vogelstein B: Variation in
cancer risk among tissues can be explained by the number of stem
cell divisions. Science. 347:78–81. 2015.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Zheng PP, Li J and Kros JM: Breakthroughs
in modern cancer therapy and elusive cardiotoxicity: Critical
research-practice gaps, challenges, and insights. Med Res Rev.
38:325–376. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Li Y, Li M, Zhang Y, Zhou J, Jiang L, Yang
C, Li G, Qu W, Li X and Chen Y: Age-stratified and gender-specific
reference intervals of six tumor markers panel of lung cancer: A
geographic-based multicenter study in China. J Clin Lab Anal.
35(e23816)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Desouki MM, Fadare O, Chamberlain B,
Shakir N and Kanbour-Shakir A: Malignancy associated with ovarian
teratomas: Frequency, histotypes, and diagnostic accuracy of
intraoperative consultation. Ann Diagn Pathol. 19:103–106.
2015.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Hackethal A, Brueggmann D, Bohlmann MK,
Franke FE, Tinneberg HR and Münstedt K: Squamous-cell carcinoma in
mature cystic teratoma of the ovary: Systematic review and analysis
of published data. Lancet Oncol. 9:1173–1180. 2008.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Yamanaka Y, Tateiwa Y, Miyamoto H, Umemoto
Y, Takeuchi Y, Katayama K and Hashimoto K: Preoperative diagnosis
of malignant transformation in mature cystic teratoma of the ovary.
Eur J Gynaecol Oncol. 26:391–392. 2005.PubMed/NCBI
|
|
77
|
Mahtate M, Talib S, Slaoui A, Zeraidi N,
Lakhdar A, Rhrab B and Baydada A: Malignant degeneration of a
mature ovarian teratoma. Case Rep Obstet Gynecol.
2021(5527467)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Dede M, Gungor S, Yenen MC, Alanbay I,
Duru NK and Haşimi A: CA19-9 may have clinical significance in
mature cystic teratomas of the ovary. Int J Gynecol Cancer.
16:189–193. 2006.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Frimer M, Seagle BL, Chudnoff S, Goldberg
GL and Shahabi S: Role of elevated cancer antigen 19-9 in women
with mature cystic teratoma. Reprod Sci. 21:1307–1311.
2014.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Yesilyurt H, Seckin B, Aktulay A and Ozyer
S: Age-stratified analysis of tumor markers and tumor
characteristics in adolescents and young women with mature cystic
teratoma. J Chin Med Assoc. 81:499–504. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Starkweather AR, Alhaeeri AA, Montpetit A,
Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE and
Jackson-Cook CK: An integrative review of factors associated with
telomere length and implications for biobehavioral research. Nurs
Res. 63:36–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Fasching CL: Telomere length measurement
as a clinical biomarker of aging and disease. Crit Rev Clin Lab
Sci. 55:443–465. 2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Limanaqi F, Biagioni F, Gambardella S,
Familiari P, Frati A and Fornai F: Promiscuous roles of autophagy
and proteasome in neurodegenerative proteinopathies. Int J Mol Sci.
24(3028)2020.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Kumari R and Jat P: Mechanisms of cellular
senescence: Cell cycle arrest and senescence associated secretory
phenotype. Front Cell Dev Biol. 9(645593)2021.PubMed/NCBI View Article : Google Scholar
|