Introduction
Lung cancer is one of the most common causes of
cancer-related mortality worldwide and approximately 80% of all
lung cancers are of the non-small cell lung carcinoma (NSCLC) type
(1). Despite continuous efforts to
improve conventional cancer treatments, the long-term outcome for
many patients treated with surgery, chemotherapy and/or
radiotherapy remains suboptimal. The standard treatment of these
tumors has only a 20–30% positive clinical response due to the
resistance of the tumors to therapeutic drugs (2). Therefore, in order to develop new
therapeutic strategies to improve the treatment of NSCLC, it is
important to understand the molecular mechanisms involved in the
resistance of the cells to death.
The Apo2 ligand or tumor necrosis factor
(TNF)-related apoptosis-inducing ligand (Apo2L/TRAIL) was
identified and cloned by its sequence homology to TNF and CD95L
(3,4). As a member of the TNF family, TRAIL
specifically activates extrinsic apoptotic pathways in cancer cells
by binding to death receptors. TRAIL is a promising candidate for
cancer therapeutics that exhibits the ability to preferentially
induce apoptosis in malignant cells (5–7).
Significantly, TRAIL selectively promotes the apoptosis of tumor
cells but has no effect on normal human reproductive tract cells,
including those in the endometrium, ovary, cervix and fallopian
tube (8). While many studies have
shown TRAIL to be a potent inducer of apoptosis in a wide variety
of human and mouse tumor cell lines, many primary tumors are
resistant to TRAIL-induced apoptosis and several mechanisms
underlying the TRAIL sensitivity/resistance have been proposed
(9).
The synthetic integrin-binding peptide, GRGDNP
(gly-arg-gly-asp-asn-pro), is an integrin-recognition motif found
in many ligands. The integrins, ανβ3 and ανβ5, serve as receptors
for a variety of extracellular matrix proteins having the exposed
RGD peptide sequence (10).
Integrins ανβ3 and ανβ5 are overexpressed in multiple tumor cell
types and in the activated endothelial cells of tumor
neovasculature (11–13). The highly restricted expression of
integrins ανβ3 and ανβ5 correlates well with tumor growth,
progression, invasion and metastasis. When coupled to an anticancer
drug, the RGD peptide may enable targeting of the drug and enhance
its antitumor efficacy. Moreover, RGD-containing peptides are able
to directly induce apoptosis by triggering conformational changes
that promote pro-caspase-3 autoprocessing and activation (14).
In this study, the TRAIL mutant, RGD-TRAIL, was
constructed by inserting a GRGDNP peptide before wild-type TRAIL
(wtTRAIL). The ability of RGD-TRAIL to mediate cancer-selective
cell inhibition and its targeted antitumor efficacy in three lung
tumor cell lines (A549, NCI-H1299 and HCC827) were assessed.
Moreover, the mechanism of the RGD-TRAIL-induced apoptosis was
investigated.
Materials and methods
Cell line and culture
The human lung cancer cell lines, A549, NCI-H1299
and HCC827, and the normal human lung cell line, WI-38, were
purchased from the Cell Bank of the Chinese Academy of Sciences.
All cell lines were maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS; Gibco-BRL, Carlsbad, CA, USA) and
1% K-glutamine/antimycotic solution. The cells were grown at 37°C
in a humidified atmosphere containing 5% CO2.
Construction of expression vectors for
TRAIL and mutant RGD-TRAIL
The whole cDNA coding region of the TRAIL gene was
cloned from the WI-38 cell line and used as the template for an
overlapping polymerase chain reaction (PCR). Three primers for PCR
were designed as follows based on the TRAIL sequence and the
pET-28a(+) vector: P1, 5′-CGGATC
CGTGAGAGAAAGAGGTCCTCAGAGAGT-3′; P2, 5′-CGT
CGACTTAGCCAACTAAAAAGGCCCCGAA-3′; and P3, 5′-CGGATCCGGGCGCGGCGATAACCCTGTGAGAGA
AAGAGGTCCTCAGAGAGT-3′. P1: the underlined sequence is BamHI; P2 is
SalI; P3 is Bam HI. P1/P3 and P2 had BamHI and SalI
recognition sites, respectively, for directed cloning into the
vector. Using P1/P2 and P2/P3 primers for PCR, agarose gel
purification was performed for the two products to obtain the
fragments encoding TRAIL and RGD-TRAIL, respectively. The fragments
were digested with BamHI and SalI and then inserted
inframe into the pET-28a(+) expression vector to construct the
pET28-TRAIL and pET28-RGD-TRAIL plasmids. The plasmids were
confirmed by nucleotide sequencing. The pET-28a(+) expression
vector contains a 6×His tag to allow immobilized metal ion affinity
purification.
Expression and purification of wtTRAIL
and RGD-TRAIL proteins
The plasmids were used to transform Escherichia
coli (E. coli) strain BL21 (DE3) (resistant to 15 μg/ml
kanamycin) competent cells. Positive clones were confirmed by
restriction analysis. BL21 (DE3) competent cells were transformed
with the RGD-TRAIL construct and the expression of recombinant
RGD-TRAIL was determined following induction with 1 mM IPTG. To
purify the RGD-TRAIL protein, cultures were grown in shaker flasks
at 22°C for 16 h and, following induction, the cells were pelleted.
The bacterial pellets were resuspended in lysis buffer (50 mM Tris
pH 7.4, 500 mM NaCl and 0.1% Triton X-100) and sonicated in
ice-water baths (15 sec bursts, 30 sec cooling at 200–300 W) for 50
cycles. The lysate was centrifuged at 10,000 × g for 30 min. The
supernatant was mixed with Ni-NTA agarose beads for 1 h to allow
the protein to bind to the beads. After binding, the beads were
packed into a column and washed with 100 ml Tris-buffered saline.
The RGD-TRAIL protein was eluted with 250 mM imidazole in
Tris-buffered saline and dialyzed against phosphate-buffered saline
(PBS) followed by DMEM. The protein was passed through a 0.2 μm
filter, quantified, aliquoted and stored in ice at −80°C.
Recombinant TRAIL was purified similarly. Protein purity and
identity were determined by sodium dodecyl sulfate-polyacrylamide
gel electrophoresis (SDS-PAGE) and western blot analysis.
Immunofluorescence analyses
A549 cells were grown on chamber slides (BD Falcon™;
BD Biosciences, San Jose, CA, USA) fixed with 2% paraformaldehyde,
permeabilized by 0.1% Triton X-100 and then incubated with the
primary antibodies anti-rabbit TRAIL and TRITC-conjugated goat
anti-rabbit IgG (Molecular Probes, Eugene, OR, USA). Sections were
stained for 5 min with DAPI, washed an additional three times with
PBS and then visualized under a Leica DMI4000B fluorescence
microscope (Leica Microsystems GmbH Wetzlar, Germany).
Cell viability assay
The cells were plated in 96-well plates and treated
with the RGD-TRAIL and wtTRAIL proteins at the concentrations
designated in Fig. 2. At the
indicated times, the medium was removed and fresh medium containing
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
0.5 mg/ml; Sigma, St. Louis, MO, USA) was added to each well. The
cells were incubated at 37°C for 4 h, then the medium was removed
and 150 μl solubilization solution (DMSO) was added and mixed in
thoroughly. The absorbance of the plates at 490 nm was read using a
Safire II spectrometer reader (Tecan Trading AG, Männedorf,
Switzerland).
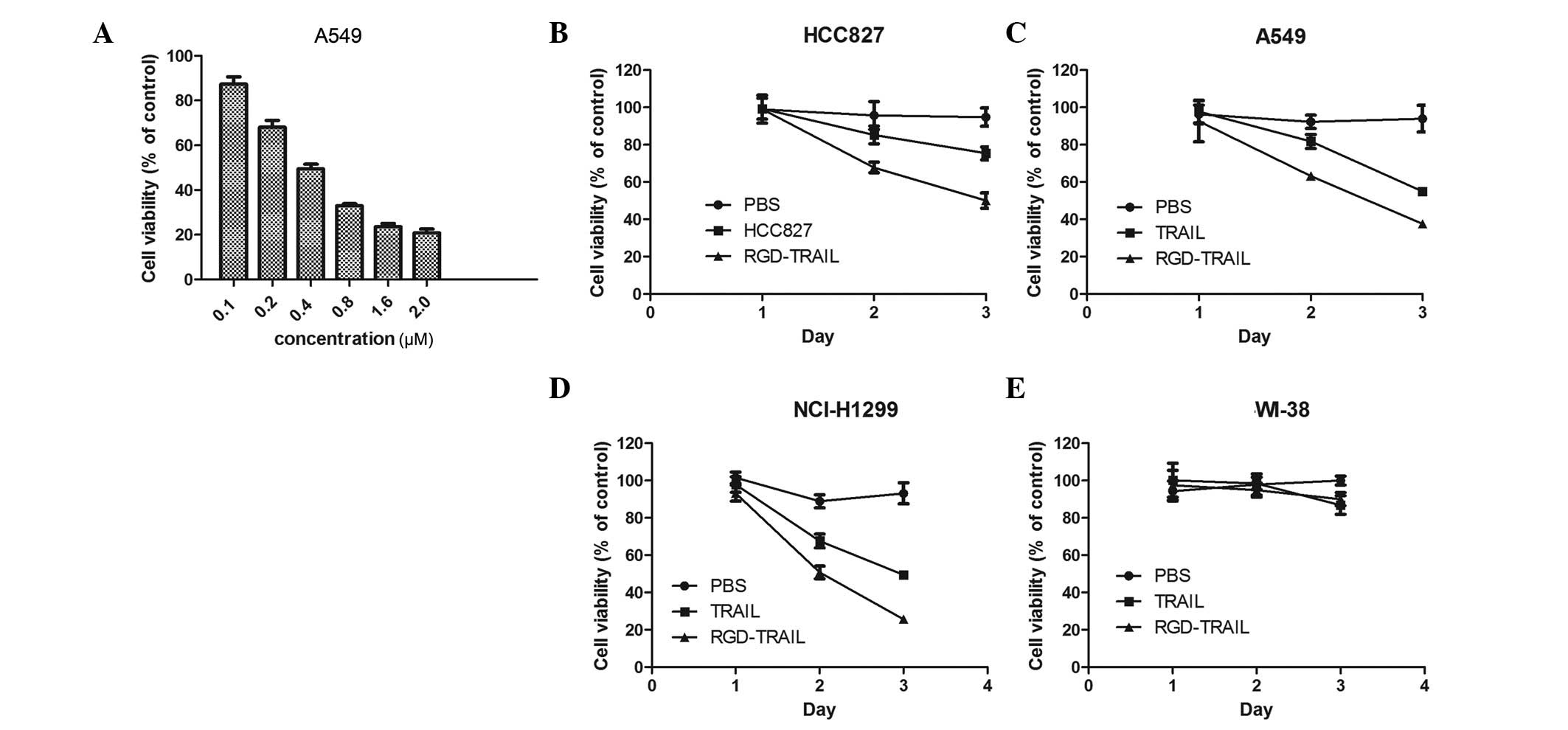 | Figure 2Effect of RGD-TRAIL on the viability
of tumor cells. (A) Tumor cells were treated with RGD-TRAIL for 48
h, at concentrations of 0.1, 0.2, 0.4, 0.8, 1.6 and 2 μM. Cell
viability was determined by the MTT assay. (B-E) Tumor and normal
cells were treated with RGD-TRAIL, TRAIL and PBS at a concentration
of 0.8 μM. On days 1, 2 and 3 post-treatment, cell viability was
determined by the MTT assay. p<0.05 (RGD-TRAIL vs. wtTRAIL).
TRAIL, tumor necrosis factor-related apoptosis-inducing ligand;
MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
PBS, phosphate-buffered saline. |
Hoechst 33342 staining
The nuclear DNA in the treated cells contained in
24-well plates was visualized by staining with the DNA-specific dye
Hoechst 33342 at a final concentration of 5 μg/ml. The cells were
observed immediately with filters for blue fluorescence.
Motility assays
For the wound healing assay, A549 cells plated on
fibronectin-coated 24-well plates were grown to confluence. The
cells were then scratched with the side corner of a cell lifter,
washed three times with DMEM and incubated in DMEM supplemented
with 2% FBS for 11 h. Images of the same fields were captured with
a Leica microscope following the scratch and at the end of the
incubation time. The covered area in each sample was calculated and
compared with the covered area in the control sample. The results
presented are an average of three repetitions and the statistical
significance was determined by the Student’s t-test.
Western blot analysis
Cell lines were grown on 10-cm plates and protein
extracts were prepared using a RIPA buffer containing a cocktail of
protease inhibitors. A 50-μg sample of the protein was applied to
15% SDS-PAGE and transferred to nitrocellulose membranes. The
membranes were probed with polyclonal or monoclonal antibodies to
TRAIL and cleaved PARP, a general marker of apoptosis.
Statistical analysis
All values are expressed as the means ± SD, and
statistical analysis of the results was carried out by one-way
analysis of variance followed by Duncan’s new multiple range method
or Newman-Keuls test. A value of p<0.05 was considered to
indicate a statistically significant result.
Results
Expression and purification of the
recombinant wild-type (wt) and RGD-TRAIL proteins
By means of overlapping PCR, an RGD-TRAIL mutant was
created. The RGD-TRAIL was cloned into the pET-28a(+) vector and
transformed into DH5α competent cells. The expected mutation was
confirmed by DNA sequencing. The transformed BL21 (DE3) cells were
inoculated into Overnight Express Instant LB medium at 22°C for 16
h following the addition of 1 mM IPTG. After induction, the cells
were dissolved in SDS-PAGE sample buffer and subjected to SDS-PAGE.
The results revealed that the recombinant wtTRAIL and RGD-TRAIL
proteins were efficiently expressed, with yields accounting for 30%
of total bacterial proteins (Fig.
1A).
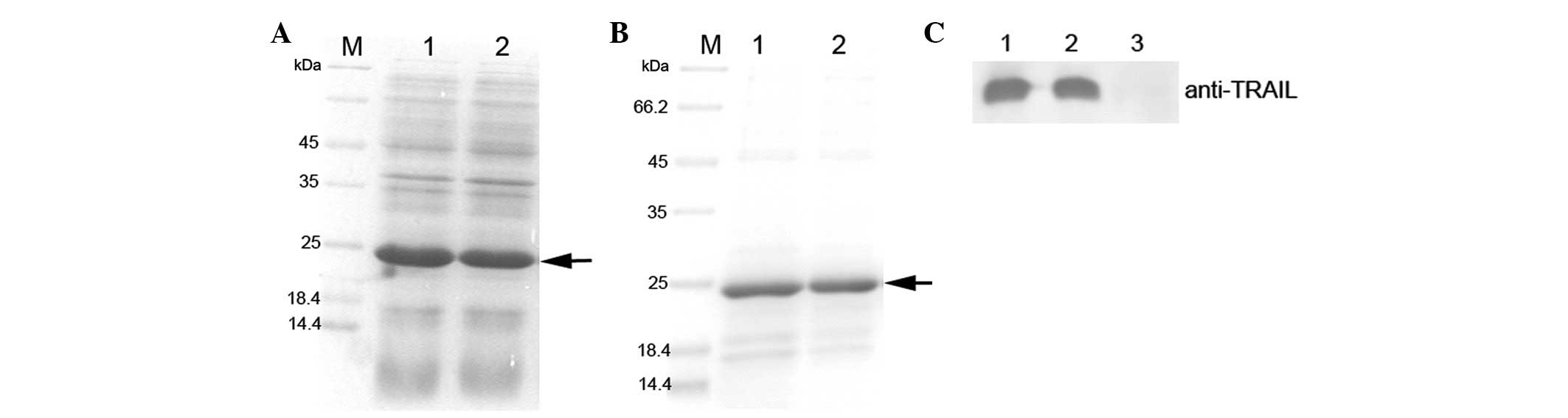 | Figure 1Expression, purification, and
identification of RGD-TRAIL and wtTRAIL. (A) SDS-PAGE gel analysis
of TRAIL. M, protein marker; lane 1, pET-28a-TRAIL, whole cell
lysate induced by IPTG; lane 2, pET-28a-RGD-TRAIL, whole cell
lysate induced by IPTG. (B) SDS-PAGE gel analysis of TRAIL. M,
protein marker; lane 1, purified wtTRAIL; lane 2, purified
RGD-TRAIL. (C) Western blot analysis of TRAIL protein. Lane 1,
wtTRAIL; lane 2, RGD-TRAIL; lane 3, blank control without IPTG
induction. TRAIL, tumor necrosis factor-related apoptosis-inducing
ligand; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel
electrophoresis. |
To investigate the apoptotic activities of the
recombinant wtTRAIL and RGD-TRAIL proteins, we purified TRAIL
protein from E. coli using the non-denaturing method as
described in Materials and methods. SDS-PAGE gel analysis confirmed
that the recombinant wtTRAIL and RGD-TRAIL proteins were purified,
respectively (Fig. 1B). Due to the
removal of the signal peptides of TRAIL, the soluble protein
activities of the purified target proteins were improved. We
obtained ~1.0 mg wtTRAIL and RGD-TRAIL, respectively, per liter of
bacterial culture. Western blot analysis confirmed that the
RGD-TRAIL and wtTRAIL proteins were recognized by the anti-TRAIL
monoclonal antibody (Fig. 1C).
Inhibition of cell viability is induced
by RGD-TRAIL in tumor cells but not in normal lung cells
To assess the cytopathic effect of RGD-TRAIL, an MTT
assay was performed to examine cell viability. A549 cells were
plated on 96-well plates and treated with RGD-TRAIL at various
concentrations. As shown in Fig.
2A, the treatment of the A549 cells with RGD-TRAIL resulted in
a dose-dependent cytopathic effect at 48 h. As expected, the tumor
cells were inhibited by RGD-TRAIL more powerfully than by wtTRAIL.
The treatment of the HCC827, A549 and NCI-H1299 tumor cells with
RGD-TRAIL resulted in a significant decrease in cell viability
compared with the cells treated with wtTRAIL (Fig. 2B-D). In contrast, normal cells
(WI-38) treated with either RGD-TRAIL or wtTRAIL did not
demonstrate any change in cell viability (Fig. 2E).
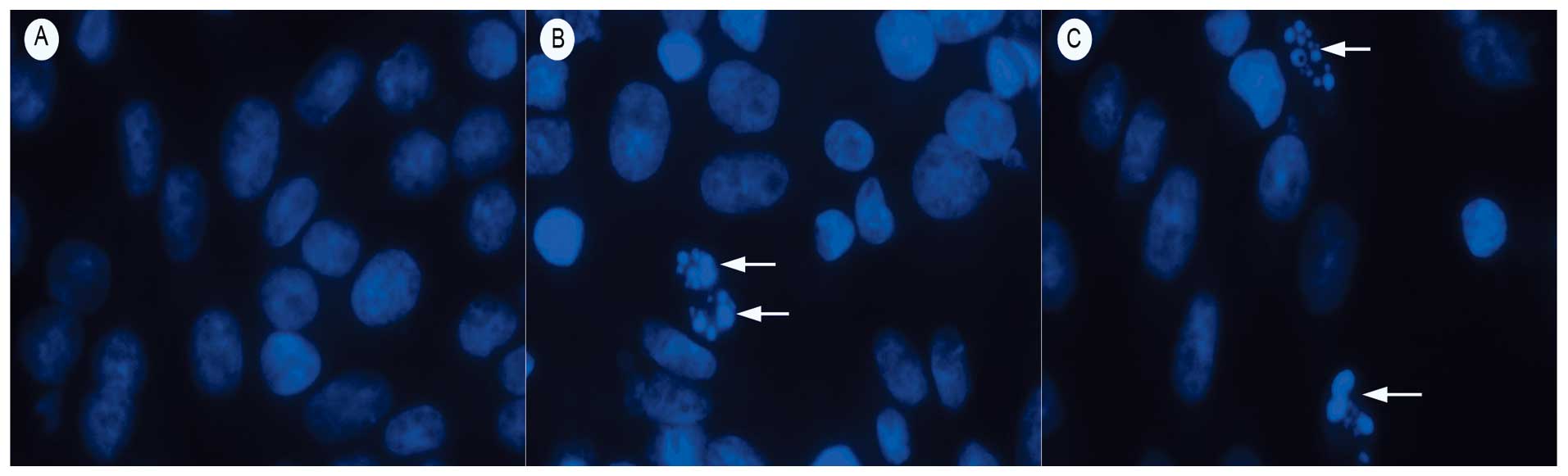
RGD-TRAIL induces apoptosis in tumor
cells
Apoptotic cells demonstrating nuclear condensation
and DNA fragmentation were detected by Hoechst 33342 staining and
fluorescence microscopy. As illustrated in Fig. 3, the lung cancer cells incubated
with TRAIL and RGD-TRAIL for 24 h exhibited many more cells with
condensed and fragmented nuclei than those treated with PBS alone
(Fig. 3).
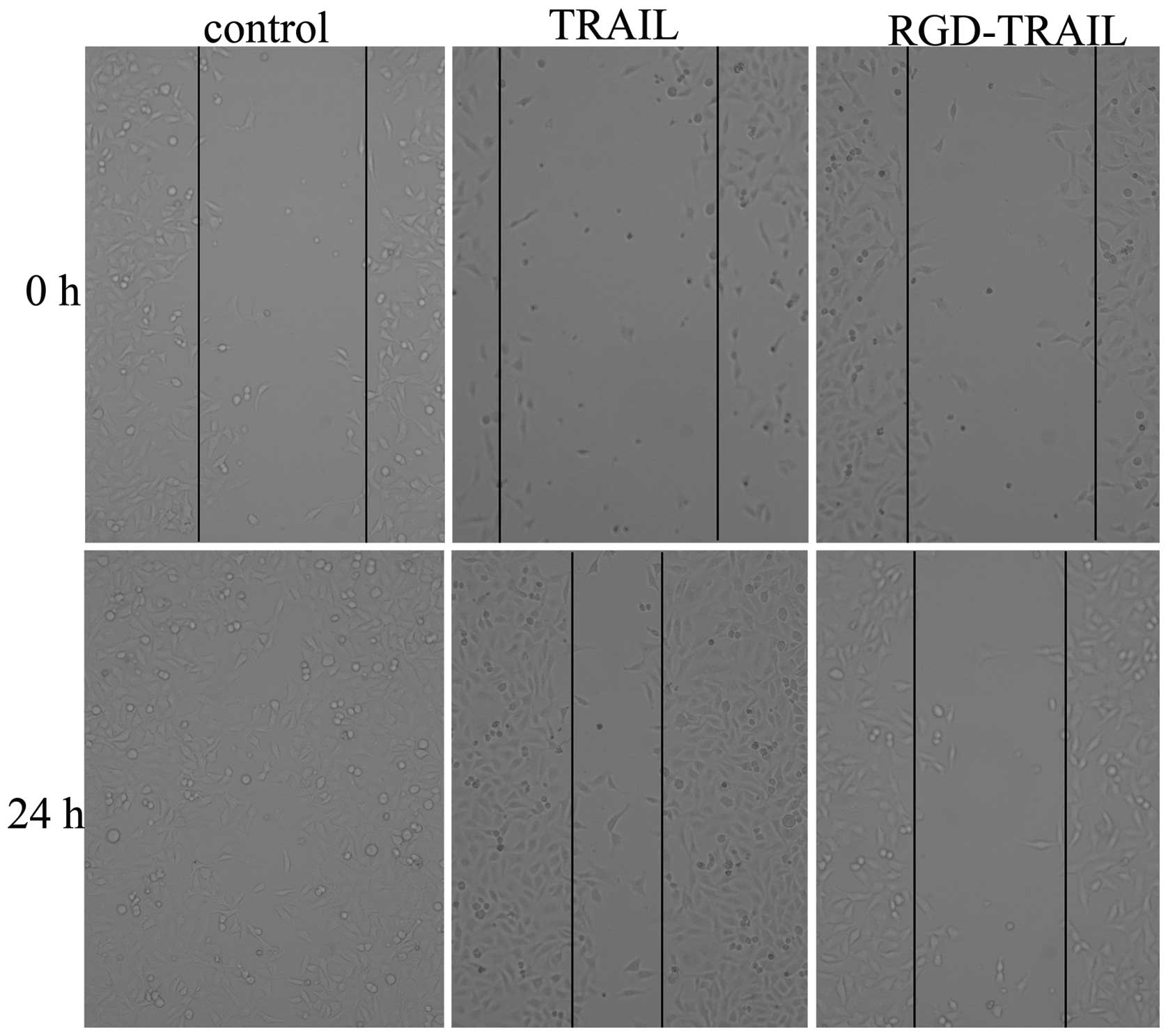
To further investigate the effect of the TRAIL
treatment on A549 cells, we subjected the cells to the scratch
wound assay of in vitro repair. The same fields of confluent
cells were pictured immediately following the scratch (time 0) and
following 24 h of incubation. We examined the migration rates of
the A549 cells treated with PBS, TRAIL and RGD-TRAIL proteins. The
RGD-TRAIL protein treatment lowered the level of the wound closure
to 70% of the control sample (Fig.
4).
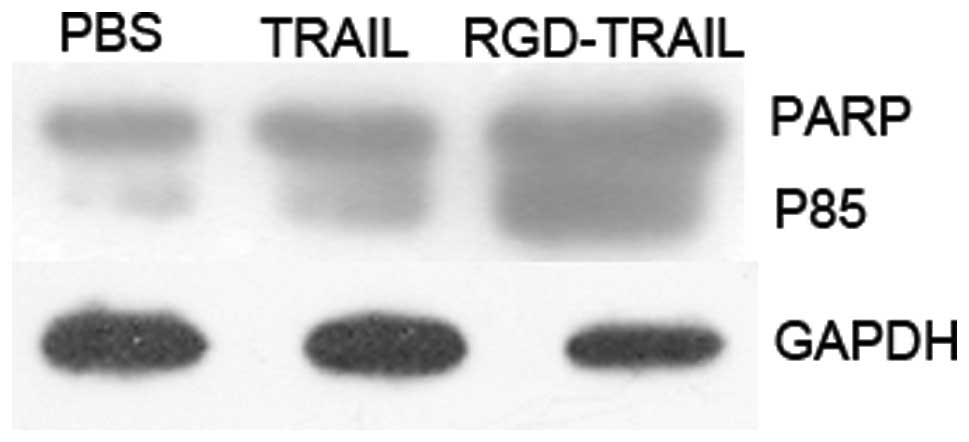
Previous reports have demonstrated that
TRAIL-induced cell death occurs through an apoptotic mechanism
characterized by the activation of a cascade of intracellular
proteases and the cleavage of numerous intracellular proteins
(15,16). To confirm that the tumor cell death
following wtTRAIL and RGD-TRAIL treatment was mediated through an
apoptotic mechanism, nuclear fragmentation and cellular protein
cleavage were examined. A549 cells were incubated with RGD-TRAIL,
wtTRAIL and PBS for 24 h, cell lysates were prepared at various
times following incubation and the cellular proteins were separated
by SDS-PAGE for western blot analysis of the cleavage of PARP, a
substrate for caspase-3 (Fig. 5).
These results demonstrated that treatment with the TRAIL gene
effectively elicited apoptosis in the cultured lung tumor
cells.
Discussion
The specific induction of apoptosis in tumor cells
is a promising therapeutic approach for cancer. In this study, we
showed that the RGD-TRAIL protein selectively localized in tumors
and resulted in the apoptosis of the tumor cells. This treatment
combines the advantages of RGD and the potent antitumor effects of
TRAIL. The TRAIL protein in various forms has been demonstrated to
induce apoptosis in many cancer cells in vitro and to
inhibit the growth of many tumors in rodent models. The TRAIL
protein acts by activating the cell surface receptors DR4 and DR5
(17,18). In our study, the RGD-TRAIL protein
mediated the inhibition of tumor growth and induced A549 cell
apoptosis in vitro.
The combination of direct cancer-specific apoptosis
induction and indirect antitumor properties makes TRAIL an ideal
cancer therapeutic. A significant feature of TRAIL is its
non-toxicity toward normal cells that may be exploited to target
the expression of TRAIL to cancer and normal cells, the latter
serving as a reservoir for the continuous secretion of TRAIL
protein, thereby exerting profound bystander antitumor effects. It
is imperative that TRAIL be delivered by a vector system that
employs a universal entry system into the cells and is also
shielded from immunologic clearance from the circulation. In the
present study, an RGD-TRAIL mutant was constructed and its ability
to mediate cancer-selective cell inhibition and to target tumor
cells was assessed. The recombinant protein was efficiently
expressed in E. coli strain BL21 (DE3). The integrins, ανβ3
and ανβ5, are receptors for the RGD tripeptide sequence and play a
significant role in tumor progression, invasion and metastasis
(19). In the present study, a
significant cytopathic effect was observed by the MTT assay in all
tumor cell lines treated with RGD-TRAIL, compared with wtTRAIL.
This cytopathic effect was time- and dosage-dependent. The superior
antitumoral efficacy of RGD-TRAIL may be due to the interaction of
the RGD sequence with integrins ανβ3 and ανβ5 in the tumor cell
membrane. These results suggest that the insertion of Gly into
wtTRAIL does not disrupt its biological function and selective
inhibitory effects in tumor cells and that RGD-TRAIL inhibits the
growth of tumor cells more effectively than wtTRAIL.
As shown in Fig. 3,
RGD-TRAIL elicited typical apoptotic morphological changes in the
A549 tumor cells, including chromatin condensation and apoptotic
bodies. By contrast, no significant morphological changes were
found in the WI-38 cell control group. Moreover, statistical
analysis revealed that the apoptosis-inducing activity of RGD-TRAIL
in the tumor cells was much stronger than that of wtTRAIL. An
explanation for the difference is that RGD-TRAIL enhanced the
antitumor potency of wtTRAIL. A number of studies have sought to
identify the molecules involved in the TRAIL-induced apoptotic
signaling in tumor cells. It is evident, based on existing data,
that the tumor-specific apoptotic activity of TRAIL involves
multiple pathways. These pathways appear to be regulated only in
tumor cells. Our results revealed that the activation of PARP
occurred in tumor cells treated with RGD-TRAIL and wtTRAIL but not
in untreated control cells (Fig.
5). These results demonstrate that RGD-TRAIL induced apoptosis
and activated PARP.
In conclusion, we have successfully prepared
RGD-TRAIL, and confirmed that it has much stronger anticancer
efficacy than TRAIL or PBS. This study provides an effective model
drug for subsequent studies concerning lung cancer treatment. The
fusion of RGD with TRAIL to construct RGD-TRAIL not only does not
disrupt the biological function of TRAIL but also enhances its
growth suppression and apoptosis-inducing effects in multiple human
tumor cell lines, but not in normal cells (WI-38). Although the
antitumor mechanism of RGD-TRAIL requires further elucidation, the
preliminary data from this study indicate that the RGD-modification
of TRAIL is an effective approach to enhance the antitumoral
potency of TRAIL.
Acknowledgements
This study is part of a project financed by a grant
from Shandong Tackle Key Problems in Science and Technology
(2010GSF10245) and Shandong Excellent Young Scientist Research
Award Fund Project (BS2010YY013).
References
|
1
|
Travis WD: Pathology of lung cancer. Clin
Chest Med. 23:65–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zaba O, Grohe C and Merk J: Novel
therapies in non-small cell lung cancer. Minerva Chir. 66:235–244.
2011.PubMed/NCBI
|
|
3
|
Call JA, Eckhardt SG and Camidge DR:
Targeted manipulation of apoptosis in cancer treatment. Lancet
Oncol. 9:1002–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Walczak H, Miller RE, Ariail K, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amm HM, Oliver PG, Lee CH, Li Y and
Buchsbaum DJ: Combined modality therapy with TRAIL or agonistic
death receptor antibodies. Cancer Biol Ther. 11:431–449. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szliszka E and Krol W: The role of dietary
polyphenols in tumor necrosis factor-related apoptosis inducing
ligand (TRAIL)-induced apoptosis for cancer chemoprevention. Eur J
Cancer Prev. 20:63–69. 2011. View Article : Google Scholar
|
|
7
|
Voelkel-Johnson C: TRAIL-mediated
signaling in prostate, bladder and renal cancer. Nat Rev Urol.
8:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadarangani A, Kato S, Espinoza N, et al:
TRAIL mediates apoptosis in cancerous but not normal primary
cultured cells of the human reproductive tract. Apoptosis.
12:73–85. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dyer MJ, MacFarlane M and Cohen GM:
Barriers to effective TRAIL-targeted therapy of malignancy. J Clin
Oncol. 25:4505–4506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu S: Radiolabeled multimeric cyclic RGD
peptides as integrin alphavbeta3 targeted radiotracers for tumor
imaging. Mol Pharm. 3:472–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Falcioni R, Cimino L, Gentileschi MP, et
al: Expression of beta 1, beta 3, beta 4, and beta 5 integrins by
human lung carcinoma cells of different histotypes. Exp Cell Res.
210:113–122. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumar CC: Integrin alpha v beta 3 as a
therapeutic target for blocking tumor-induced angiogenesis. Curr
Drug Targets. 4:123–131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zitzmann S, Ehemann V and Schwab M:
Arginine-glycine-aspartic acid (RGD)-peptide binds to both tumor
and tumor-endothelial cells in vivo. Cancer Res. 62:5139–5143.
2002.PubMed/NCBI
|
|
14
|
Buckley CD, Pilling D, Henriquez NV, et
al: RGD peptides induce apoptosis by direct caspase-3 activation.
Nature. 397:534–539. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Griffith TS, Chin WA, Jackson GC, Lynch DH
and Kubin MZ: Intracellular regulation of TRAIL-induced apoptosis
in human melanoma cells. J Immunol. 161:2833–2840. 1998.PubMed/NCBI
|
|
16
|
Schneider P, Thome M, Burns K, et al:
TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis
and activate NF-kappaB. Immunity. 7:831–836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan G, O’Rourke K, Chinnaiyan AM, et al:
The receptor for the cytotoxic ligand TRAIL. Science. 276:111–113.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walczak H, Degli-Esposti MA, Johnson RS,
et al: TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL.
EMBO J. 16:5386–5397. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reardon DA, Neyns B, Weller M, Tonn JC,
Nabors LB and Stupp R: Cilengitide: an RGD pentapeptide αvβ3 and
αvβ5 integrin inhibitor in development for glioblastoma and other
malignancies. Future Oncol. 7:339–354. 2011.
|