Introduction
Hepatitis E virus (HEV) infection occurs in an
estimated 20 million individuals, leading to an estimated 3.3
million symptomatic cases in developing and developed countries
worldwide (1). Infection by HEV
genotypes 1 and 2, which infect humans via the fecal-oral routes
through the intake of contaminated foods and water, has mainly been
reported in Southeast Asia and Mexico (2-5).
HEV genotypes 3 and 4 are associated with zoonotic infection and
are observed worldwide (6-9).
Acute hepatic insult manifesting as jaundice and
coagulopathy, and complicated within 4 weeks by ascites and/or
encephalopathy in patients with previously diagnosed or undiagnosed
chronic liver disease is defined as acute-on-chronic liver failure
(ACLF) (10). HEV genotype 1 plays a
crucial role in acute viral hepatitis and ACLF in developing
countries (11). Acute HEV genotype
1 infection is a leading cause of ACLF in Bangladesh and India
(12,13).
HEV genotype 3 can also induce chronic infection in
immunocompromised individuals and ACLF in patients with underlying
liver disease (14-16).
It has been reported that patients with HEV genotype 3 or 4 are
susceptible to treatment with ribavirin (16).
Recently, the authors treated a Japanese male
patient with severe acute HEV genotype 3b infection and
alcohol-associated liver disease. The present study reports this
case and discusses the possibility of ACLF induced by HEV genotype
3 infection and its treatment.
Case report
A patient male in his 70s who consumed alcohol
experienced abdominal distention, loss of appetite, epigastric pain
and dark urine (jaundice). After 5 days, he visited the local
clinic near his residence, and the worsening of his liver function
was observed by the obtained test results. The following day, he
was referred and admitted to Nihon University Itabashi Hospital
(Tokyo, Japan).
Due to the patient's history of cerebral infarction,
hypertension, diabetes mellitus and hyperuricemia, he regularly
visited the local clinic. Aspirin, valsartan, amlodipine besylate,
furosemide, sitagliptin phosphate hydrate, ipragliflozin L-proline,
febuxostat and magnesium oxide were prescribed. He had also
undergone surgery for his springer finger, and cefaclor, loxoprofen
sodium salt and lebamipide were prescribed. He began to consume
alcohol at 12 years of age. He had also consumed horse sashimi 1
month prior. He had no history of transfusion, tattooing, drug
abuse or drug allergies, and had not recently traveled abroad. He
had no family history of liver disease.
The height of the patient was 161 cm and his body
weight was 69 kg. His blood pressure, pulse rate and body
temperature were 157/93 mmHg, 71/min and 36.1˚C, respectively. A
physical examination revealed that he was conscious; he had hepatic
encephalopathy grade <2, and he had conjunctival icterus.
Abdominal distention was observed.
The laboratory data of the patient obtained upon
admission are presented in Table I,
indicating marked liver dysfunction and a history of hepatitis B
virus (HBV) infection. At 2 weeks following admission, positivity
for anti-HEV immunoglobulin A (IgA) antibody (Institute of
Immunology, Co. Ltd., Tokyo, Japan) was revealed. The patient was
found to be HEV RNA-positive and he had acute HEV genotype 3b
infection, as determined according to previously described methods
(17). Furthermore, using stored
serial serum samples, the IgG, IgM and IgA classes of HEV
antibodies were determined as previously described (18), and all these HEV antibodies tested
positive until the end of the observation period (day 83) (Table II). He also had chronic kidney
disease with a severely decreased estimated glomerular filtration
rate (category G4) (19) and type 2
diabetes mellitus.
 | Table ILaboratory data of the patient upon
admission (on day 0). |
Table I
Laboratory data of the patient upon
admission (on day 0).
| Item | Values | Item | Values | Item | Values |
|---|
| WBC | 5,600 /µl | AST | 708 IU/l | NH3 | 43 µg/dl |
| RBC |
479x104/µl | ALT | 1,067
IU/l | anti-HIV | Negative |
| Hemoglobin | 14.5 g/dl | LDH | 410 IU/l | HBsAg | Negative |
| Platelets |
132x103/µl | ALP | 491
IU/l |
anti-HBc |
Positive |
| Neutrophils | 70.1% | γ-GTP | 443
IU/l | anti-HBc IgM | Negative |
| Basophils | 0.4% | CPK | 66 U/ml | anti-HCV | Negative |
| Eosinophils | 1.1% | T. Bil | 6.6
mg/dl | anti-HAV IgM | Negative |
| Monocytes | 7.7% | D. Bil | 5.3
mg/dl | anti-HEV
IgA |
Positive |
| Lymphocytes | 20.6% | TP | 6.5 g/dl | HEV RNA |
Positive |
| PT, INR | 94%, 1.04 | Albumin | 3.4 g/dl | ANA | Negative |
| T. CHO | 130 mg/dl | BUN | 48.7
mg/dl | AMA M2 | Negative |
| TG | 194 mg/dl |
Creatinine | 2.2
mg/dl | IgG | 1,391 mg/dl |
| Glucose | 161 mg/dl | eGFR | 24.2 ml/min/1.73
m2 | IgA | 336 mg/dl |
| HbA1c | 8.6% | CRP | 3.4
mg/dl | IgM | 143 mg/dl |
 | Table IIChanges in biochemical and
virological parameters of the patient following admission. |
Table II
Changes in biochemical and
virological parameters of the patient following admission.
| Day | AST (IU/l) | ALT (IU/l) | T. Bil (mg/dl) | Cre (mg/dl) | Anti-HEV IgG | COI | Anti-HEV IgM | COI | Anti-HEV IgA | COI | HEV RNA |
|---|
| 0 | 708 | 1,067 | 6.6 | 2.2 | 1.724 | + | 2.728 | + | 1.963 | + | + |
| 1 | 533 | 849 | 6.2 | 1.9 | 1.766 | + | 2.517 | + | 2.135 | + | + |
| 5 | 145 | 315 | 10.3 | 1.4 | | | | | | | |
| 7 | 181 | 270 | 15.4 | 1.3 | | | | | | | |
| 8 | 192 | 247 | 15.6 | 1.2 | | | | | | | |
| 11 | 273 | 294 | 22.7 | 1.3 | | | | | | | |
| 13 | 287 | 320 | 25.9 | 1.4 | 2.680 | + | 2.099 | + | 2.279 | + | + |
| 14 | 246 | 285 | 24.2 | 1.4 | 2.617 | + | 1.901 | + | 2.264 | + | + |
| 15 | 221 | 277 | 26.3 | 1.4 | 2.630 | + | 1.838 | + | 2.218 | + | + |
| 18 | 92 | 141 | 24.7 | 1.5 | 2.616 | + | 1.777 | + | 2.203 | + | + |
| 20 | 68 | 97 | 23.5 | 1.6 | 2.583 | + | 1.741 | + | 2.207 | + | + |
| 22 | 66 | 72 | 22.3 | 1.6 | 2.502 | + | 1.718 | + | 2.236 | + | + |
| 25 | 62 | 56 | 18.1 | 1.5 | 2.476 | + | 1.748 | + | 2.236 | + | + |
| 26 | 54 | 48 | 15.2 | 1.5 | 2.482 | + | 1.688 | + | 2.163 | + | + |
| 27 | 55 | 47 | 13.7 | 1.4 | 2.562 | + | 1.679 | + | 2.053 | + | + |
| 29 | 69 | 51 | 13.1 | 1.4 | 2.562 | + | 1.720 | + | 2.061 | + | + |
| 32 | 58 | 49 | 9.3 | 1.3 | 2.582 | + | 1.595 | + | 1.848 | + | + |
| 34 | 62 | 54 | 8.2 | 1.4 | 2.628 | + | 1.577 | + | 1.840 | + | - |
| 36 | 56 | 52 | 7.1 | 1.4 | 2.562 | + | 1.420 | + | 1.771 | + | - |
| 39 | 60 | 61 | 6.5 | 1.4 | 2.514 | + | 1.508 | + | 1.845 | + | - |
| 41 | 43 | 49 | 5.1 | 1.4 | 2.556 | + | 1.440 | + | 1.710 | + | - |
| 43 | 41 | 48 | 4.3 | 1.3 | 2.814 | + | 1.453 | + | 1.672 | + | - |
| 48 | 51 | 48 | 3.7 | 1.3 | 2.812 | + | 1.456 | + | 1.564 | + | - |
| 61 | 37 | 37 | 2.3 | 1.3 | 2.751 | + | 1.592 | + | 1.265 | + | - |
| 83 | 30 | 32 | 1.4 | 1.2 | 2.770 | + | 1.026 | + | 0.992 | + | - |
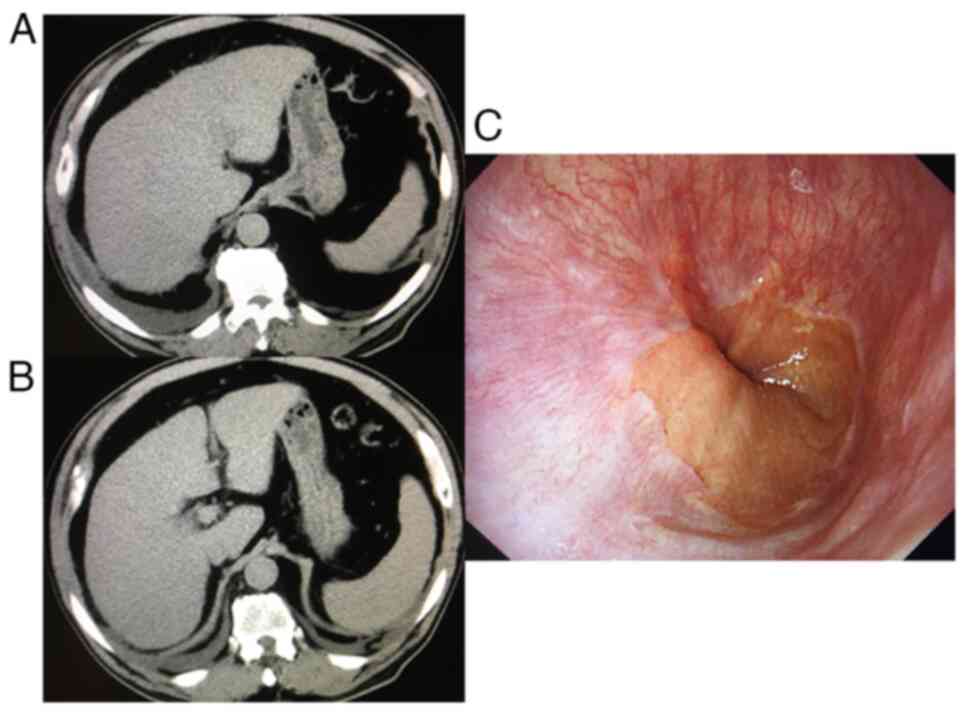
An abdominal computed tomography scan indicated an
atrophic liver and cirrhosis, although no gastrointestinal varices
were present, according to the endoscopy (Fig. 1). An abdominal ultrasound sonography
revealed collateral veins and splenomegaly; his liver stiffness was
41.2 kPa according to transient elastography, indicating liver
cirrhosis and inflammation, although he never experienced any
episodes of ascites, jaundice, hepatic encephalopathy, or variceal
bleeding (20). The patient was
diagnosed with severe acute HEV genotype 3b and alcohol-associated
liver cirrhosis.
Due to his alcohol consumption shortly prior,
indications for liver transplantation were not assessed. As
ribavirin could not be used due to renal dysfunction (21), only conservative treatment was
administered. However, his abnormal liver function tests gradually
improved, although his HEV RNA was detectable by the highly
sensitive nested reverse transcription-polymerase chain reaction
with primers targeting the ORF2/ORF3 overlapping region (22) until day 32 after admission. The peak
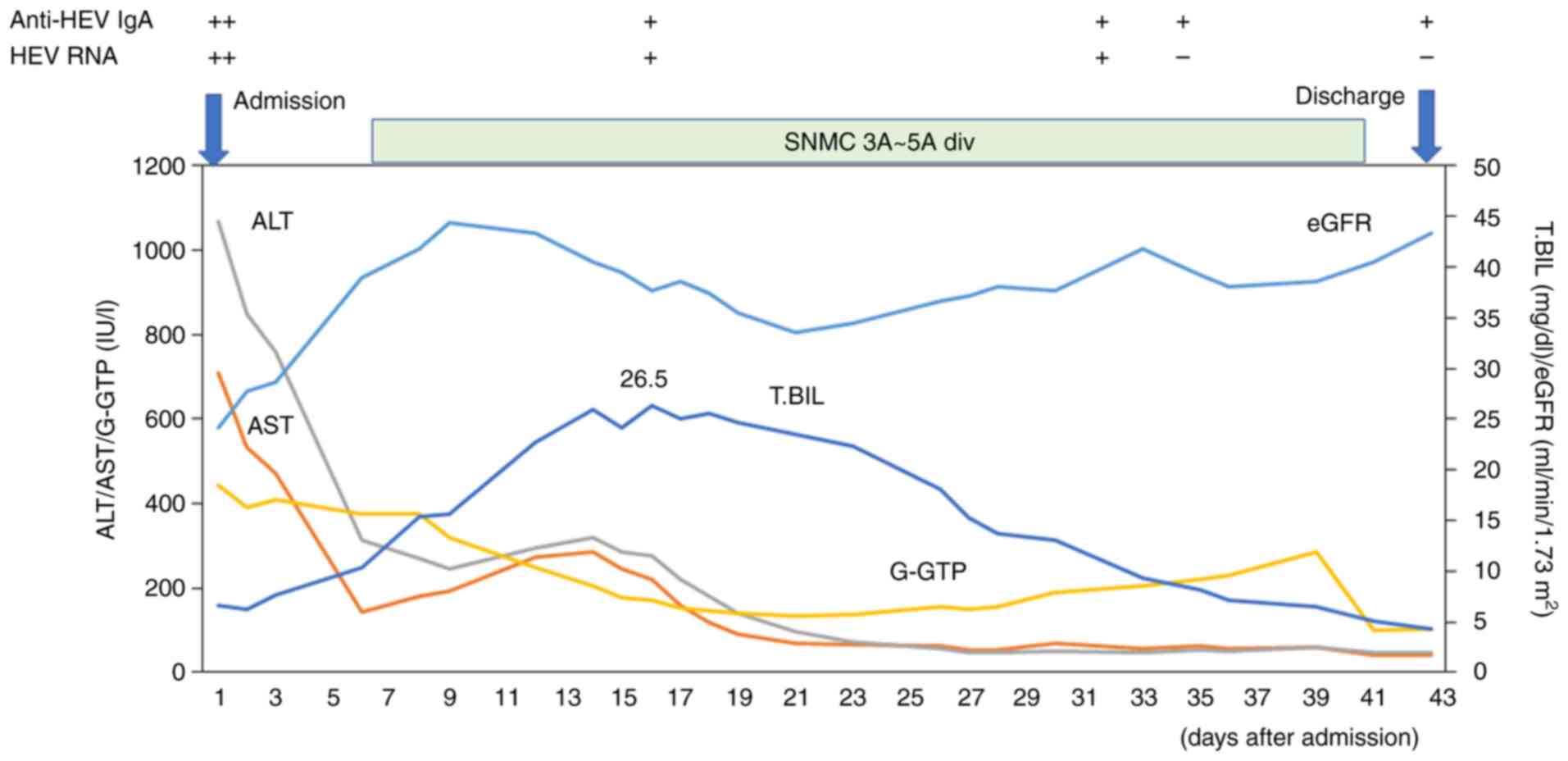
alanine aminotransferase (ALT) and total bilirubin levels were
1,067 IU/l and 26.3 mg/dl, respectively. He was ultimately
discharged, and he left the hospital on foot on day 43 (Table II and Fig. 2).
Discussion
Barbosa et al (21) reported four ACLF/death patients with
an HEV genotype 3 infection, and HEV should be considered an acute
insult in the acute decompensation of cirrhosis and ACLF. The
present study also observed ACLF, which was associated with HEV
genotype 3b and alcohol-induced acute insult and chronic liver
disease in a patient in Japan.
Barbosa et al (21) reported that 50% of the patients with
HEV genotype 3 infection were male, the median age was 63 years
(range, 51-76 years), and the median ALT level at presentation was
2,486 U/l (range, 1,146-3,134 U/l) in the majority of cases of
HEV-related ACLF. Among the causes of cirrhosis in these 4
patients, in 1 and 3 patients, this was caused by non-alcoholic
steatohepatitis and alcohol-use, respectively (21). The data of the patient described in
the present study were in agreement with this previous report
(21).
The peak ALT level was 1,067 IU/l in the present
case. High ALT levels may provide an indication for the diagnosis
of acute HEV infection (21,23). Barbosa et al (21) also used ribavirin in 3 patients;
however, in the present study, ribavirin could not be used due to
renal dysfunction. In general, pregnancy, severe anemia or renal
dysfunction prohibit the use of ribavirin.
Although the present study did not measure HEV viral
loads, HEV RNA became undetectable on day 34 in the present case
(Table II). It appears to be more
beneficial for patients to eradicate the hepatitis virus causing
the hepatitis. More effective anti-HEV drugs need to be developed
for severe HEV infection, particularly, in pregnant females
(24). HEV-infected patients with
cirrhosis with or without HBV infection may develop ACLF, which is
associated with a high mortality rate (~70%) (25-27).
The patient in the present study had consumed horse
sashimi approximately 1 month prior to the onset of his symptoms,
such as abdominal distention, loss of appetite, epigastric pain and
dark urine (jaundice). It has been reported that anti-HEV IgG
antibody and/or HEV RNA are positive in workhorses or horses in
Egypt (28), China (29), the Netherlands (30), Bulgaria (31) and Germany (32). However, since whether horses play a
role in the transmission of HEV remains unknown, further studies
are warranted in this regard.
HEV has been reported to be the most common cause of
infection in 95 (46.1%) of 206 patients with acute sporadic viral
hepatitis and 60 (67.4%) of 89 patients with ACLF in India, an
endemic country of HEV genotype 1 infection (11). Lim et al (33) reported that a 59-year-old Caucasian
male acquired HEV infection and fatal hepatic decompensated
alcohol-associated liver cirrhosis in the United Kingdom, where the
HEV genotype 3 is a major genotype. Fantilli et al (34) reported that HEV genotype 3 infection
in a patient with alcohol-associated liver disease developed ACLF
in Argentina.
To the best of our knowledge, there are two reported
cases from Japan, where patients with primary biliary cholangitis
were infected with HEV genotype 3b (35,36). Of
these 2 patients, 1 patient succumbed due to the rupture of
hepatocellular carcinoma (36).
Another study reported that a 49-year-old male with excessive
alcohol consumption and acute HEV genotype 4 infection developed
acute liver failure (37). Thus, HEV
genotype 3 or HEV genotype 4 is also a key acute insult in ACLF. Of
interest, among patients with alcohol-associated liver cirrhosis, a
higher prevalence of anti-HEV IgG has been observed in Poland
(38) and Argentina (39).
Pegylated interferon-α with or without ribavirin may
play a role in eradicating HEV in some patients, although pegylated
interferon-α has more side-effects (40). Several drugs, such as interferon-λ,
sofosbuvir, azithromycin and ritonavir, exert anti-viral effects on
HEV replication in vitro (41,42).
Further studies are required for the development of drugs against
HEV infection.
In conclusion, careful attention should be paid to
viral hepatitis, including hepatitis E, in patients with
alcohol-associated liver disease. Further research and the
development of novel drugs for HEV infection are required for the
prevention of severe HEV infections.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Japan Agency for
Medical Research and Development (AMED) under the grant no.
JP23fk0210132.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TK, RST, MTa and HO conceptualized the study, and
collected and analyzed the patient's data. TK, SA, MTo, MH, RM, NM,
MO and HK saw and examined the patient described in the present
case report. MTa and HO analyzed the serum anti-HEV and serum HEV
RNA levels of the patient. NM, MO and HK advised on medical images.
TK, SA, RM, MTa and HO confirm the authenticity of all the raw
data. TK, RST, MTa, HO and HK drafted the initial manuscript and
revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and approved by the Ethics Committee of
Nihon University Itabashi Hospital (protocol code: RK-180911-12;
dates of approval: October 5, 2018 and September 13, 2023) for
studies involving human participants. Participation in the study
was posted on the website of Nihon University Itabashi Hospital,
and informed consent was obtained from the patient described
herein.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization: Hepatitis E.
WHO, Geneva, 2023. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
Accessed on December 25, 2023.
|
|
2
|
Balayan MS, Andjaparidze AG, Savinskaya
SS, Ketiladze ES, Braginsky DM, Savinov AP and Poleschuk VF:
Evidence for a virus in non-A, non-B hepatitis transmitted via the
fecal-oral route. Intervirology. 20:23–31. 1983.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reyes GR, Purdy MA, Kim JP, Luk KC, Young
LM, Fry KE and Bradley DW: Isolation of a cDNA from the virus
responsible for enterically transmitted non-A, non-B hepatitis.
Science. 247:1335–1339. 1990.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ray R, Aggarwal R, Salunke PN, Mehrotra
NN, Talwar GP and Naik SR: Hepatitis E virus genome in stools of
hepatitis patients during large epidemic in north India. Lancet.
338:783–784. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Huang CC, Nguyen D, Fernandez J, Yun KY,
Fry KE, Bradley DW, Tam AW and Reyes GR: Molecular cloning and
sequencing of the Mexico isolate of hepatitis E virus (HEV).
Virology. 191:550–558. 1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Meng XJ, Purcell RH, Halbur PG, Lehman JR,
Webb DM, Tsareva TS, Haynes JS, Thacker BJ and Emerson SU: A novel
virus in swine is closely related to the human hepatitis E virus.
Proc Natl Acad Sci USA. 94:9860–9865. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Schlauder GG, Dawson GJ, Erker JC, Kwo PY,
Knigge MF, Smalley DL, Rosenblatt JE, Desai SM and Mushahwar IK:
The sequence and phylogenetic analysis of a novel hepatitis E virus
isolated from a patient with acute hepatitis reported in the United
States. J Gen Virol. 79 (Pt 3):447–456. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Okamoto H, Takahashi M, Nishizawa T, Fukai
K, Muramatsu U and Yoshikawa A: Analysis of the complete genome of
indigenous swine hepatitis E virus isolated in Japan. Biochem
Biophys Res Commun. 289:929–936. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takahashi K, Kang JH, Ohnishi S, Hino K,
Miyakawa H, Miyakawa Y, Maekubo H and Mishiro S: Full-length
sequences of six hepatitis E virus isolates of genotypes III and IV
from patients with sporadic acute or fulminant hepatitis in Japan.
Intervirology. 46:308–318. 2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sarin SK, Kumar A, Almeida JA, Chawla YK,
Fan ST, Garg H, de Silva HJ, Hamid SS, Jalan R, Komolmit P, et al:
Acute-on-chronic liver failure: Consensus recommendations of the
Asian Pacific Association for the study of the liver (APASL).
Hepatol Int. 3:269–282. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gupta E, Ballani N, Kumar M and Sarin SK:
Role of non-hepatotropic viruses in acute sporadic viral hepatitis
and acute-on-chronic liver failure in adults. Indian J
Gastroenterol. 34:448–452. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mahtab MA, Rahman S, Khan M and Karim MF:
Hepatitis E virus is a leading cause of acute-on-chronic liver
disease: Experience from a tertiary centre in Bangladesh.
Hepatobiliary Pancreat Dis Int. 8:50–52. 2009.PubMed/NCBI
|
|
13
|
Krishna YA, Saraswat VA, Das K, Himanshu
G, Yachha SK, Aggarwal R and Choudhuri G: Clinical features and
predictors of outcome in acute hepatitis A and hepatitis E virus
hepatitis on cirrhosis. Liver Int. 29:392–398. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Péron JM, Bureau C, Poirson H, Mansuy JM,
Alric L, Selves J, Dupuis E, Izopet J and Vinel JP: Fulminant liver
failure from acute autochthonous hepatitis E in France: Description
of seven patients with acute hepatitis E and encephalopathy. J
Viral Hepat. 14:298–303. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Blasco-Perrin H, Madden RG, Stanley A,
Crossan C, Hunter JG, Vine L, Lane K, Devooght-Johnson N,
Mclaughlin C, Petrik J, et al: Hepatitis E virus in patients with
decompensated chronic liver disease: A prospective UK/French study.
Aliment Pharmacol Ther. 42:574–581. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Denner J, Pischke S, Steinmann E, Blümel J
and Glebe D: Why all blood donations should be tested for hepatitis
E virus (HEV). BMC Infect Dis. 19(541)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mizuo H, Suzuki K, Takikawa Y, Sugai Y,
Tokita H, Akahane Y, Itoh K, Gotanda Y, Takahashi M, Nishizawa T
and Okamoto H: Polyphyletic strains of hepatitis E virus are
responsible for sporadic cases of acute hepatitis in Japan. J Clin
Microbiol. 40:3209–3218. 2002.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Takahashi M, Kusakai S, Mizuo H, Suzuki K,
Fujimura K, Masuko K, Sugai Y, Aikawa T, Nishizawa T and Okamoto H:
Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies
against hepatitis E virus (HEV) Is highly specific for diagnosis of
acute HEV infection. J Clin Microbiol. 43:49–56. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Murton M, Goff-Leggett D, Bobrowska A,
Sanchez JJ, James G, Wittbrodt E, Nolan S, Sörstadius E,
Pecoits-Filho R and Tuttle K: Burden of chronic kidney disease by
KDIGO categories of glomerular filtration rate and albuminuria: A
systematic review. Adv Ther. 38:180–200. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ikegami C, Kanda T, Ishii T, Honda M,
Yamana Y, Tanaka RS, Kumagawa M, Kanezawa S, Mizutani T, Yamagami
H, et al: COVID-19 after treatment with direct-acting antivirals
for HCV infection and decompensated cirrhosis: A case report. In
Vivo. 36:1986–1993. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barbosa JV, Müllhaupt B, Brunner F,
Sinnreich MF, Semela D, Montani M, Cathomas G, Neuweiler J,
Gouttenoire J, Artru F, et al: Autochthonous hepatitis E as a cause
of acute-on-chronic liver failure and death: Histopathology can be
misleading but transaminases may provide a clue. Swiss Med Wkly.
151(w20502)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Inoue J, Takahashi M, Yazaki Y, Tsuda F
and Okamoto H: Development and validation of an improved RT-PCR
assay with nested universal primers for detection of hepatitis E
virus strains with significant sequence divergence. J Virol
Methods. 137:325–333. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hirano R, Kanda T, Honda M, Arima S,
Totsuka M, Masuzaki R, Kanezawa S, Sasaki-Tanaka R, Matsumoto N,
Yamagami H, et al: Hepatitis E virus infection caused elevation of
alanine aminotransferase levels in a patient with chronic hepatitis
B and choledocholithiasis. Reports. 6(55)2023.
|
|
24
|
Miyoshi M, Kakinuma S, Tanabe Y, Ishii K,
Li TC, Wakita T, Tsuura Y, Watanabe H, Asahina Y, Watanabe M and
Ikeda T: Chronic hepatitis E infection in a persistently
immunosuppressed patient unable to be eliminated after ribavirin
therapy. Intern Med. 55:2811–2817. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Frias M, López-López P, Rivero A and
Rivero-Juarez A: Role of hepatitis E virus infection in
acute-on-chronic liver failure. Biomed Res Int.
2018(9098535)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Choi JW, Son HJ, Lee SS, Jeon H, Cho JK,
Kim HJ, Cha RR, Lee JM, Kim HJ, Jung WT and Lee OJ: Acute hepatitis
E virus superinfection increases mortality in patients with
cirrhosis. BMC Infect Dis. 22(62)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao H, Ye W, Yu X, Hu J, Zhang X, Yang M,
Sheng J and Shi Y: Hepatitis E virus superinfection impairs
long-term outcome in hospitalized patients with hepatitis B
virus-related decompensated liver cirrhosis. Ann Hepatol.
28(100878)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Saad MD, Hussein HA, Bashandy MM, Kamel
HH, Earhart KC, Fryauff DJ, Younan M and Mohamed AH: Hepatitis E
virus infection in work horses in Egypt. Infect Genet Evol.
7:368–373. 2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhang W, Shen Q, Mou J, Gong G, Yang Z,
Cui L, Zhu J, Ju G and Hua X: Hepatitis E virus infection among
domestic animals in eastern China. Zoonoses Public Health.
55:291–298. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li Y, Qu C, Spee B, Zhang R, Penning LC,
de Man RA, Peppelenbosch MP, Fieten H and Pan Q: Hepatitis E virus
seroprevalence in pets in the Netherlands and the permissiveness of
canine liver cells to the infection. Ir Vet J. 73(6)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tsachev I, Gospodinova K, Pepovich R,
Takova K, Kundurzhiev T, Zahmanova G, Kaneva K and Baymakova M:
First insight into the seroepidemiology of hepatitis E Virus (HEV)
in dogs, cats, horses, cattle, sheep, and goats from Bulgaria.
Viruses. 15(1594)2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pischke S, Knoop EV, Mader M, Kling L,
Wolski A, Wagner A, Mueller K, Horvatits T, Stiller J, Wisnewski K,
et al: Anti-HEV seroprevalence and rate of viremia in a German
cohort of dogs, cats, and horses. Sci Rep. 13(19240)2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lim KH, Howard M, Jackson N and Verma S:
Long-term outcomes after hospitalization with spontaneous bacterial
peritonitis. BMJ Case Rep. 2014(bcr2013202561)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fantilli A, Villa SD, Zerega A, Di Cola G,
López L, Martínez MW, Pisano MB and Ré VE: Hepatitis E virus
infection in a patient with alcohol related chronic liver disease:
A case report of acute-on-chronic liver failure. Virol J.
18(245)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Inagaki K, Takaki S, Honda Y, Inoue M,
Mori N, Kawakami H, Kawakami Y, Kawakami M, Okamoto H, Tuji K, et
al: A case of hepatitis E virus infection in a patient with primary
biliary cholangitis. Kanzo. 58:183–190. 2017.(In Japanese).
|
|
36
|
Okano H, Asakawa H, Tsuruga S, Nose K,
Tochio T, Kumazawa H, Isono Y, Tanaka H, Matsusaki S, Sase T, et
al: A fatal case of exacerbated liver cirrhosis caused by acute
hepatitis E virus infection: Atypical dynamics of anti-hepatitis E
virus antibody titers. Kanzo. 61:326–334. 2020.(In Japanese).
|
|
37
|
Kobayashi T, Tanaka S, Yokoyama T,
Matsumoto S, Sarashina K and Kawahata S: A case of acute liver
failure induced by hepatitis E virus in a patient with chronic
alcoholic liver dysfunction. Kanzo. 61:376–381. 2020.(In
Japanese).
|
|
38
|
Parfieniuk-Kowerda A, Jaroszewicz J,
Łapiński TW, Łucejko M, Maciaszek M, Świderska M, Grzeszczuk A,
Naumnik B, Rowiński M and Flisiak R: High prevalence of anti-HEV
antibodies among patients with immunosuppression and hepatic
disorders in eastern Poland. Arch Med Sci. 17:675–681.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fantilli AC, Trinks J, Marciano S, Zárate
F, Balderramo DC, Wassaf MGM, Haddad L, Gadano A, Debes JD, Pisano
MB and Ré VE: Unexpected high seroprevalence of hepatitis E virus
in patients with alcohol-related cirrhosis. PLoS One.
14(e0224404)2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.;
European Association for the Study of the Liver. EASL clinical
practice guidelines on hepatitis E virus infection. J Hepatol.
68:1256–1271. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nishiyama T, Kobayashi T, Jirintai S,
Nagashima S, Primadharsini PP, Nishizawa T and Okamoto H: Antiviral
candidates against the hepatitis E virus (HEV) and their
combinations inhibit HEV growth in in vitro. Antiviral Res.
170(104570)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Primadharsini PP, Nagashima S, Nishiyama
T, Takahashi M, Murata K and Okamoto H: Development of recombinant
infectious hepatitis E virus harboring the nanoKAZ gene and its
application in drug screening. J Virol. 96(e0190621)2022.PubMed/NCBI View Article : Google Scholar
|