Introduction
Plumbum (Pb), one of the most widespread pollutants
in the world, induces oxidative stress and dysfunction in many cell
types (1). For humans, the major
sources of exposure to lead compounds include air, dust, food,
water and batteries (2–5). Lead has been found to cause
neurotoxicity, reproductive toxicity, liver toxicity, kidney
damage, hematological dysfunctions and immunotoxicity (3). Among all of these, the hematological
system has been proposed as an important target for lead-induced
toxicity. Approximately 99% of the lead present in the blood is
bound to erythrocytes. Moreover, erythrocytes may spread lead to
different organs of the body. The effect of lead on health depends
not only on its total dose and the length of exposure but also on
the physical and chemical state of the element and the
physiological status and age of the individual (6,7). The
most vulnerable groups at risk of harm from lead exposure are
fetuses and children of preschool age (6,8). A
previous report demonstrated that iron deficiency was associated
with higher blood lead levels in children (9).
In the current study, we used inorganic lead and
lead acetate to investigate the effects of lead on the
proliferation, osteogenic differentiation and
hematopoiesis-supportive function of mesenchymal stem cells (MSCs).
Stem cells may provide a model of cells undergoing differentiation
and proliferation for toxicology assays (10). For example, embryonic stem cells
have already provided new assays to predict embryo-fetal
developmental toxicity in vitro(11). However, certain problems are
encountered with the application of embryonic stem cells, including
complicated culture methods, high costs and ethical concerns. MSCs,
a type of adult stem cell, are capable of self-renewal, have
multipotent differentiation potential (12,13),
may be isolated from different tissue sources, and are easily
isolated from bone marrow (BM) and expanded in
vitro(14–17). They are capable of supporting
hematopoiesis (18), enhancing
angiogenesis (19) and repairing
tissue (20). Thus, it is clear
that any cellular stress in MSCs is capable of markedly diminishing
the hematopoietic system. The hematopoietic system is vulnerable to
environmental toxins and heavy metals such as cadmium (21) and lead (22). They interfere in the hematopoietic
system through the damage of genetic material of stromal and
hematopoietic cells and the alteration of cytokine homeostasis. It
is, therefore, necessary to explore the lead-induced response of
MSCs.
Materials and methods
Cell culture
Human umbilical cord mesenchymal stem cells (UCMSCs)
were isolated from umbilical cord Wharton’s jelly. Umbilical cords
were obtained from the Fourth Hospital of Zhenjiang, China.
Umbilical cords were rinsed twice by phosphate-buffered saline
(PBS) in penicillin and streptomycin. The tissues were minced into
1–2 mm3 fragments. They were cultured in a 37°C
incubator in plates with low-glucose Dulbecco’s modified Eagle’s
medium (L-DMEM) (Gibco, USA) supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 U/ml streptomycin. The
medium was changed every 3 days. After approximately two weeks,
there were cells at the edge of the tissue fragments. When UCMSCs
grew to 70% confluence, they were detached by 0.25% Trypsin-EDTA
and reseeded in a flask of 5,000 cells/cm2 for optimal
proliferation. The experimental protocol was approved by the
Jiangsu University ethics committee and informed consent was
obtained.
Cell count
UCMSCs were seeded at 1×104
cells/cm2 in 24-well plates with L-DMEM containing 10%
FBS. After one day for attachment and growth, the medium was
replaced with fresh L-DMEM supplemented with 10% FBS containing 0,
20, 40, 60, 80 and 100 μM lead acetate for 24, 48 and 72 h. Cells
were harvested and counted by quantitative cytometry.
Quantifying cell viability by MTT
assay
UCMSCs were grown in 96-well culture plates. After
one day for attachment and growth, cells grown in 96-well plates
with 200 μl L-DMDM were exposed to various concentrations of lead
acetate (0, 20, 40, 60, 80 and 100 μM) for 72 h. MTT (20 μl) was
added to each well for the final 4 h. When the reaction was
terminated, all the solution was discarded and 150 μl DMSO was
added to each well. The 96-well plate was agitated to ensure that
complete solubilization of the purple formazan crystals occurred.
Absorbance at 490 nm was measured using an ELISA reader.
Cell cycle assay
UCMSCs were treated with lead acetate for 24, 48 and
72 h. Cells were harvested and washed twice with PBS and stained
with propidium iodide (PI) (Sigma-Aldrich, USA) for 30 min in dark
conditions. The stained cells were analyzed by flow cytometry.
Apoptosis assay
UCMSCs were treated with lead acetate for 48 h.
Following treatment, the cells were trypsinized with 0.25%
trypsin-EDTA, washed twice with PBS and stained with PI and
annexin-V-FITC according to the manufacturer’s instructions. The
stained cells were analyzed by flow cytometry.
Osteogenic differentiation
UCMSCs were seeded at a density of 3×104
cells/cm2 and stimulated with osteogenic induction
medium for 14 days. The osteogenic medium consisted of low-glucose
DMEM, 10% FBS, 100 nM dexamethasone, 10 mM sodium
β-glycerophosphate and 0.05 mM-ascorbic acid 2-phosphate (all from
Sigma), and was replaced every 3 days.
Cell histochemical staining
In the differentiated UCMSCs, osteogenic
characteristics were confirmed by alkaline phosphatase (ALP)
expression using histochemical staining and the observation of the
hydroxyapatite matrix using Von Kossa staining. Cells were fixed
with fixing agent and stained with ALP according to the
manufacturer’s instructions. The Von Kossa stain was performed
according to the protocol described previously (23,24)
with a few modifications. The cells were fixed with 4%
paraformaldehyde for 5 min at room temperature, stained for 20–60
min with 2% silver nitrate (Sigma-Aldrich) and then treated with 5%
sodium thiosulfate (Sigma-Aldrich) for 2 min.
RT-PCR
Total RNA was extracted from UCMSCs and UCMSCs
treated with lead acetate using TRIzol reagent (Invitrogen, USA).
cDNA was obtained from 1 μg total RNA using an oligo-dT primer in a
reaction volume of 20 μl, using a reverse transcription kit
according to the manufacturer’s instructions (Fermentas). The cDNA
samples were subjected to polymerase chain reaction (PCR) using
specific primers. The conditions of PCR were as follows: Initial
denaturation at 94°C for 5 min, denaturation at 94°C for 30 sec,
annealing at 55–70°C for 30 sec (see Table I for temperatures used), extension
for 30 sec at 72°C for 30 cycles, and a final polymerization at
72°C for 10 min. PCR products were analyzed by electrophoresis on a
1.5% agarose gel with ethidium bromide staining and photographed
under UV transillumination. β-actin was used as a control. The
specific primers were designed as shown in Table I.
 | Table ISpecific primers for control and
target genes. |
Table I
Specific primers for control and
target genes.
| ID | Gene | Primers sequence (5′
to 3′) | Size (bp) | Annealing temp
(°C) |
|---|
| 1 | Ang-1-F |
AGAGGCACGGAAGGAGTGTG | 249 | 57 |
| Ang-1-R |
CTATCTCCAGCATGGTAGCCG | | |
| 2 | TPO-F |
ATTGCTCCTCGTGGTCAT | 220 | 56 |
| TPO-R |
CTCCTCCATCTGGGTTTT | | |
| 3 | Flt3-ligand-F |
CTGGAGCCCAACAACCTATC | 353 | 60 |
| Flt3-ligand-R |
TCTGGACGAAGCGAAGACA | | |
| 4 | SCF-F |
TGGATAAGCGAGATGGTA | 189 | 54 |
| SCF-R |
TTCTGGGCTCTTGAATGA | | |
| 5 | Ang-2-F |
GGATCTGGGGAGAGAGGAAC | 371 | 55 |
| Ang-2-R |
CTCTGCACCGAGTCATCGTA | | |
| 6 | VEGF-F |
CCTTGCTCTACCTCCAC | 280 | 61 |
| VEGF-R |
ATCTGCATCCTGTTGGA | | |
| 7 | ALP-F |
AGCTTCAAACCGAGATACAA | 220 | 56.5 |
| ALP-R |
ATTCTGCCTCCTTCCACC | | |
| 8 | β-actin-F |
CACGAAACTACCTTCAACTC | 256 | 56 |
| β-actin-R |
CATACTCCTGCTTGCTGATC | | |
Colony formation assay
UCMSCs were seeded in a 6-well plate with L-DMEM
medium supplemented with 10% FBS. After one day for attachment and
growth, the medium was replaced with fresh L-DMEM supplemented with
10% FBS containing 60 μM lead acetate for 72 h. There was no lead
acetate in the control group. Following treatment, the medium was
removed and cells were washed with PBS 2–3 times. Sterile 3% agar
was prepared and incubated at 65°C in a waterbath. Bone marrow
cells were added to a 10 ml tube with 9 ml L-DMEM medium
supplemented with 10% FBS and incubated at 37°C. In total, 1 ml 3%
agar was added to 9 ml cell suspension and mixed gently by
swirling, and 1 ml was quickly added to each of the wells of the
prepared 6-well plate. Plates were incubated at 37°C for 14 days.
Colonies were counted using a microscope.
Results
Effect of lead acetate on cell
proliferation
UCMSCs were exposed to various concentrations of
lead acetate (0, 20, 40, 60, 80 and 100 μM) for 24, 48 and 72 h,
and counted with a cytometer. The number of cells was significantly
decreased in a dose-dependent manner following lead acetate
treatment for 24, 48 and 72 h (Fig.
1A). To further investigate the cytotoxic effects of lead
acetate on cell proliferation, we used the MTT assay to test lead
acetate-induced toxicity in UCMSCs. After 72 h of lead acetate
treatment, cell viability was markedly decreased in a
dose-dependent manner (Fig.
1B).
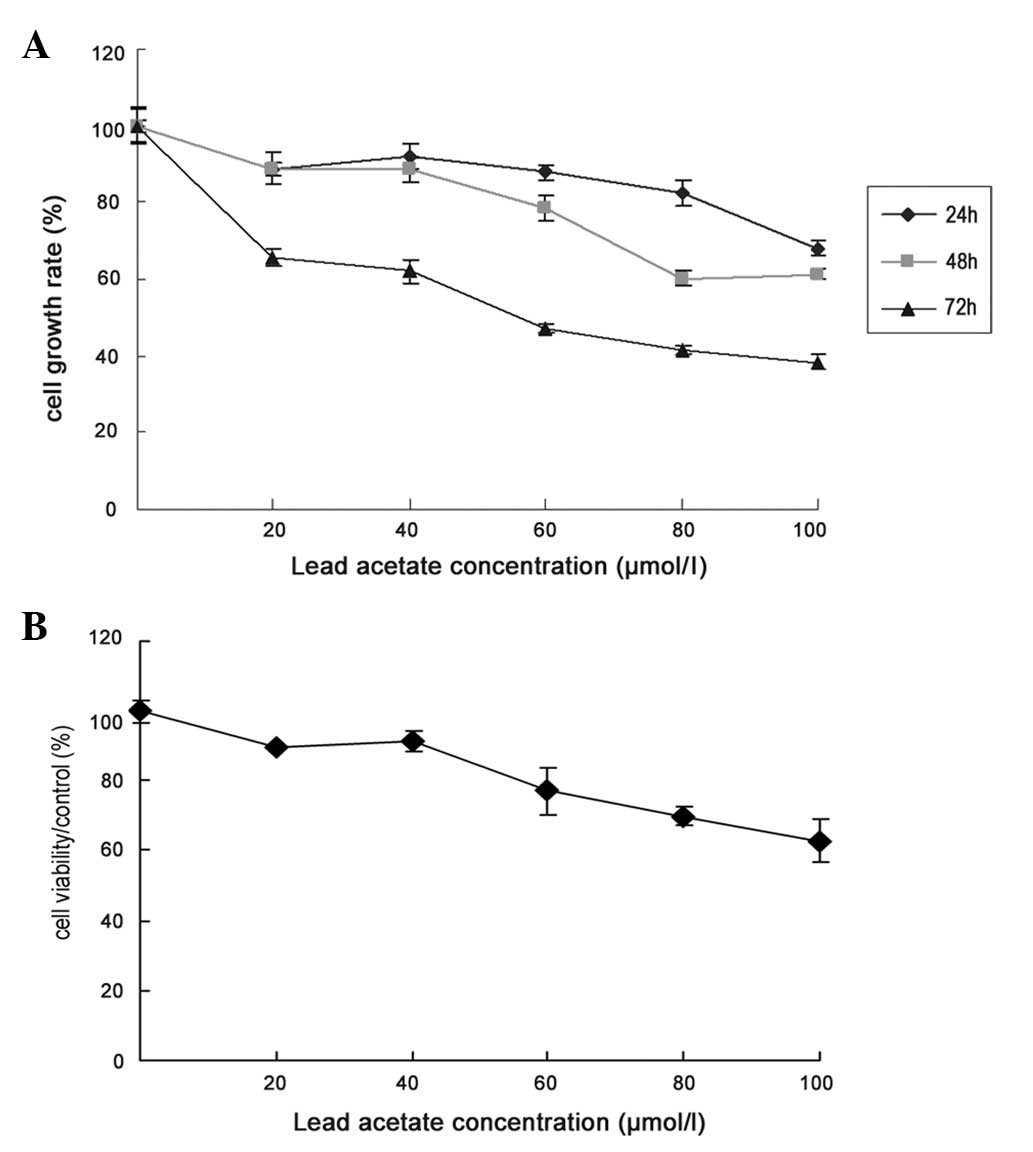 | Figure 1Effect of lead acetate on the growth
rate of MSCs. (A) Cell growth rate was assessed by cell count. The
cells were treated by lead acetate (20, 40, 60, 80 and 100 μM) for
24, 48 and 72 h. The number of untreated cells were as 100% and the
bars represent the means ± SE. (B) Cells were treated with lead
acetate (20, 40, 60, 80 and 100 μM) for 72 h, and cell viability
was determined by MTT (3-(4,5-dimethylthiazol-2-yl-)-2,5-diphenyl
tetrazolium bromide) assay. MSCs, mesenchymal stem cells. |
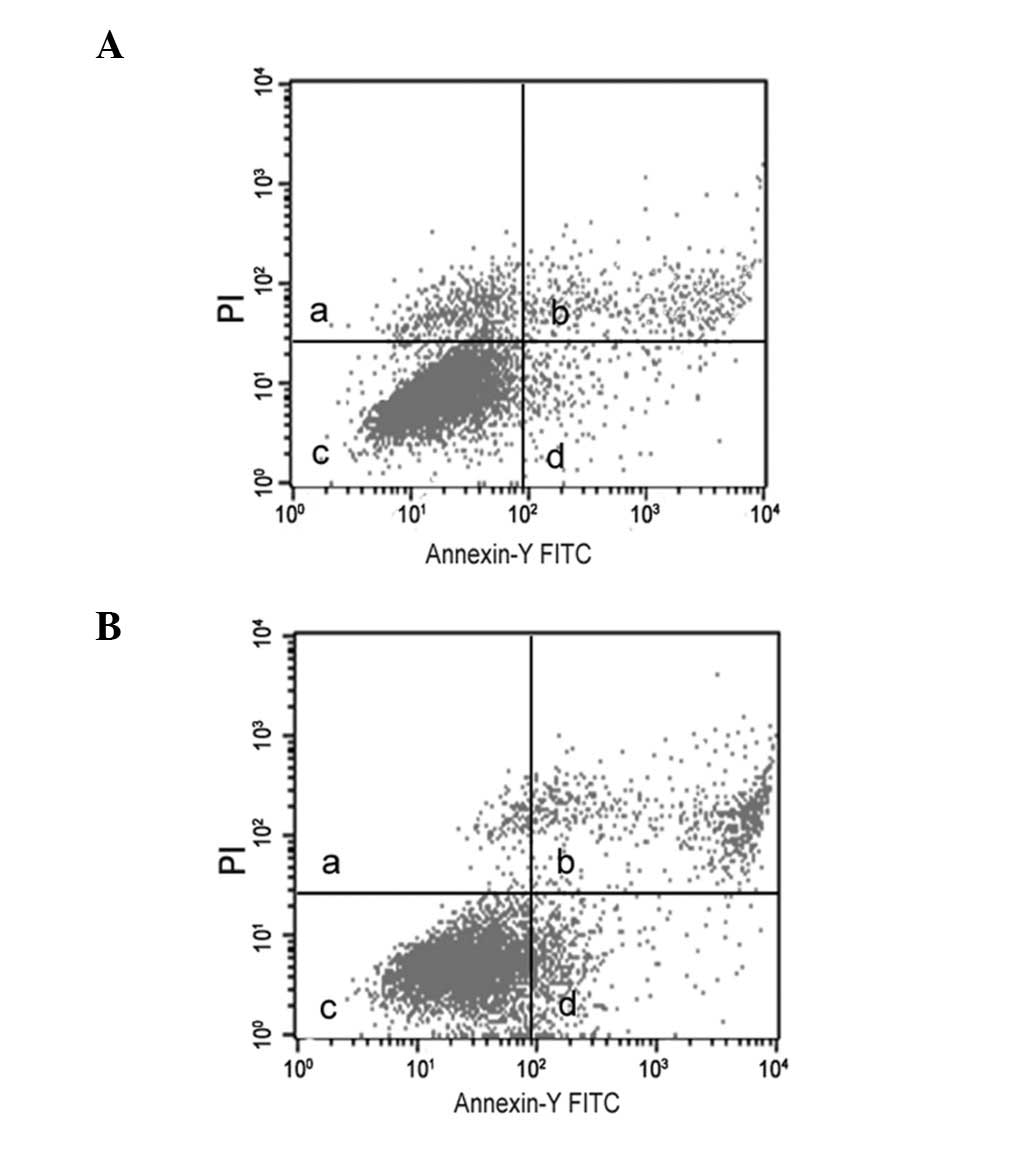
Effect of lead acetate-induced apoptosis
in human UMSCs
Treatment of lead acetate at the concentration of 60
μM showed an increase in annexin V-positive cells over the
untreated UCMSCs (Fig. 2), which
suggested that lead acetate is capable of inducing apoptosis in
UCMSCs.
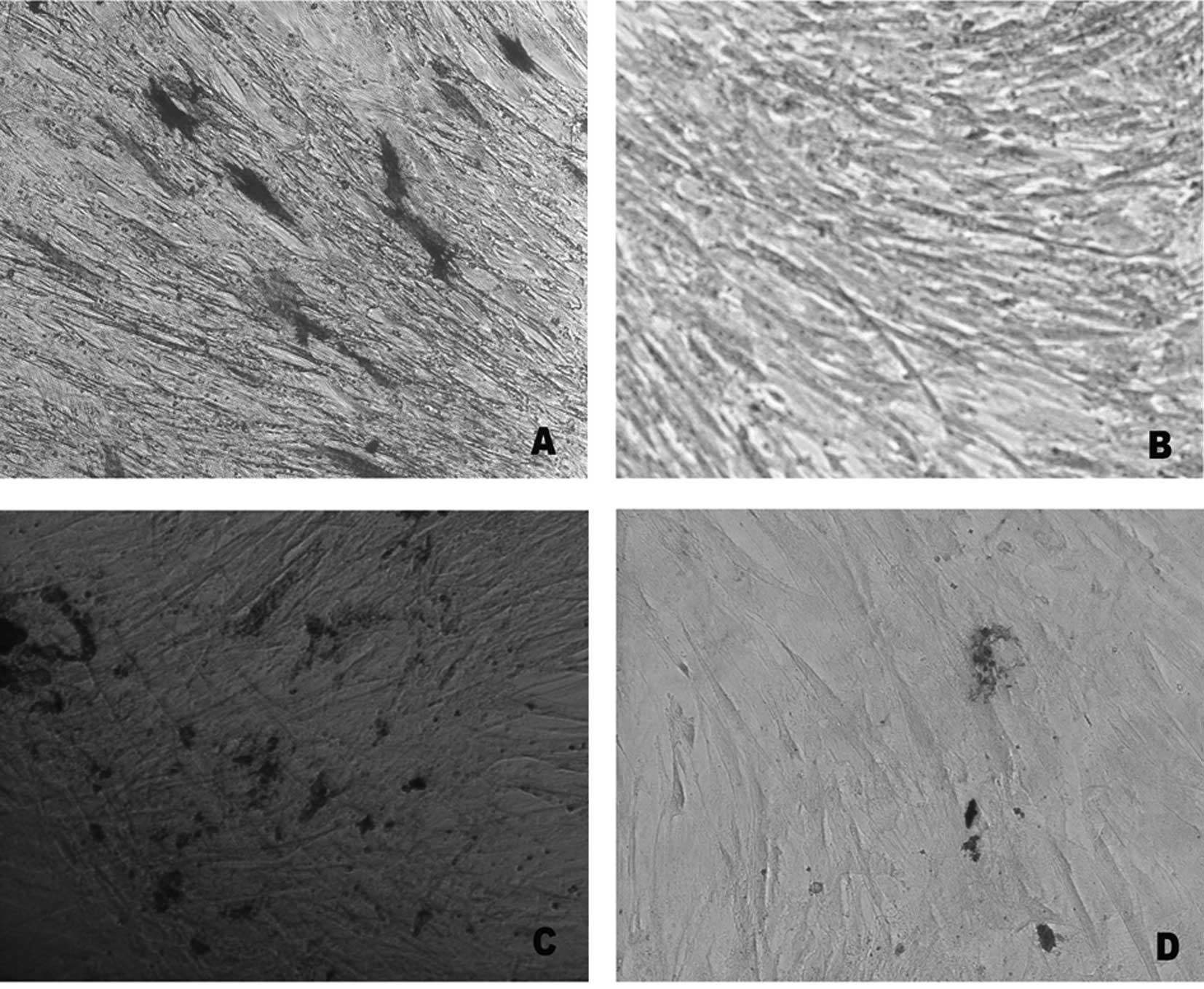
Effect of lead acetate on osteogenic
differentiation
In order to investigate the effect of lead acetate
on the differentiation potential, UCMSCs were induced to osteogenic
differentiation. Following osteogenic differentiation of UCMSCs,
the cells were stained with ALP and Von Kossa. Compared with the
control, the rates of ALP-positive staining and extracellular
matrix (ECM) calcium deposits were reduced following lead acetate
treatment (Fig. 3). The gene
expression of ALP also revealed a marked downregulation in the
experimental group (Fig. 3E).
These results indicated that lead acetate inhibited the osteogenic
differentiation of MSCs.
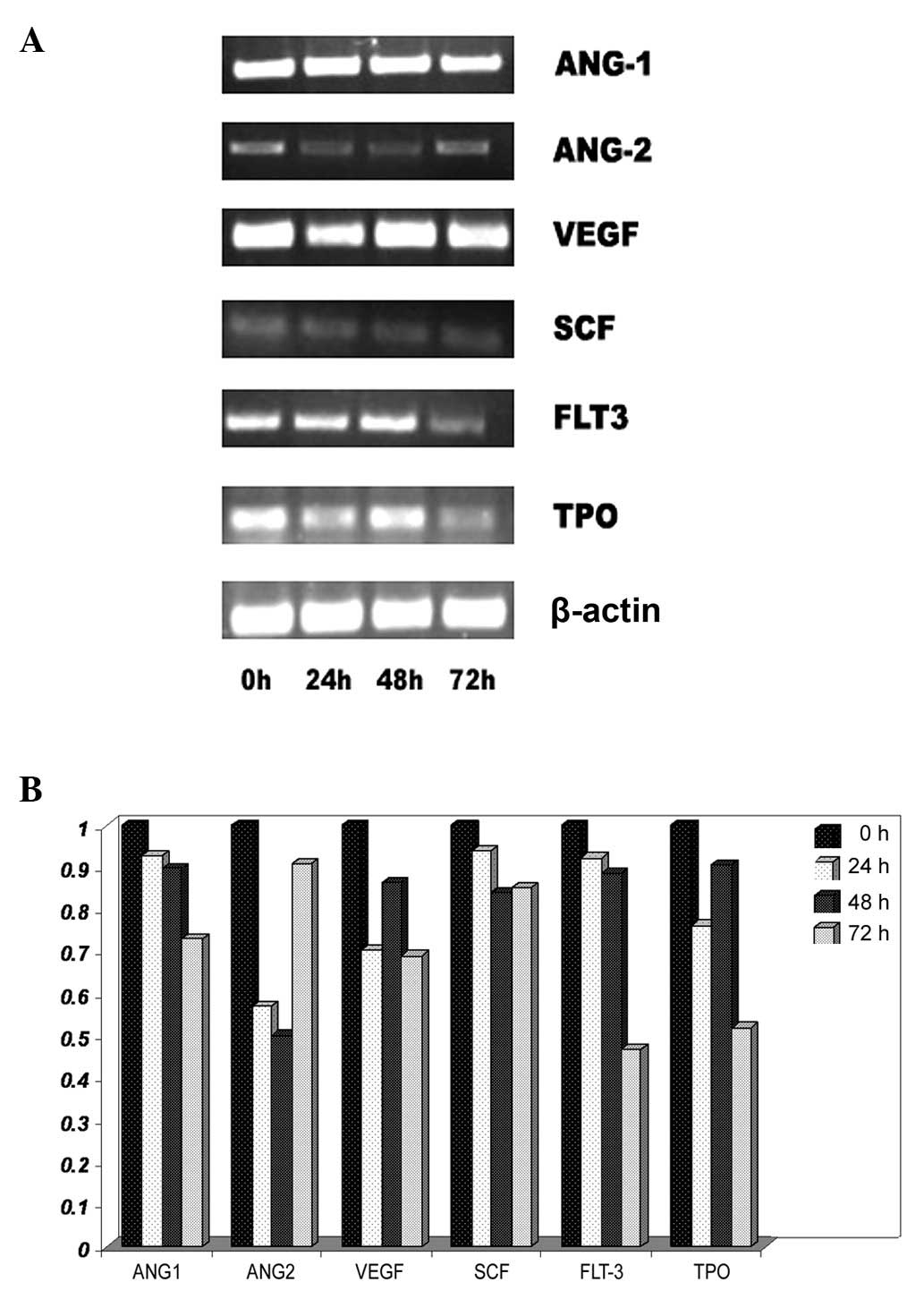
Effect of lead acetate on certain genes
of hematogenesis
To investigate the effect of lead acetate on the
hematopoietic system, the mRNA expression of cytokines was detected
in UCMSCs. These cytokines are an important component of the
hematopoietic microenvironment as well as MSCs. UCMSCs were treated
with lead acetate (60 μM) and RT-PCR was performed to analyze mRNA
expression. Following lead treatment, certain genes which were
connected with the hematopoietic system and angiopoiesis, including
Ang-1, Ang-2, VEGF, SCF, Flt3-ligand and TPO, were significantly
decreased (Fig. 4). These results
indicated that lead acetate could induce hematopoietic system
damage through inhibiting the hematopoiesis-supportive function of
MSCs.
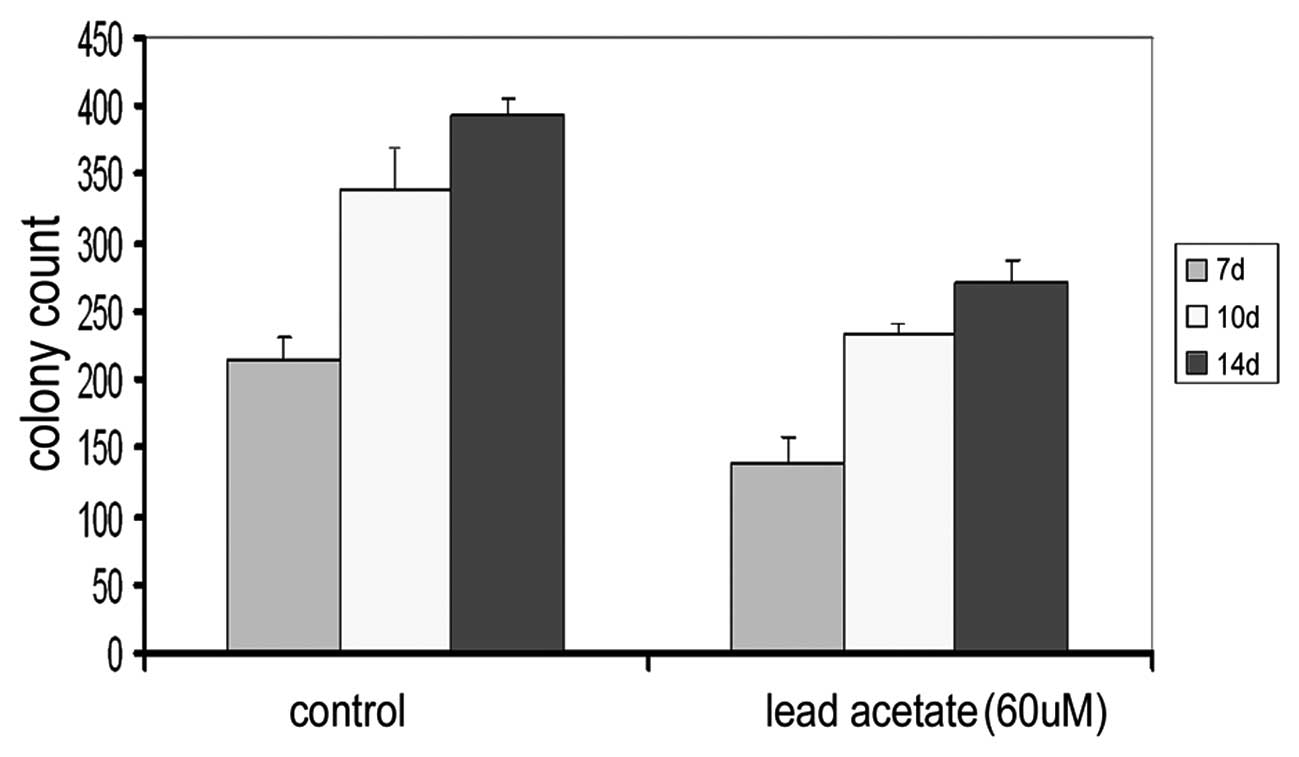
Effect of lead acetate on the
hematopoiesis-supportive function of UCMSCs
MSCs have a hematopoiesis-supportive function in
vitro. To further prove the reverse effect of lead acetate on
the hematopoiesis-supportive function of MSCs, we detected the
supportive function of UCMSCs on the colony formation ability of
bone marrow cells following treatment of lead acetate. After 7, 10
and 14 days, we counted the number of colonies in each well and
found that the number of cells in the experimental groups were all
significantly lower than that of the control group (Fig. 5 and Table II). Following treatment with lead,
the supportive function of MSCs on colony formation was
inhibited.
 | Table IIEffect of lead acetate on supportive
function of MSCs on colony formation ability. |
Table II
Effect of lead acetate on supportive
function of MSCs on colony formation ability.
| Days | Control group | Lead acetate group
(60 μM) |
|---|
| 7 | 214±9.5 | 139±10.7a |
| 10 | 340±15.6 | 233±3.7a |
| 14 | 393±6.6 | 272±9.3a |
Discussion
In previous reports (25), lead concentrations in the bone
greatly exceeded the concentrations in soft tissues and were
highest in the dense bones. Over 90% of the total body burden of
lead in adults was in the bone, of which over 70% was in dense bone
(26). Therefore, bone marrow is
an important target organ of lead toxicity. Haleagrahara et
al found that treatment with 500 ppm lead acetate in the
drinking water of Sprague-Dawley rats for 14 days showed
morphological changes in the bone marrow, and there was very little
hematopoietic tissue and a relatively increased amount of fat cells
(27). The heavy metal lead is
capable of inducing genotoxicity. Following the treatment with
lead, there were significant increases in the chromosomal
aberration of rat bone marrow cells (28). The ability of the stromal cell
layer of bone marrow to display myeloid progenitor cells in
vitro was reduced when mice were treated with lead (29). The cells and microenvironment of
the bone marrow were impaired when exposed to lead. Bone marrow is
the site where hematopoiesis occurs. Therefore, lead-induced damage
of the hematopoietic system may be associated with the bone marrow.
Lead is known to alter the functions of the hematopoietic system.
Lead toxicity induces changes in the composition of red blood cell
membrane proteins and lipids that inhibit hemoglobin synthesis,
limit erythrocyte production and reduce red cell survival (30,31).
Furthermore, lead interferes with iron utilization for heme
formation in the mitochondria, and radio-iron studies showed that
lead competes with iron for incorporation into red blood cells
(32).
Bone marrow MSCs are thought to give rise to cells
that constitute the hematopoietic microenvironment. MSCs may
produce a number of cytokines and ECM proteins and express cell
adhesion molecules, all of which are involved in the regulation of
hematopoiesis (33). Human MSCs,
when injected into the bone marrow cavity of non-obese
diabetic/severe combined immunodeficiency (NOD/SCID) mice,
differentiate into the various cellular components of the bone
marrow stroma, which constitute the functional components of the
hematopoietic microenvironment (12).
In our study, lead acetate not only damaged the
balance of cell proliferation and induced cell apoptosis, but also
affected the potential of differentiation. Following osteogenic
induction, we found that the positive rate of ALP in the treatment
group was markedly lower than that in the control group. The
underlying mechanisms may be complicated and worth further
investigation.
MSCs are one of the essential components of the
hematopoietic microenvironment. MSCs are capable of secreting
certain cytokines associated with hematogenesis. In the present
study, we found that the expression of certain UCMSC cytokines was
significantly decreased following lead acetate treatment. The
cytokine spectrum of MSCs could partly explain the
hematopoiesis-supportive function. These results suggested that one
mechanism underlying the toxic effects of lead on the hematopoietic
system may change the hematopoietic microenvironment.
To further demonstrate this hypothesis, we detected
the hematopoiesis-supportive function of MSCs through a colony
formation assay in vitro, and found that after the treatment
of lead acetate the supportive function of MSCs on colony formation
was inhibited. The result indicated that lead acetate injured the
hematopoiesis-supportive function of UCMSCs. Therefore, the
hematopoiesis-supportive function of MSCs was destroyed, which is
one of the reasons for the anemia that lead poisoning may
produce.
Based on all of the above, we concluded that lead
toxicity induces changes in the red blood cell membrane, inhibits
hemoglobin synthesis, and then leads to decreased erythrocyte
production or reduced red blood cell survival. However, lead
interferes with iron utilization for heme formation and competes
with iron for incorporation into red blood cells. Furthermore, lead
destroys the hematopoiesis-supportive function of MSCs, and all of
the above are causes of the anemia that occurs in lead
toxicity.
In conclusion, lead acetate could inhibit the
proliferation, osteogenic differentiation and
hematopoiesis-supportive function of UCMSCs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (Grant no. 30840053), the Natural
Science Foundation of Jiangsu Province (Grant no. BK2008232) and
Foundation of the Jiangsu University for senior talent (Grant no.
11JDG0089).
References
|
1
|
Srianujata S: Lead - the toxic metal to
stay with human. J Toxicol Sci. 23(Suppl 2): 237–240. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen H and Pan SS: Bioremediation
potential of spirulina: toxicity and biosorption studies of lead. J
Zhejiang Univ Sci B. 6:171–174. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mielke HW and Reagan PL: Soil is an
important pathway of human lead exposure. Environ Health Perspect.
106(Suppl 1): 217–229. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Massadeh AM, Al-Safi SA, Momani IF,
Alomary AA, Jaradat QM and AlKofahi AS: Garlic (Allium sativum
L) as a potential antidote for cadmium and lead intoxication:
cadmium and lead distribution and analysis in different mice
organs. Biol Trace Elem Res. 120:227–234. 2007.
|
|
5
|
Jeyaratnam J, Devathasan G, Ong CN, Phoon
WO and Wong PK: Neurophysiological studies on workers exposed to
lead. Br J Ind Med. 42:173–177. 1985.PubMed/NCBI
|
|
6
|
Rader JI, Peeler JT and Mahaffey KR:
Comparative toxicity and tissue distribution of lead acetate in
weanling and adult rats. Environ Health Perspect. 42:187–195. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Audesirk G, Shugarts D, Nelson G and
Przekwas J: Organic and inorganic lead inhibit neurite growth in
vertebrate and invertebrate neurons in culture. In Vitro Cell Dev
Biol. 25:1121–1128. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vigeh M, Saito H and Sawada S: Lead
exposure in female workers who are pregnant or of childbearing age.
Ind Health. 49:255–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bradman A, Eskenazi B, Sutton P,
Athanasoulis M and Goldman LR: Iron deficiency associated with
higher blood lead in children living in contaminated environments.
Environ Health Perspect. 109:1079–1084. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chapin RE and Stedman DB: Endless
possibilities: stem cells and the vision for toxicology testing in
the 21st century. Toxicol Sci. 112:17–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stummann TC, Hareng L and Bremer S: Hazard
assessment of methylmercury toxicity to neuronal induction in
embryogenesis using human embryonic stem cells. Toxicology.
257:117–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pittenger MF, Mackay AM, Beck SC, et al:
Multilineage potential of adult human mesenchymal stem cells.
Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Y, Jahagirdar BN, Reinhardt RL, et
al: Pluripotency of mesenchymal stem cells derived from adult
marrow. Nature. 418:41–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caplan AI: Mesenchymal stem cells. J
Orthop Res. 9:641–650. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rotter N, Oder J, Schlenke P, et al:
Isolation and characterization of adult stem cells from human
salivary glands. Stem Cells Dev. 17:509–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin TM, Chang HW, Wang KH, et al:
Isolation and identification of mesenchymal stem cells from human
lipoma tissue. Biochem Biophys Res Commun. 361:883–889. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fickert S, Fiedler J and Brenner RE:
Identification, quantification and isolation of mesenchymal
progenitor cells from osteoarthritic synovium by fluorescence
automated cell sorting. Osteoarthritis Cartilage. 11:790–800. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dazzi F, Ramasamy R, Glennie S, Jones SP
and Roberts I: The role of mesenchymal stem cells in haemopoiesis.
Blood Rev. 20:161–171. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu W, Xu W, Jiang R, et al: Mesenchymal
stem cells derived from bone marrow favor tumor cell growth in
vivo. Exp Mol Pathol. 80:267–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Zhao T, Huang W, et al:
Hsp20-engineered mesenchymal stem cells are resistant to oxidative
stress via enhanced activation of Akt and increased secretion of
growth factors. Stem Cells. 27:3021–3031. 2009.PubMed/NCBI
|
|
21
|
Cai Y, Aoshima K, Katoh T, Teranishi H and
Kasuya M: Renal tubular dysfunction in male inhabitants of a
cadmium-polluted area in Toyama, Japan - an eleven-year follow-up
study. J Epidemiol. 11:180–189. 2001.PubMed/NCBI
|
|
22
|
Topashka-Ancheva M, Metcheva R and
Teodorova S: Bioaccumulation and damaging action of polymetal
industrial dust on laboratory mice Mus musculus alba. II Genetic,
cell, and metabolic disturbances. Environ Res. 92:152–160. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang W, Yang N and Shi XM: Regulation of
mesenchymal stem cell osteogenic differentiation by
glucocorticoid-induced leucine zipper (GILZ). J Biol Chem.
283:4723–4729. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheng SL, Yang JW, Rifas L, Zhang SF and
Avioli LV: Differentiation of human bone marrow osteogenic stromal
cells in vitro: induction of the osteoblast phenotype by
dexamethasone. Endocrinology. 134:277–286. 1994.PubMed/NCBI
|
|
25
|
Rio B, Froquet R and Parent-massin D: In
vitro effect of leadacetate on human erythropoietic progenitors.
Cell Biol Toxicol. 17:41–50. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Barry PS: A comparison of concentrations
of lead in human tissues. Br J Ind Med. 32:119–139. 1975.PubMed/NCBI
|
|
27
|
Haleagrahara N, Jackie T, Chakravarthi S,
Rao M and Kulur A: Protective effect of Etlingera elatior
(torch ginger) extract on lead acetate - induced hepatotoxicity in
rats. J Toxicol Sci. 35:663–671. 2010.
|
|
28
|
Nehez M, Lorencz R and Desi I:
Simultaneous action of cypermethrin and two environmental pollutant
metals, cadmium and lead, on bone marrow cell chromosomes of rats
in subchronic administration. Ecotoxicol Environ Saf. 45:55–60.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Queiroz ML, Torello CO, Perhs SM, et al:
Chlorella vulgaris up-modulation of myelossupression induced
by lead: the role of stromal cells. Food Chem Toxicol.
46:3147–3154. 2008. View Article : Google Scholar
|
|
30
|
Kasperczyk A, Kasperczyk S, Horak S, et
al: Assessment of semen function and lipid peroxidation among lead
exposed men. Toxicol Appl Pharmacol. 228:378–384. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Osterode W, Barnas U and Geissler K: Dose
dependent reduction of erythroid progenitor cells and inappropriate
erythropoietin response in exposure to lead: new aspects of anaemia
induced by lead. Occup Environ Med. 56:106–109. 1999. View Article : Google Scholar
|
|
32
|
Jacob B, Ritz B, Heinrich J, Hoelscher B
and Wichmann HE: The effect of low-level blood lead on hematologic
parameters in children. Environ Res. 82:150–159. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weissman IL: Stem cells: units of
development, units of regeneration, and units in evolution. Cell.
100:157–168. 2000. View Article : Google Scholar : PubMed/NCBI
|