Introduction
Propofol (2,6-diisopropylphenol) is a widely used
intravenous sedative-hypnotic agent for the induction and
maintenance of anesthesia and the sedation of critically ill
patients (1). The advantages of
this agent over others used for similar applications include a
lower incidence of side effects (2) and improved quality of anesthesia
(3). Although its clinical
properties are well described, information concerning its potential
for abuse has been slow to emerge. However, a growing body of
literature has documented propofol abuse in humans and abuse-like
behavior in animal models (4).
Propofol directly activates the γ-aminobutyric acid (GABA)
receptors and inhibits the N-methyl-D-aspartate (NMDA) receptor in
the nervous system (5). Previous
studies also showed that the μ-opioid receptor (MOR) is
functionally linked to GABA (6,7) and
NMDA receptors in neurons (8). The
findings suggest a correlation between propofol and the MOR,
however, this association has yet to be studied.
Opioid systems are critical in the modulation of
pain behavior and antinociception. Opioid peptides and their
receptors are expressed throughout the nociceptive neural circuitry
and critical regions of the central nervous system (CNS) and are
involved in the regulation of reward-seeking behavior and emotions
(9). The opioid receptors μ, δ and
κ have been cloned. The most commonly used opioids for pain
management act on the MOR, which is expressed primarily in the CNS
and contributes significantly to the regulation of opioid-induced
analgesia, tolerance and dependence (10). In the present study, we explored
for the first time the effect of propofol on MOR expression in a
human neuronal cell line, aiming to reveal potentially new
mechanisms underlying the effects of propofol as well as its
potential for abuse.
Materials and methods
Reagents
Cell culture media and fetal calf serum were
purchased from Invitrogen (Carlsbad, CA, USA). Actinomycin D,
propofol and all other reagents were obtained from Sigma (St.
Louis, MO, USA). The selective MOR ligand [3H]DAMGO was
purchased from Perkin-Elmer (Waltham, MA, USA). TRIzol reagent for
RNA isolation and the SYBR-Green I kit were purchased from
Invitrogen and Roche Diagnostics (Indianapolis, IN, USA),
respectively. Anti-MOR antibody (sc-7489) was purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All secondary
antibodies were obtained from Jackson ImmunoResearch Laboratories,
Inc. (West Grove, PA, USA).
Cell culture and treatment
The SH-SY5Y human neuroblastoma cell line was
purchased from the American Type Culture Collection (Manassas, VA,
USA) and maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum as previously described (11). The cells were treated with various
concentrations of propofol (1, 5, 10 or 20 μM) for different time
periods (6, 12 or 24 h). Propofol was dissolved in DMSO. Cells
treated with DMSO were used as the control. The final concentration
of DMSO in all samples was <0.3% (v/v). For actinomycin D
treatment, the cells were pretreated with actinomycin D (1 mg/ml)
for 30 min and then cultured for 12 or 24 h in medium containing
actinomycin D (1 mg/ml) with or without propofol (10 μM).
Real-time quantitative RT-PCR
RNA was prepared using the TRIzol reagent followed
by purification using a Turbo DNA-free kit (Ambion, Austin, TX,
USA). cDNA was synthesized using SuperScript II reverse
transcriptase (Invitrogen). Real-time quantitative PCR was
performed using the LightCycler thermal cycler system (Roche
Diagnostics) and the SYBR-Green I kit, following the manufacturer’s
instructions. The results were normalized against those of the
housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
in the same sample. The primers used were: for human MOR,
forward, 5′-CTGGGTCAACTTGTCCCACT-3′ and reverse,
5′-TGGACTAGAGGGCCAATGATC-3′; for human GAPDH, forward,
5′-GTCAGTGGTGGACCTGACCT-3′ and reverse, 5′-TGCTGTAGCCAAATTCGTTG-3′.
The mRNA levels of the treated cells are shown as fold or
percentage changes from those of the untreated control cells
(designated as 1 or 100%). Each experiment was repeated three times
in triplicate. Results were shown as the mean ± SD.
Western blot analysis
Immunoblotting was performed as previously described
(12). Briefly, cells were
dissolved in 250 μl of 2X SDS loading buffer (62.5 mM Tris-HCl, pH
6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue and 5%
2-mercaptoethanol), and incubated at 95°C for 10 min. Equal amounts
of proteins from each sample were separated by 8% SDS-PAGE and
blotted onto a polyvinylidene difluoride microporous membrane
(Millipore, Billerica, MA, USA). The membranes were incubated for 1
h with a 1:1,000 dilution of anti-MOR antibody (sc-7489), washed
and visualized using secondary antibodies conjugated with
horseradish peroxidase (1:5,000, 1 h). Peroxidase was visualized
with an ECL kit from GE Healthcare. The proteins were quantified
prior to being loaded onto the gel and equal loading of extracts
was verified by Ponceau staining.
μ-opioid receptor binding assay
SH-SY5Y cell membranes were prepared by homogenizing
cells in 50 mM Tris-HCl buffer, pH 7.4, containing 1 mM EDTA, 1 mM
dithiothreitol and 1 mM benzamidine, with a Polytron homogenizer.
Following centrifugation (1,000 × g for 10 min at 4°C),
supernatants were centrifuged (18,000 × g for 30 min at 4°C) and
the pellets were resuspended in 50 mM Tris-HCl buffer, pH 7.4,
containing 5 mM MgCl2. The protein concentrations were
determined by bicinchoninic acid assay. For the saturation binding
experiments, cell membranes (100 μg/assay) were incubated in 100 mM
Tris-HCl, pH 7.4, containing 0.3% bovine serum albumin with
increasing concentrations of [3H]DAMGO (0.1–5 nM).
Non-specific binding was determined in the presence of DAMGO (10
μM). Following 90 min of incubation at 25°C, the bound ligand was
isolated by rapid filtration on Whatman GF/B filters (Schleicher
and Schuell, Riviera Beach, FL, USA). The filters were washed with
20 ml ice-cold 50 mM Tris-HCl buffer, pH 7.4, and left in
scintillation fluid for 8 h prior to counting. Data were fitted by
non-linear least-square regression and the Ligand program (13) was used to calculate receptor
density (Bmax), Hill slopes and ligand affinity (Kd).
Data are expressed as fmol of [3H]DAMGO bound and
normalized to cell protein content. Each experiment was repeated
three times in triplicate. Results are expressed as the mean ±
SD.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 10.0. Data values were shown as the mean ± SD. Comparisons
of means among multiple groups were performed with one-way ANOVA
followed by post hoc pairwise comparisons using the least
significant difference method. The significance level of this study
was set at two-sided α=0.05.
Results
SH-SY5Y cells were treated with various
concentrations of propofol (1, 5, 10 or 20 μM) for different time
periods (6, 12 or 24 h) and the endogenous MOR transcripts were
evaluated using real-time quantitative RT-PCR. The MOR mRNA levels
of the treated cells are shown as fold changes compared to those of
the untreated control cells (designated as 1). At a concentration
range of 1–10 μM, propofol increased the MOR mRNA levels in a
statistically significant dose- and time-dependent manner within 12
h of treatment (Table I).
 | Table IRelative μ-opioid receptor mRNA levels
in SH-SY5Y cells under propofol treatment. |
Table I
Relative μ-opioid receptor mRNA levels
in SH-SY5Y cells under propofol treatment.
| Time |
|---|
|
|
|---|
| Propofol (μM) | 6 h | 12 h | 24 h |
|---|
| 1 | 1.08±0.02 | 1.12±0.03 | 1.10±0.02 |
| 5 | 1.27±0.04a | 1.49±0.06a,c | 1.53±0.06a,c |
| 10 | 1.81±0.06a,b | 1.98±0.09a–c | 2.03±0.09a–c |
| 20 | 2.56±0.06a,b | 2.86±0.09a–c | 2.92±0.09a–c |
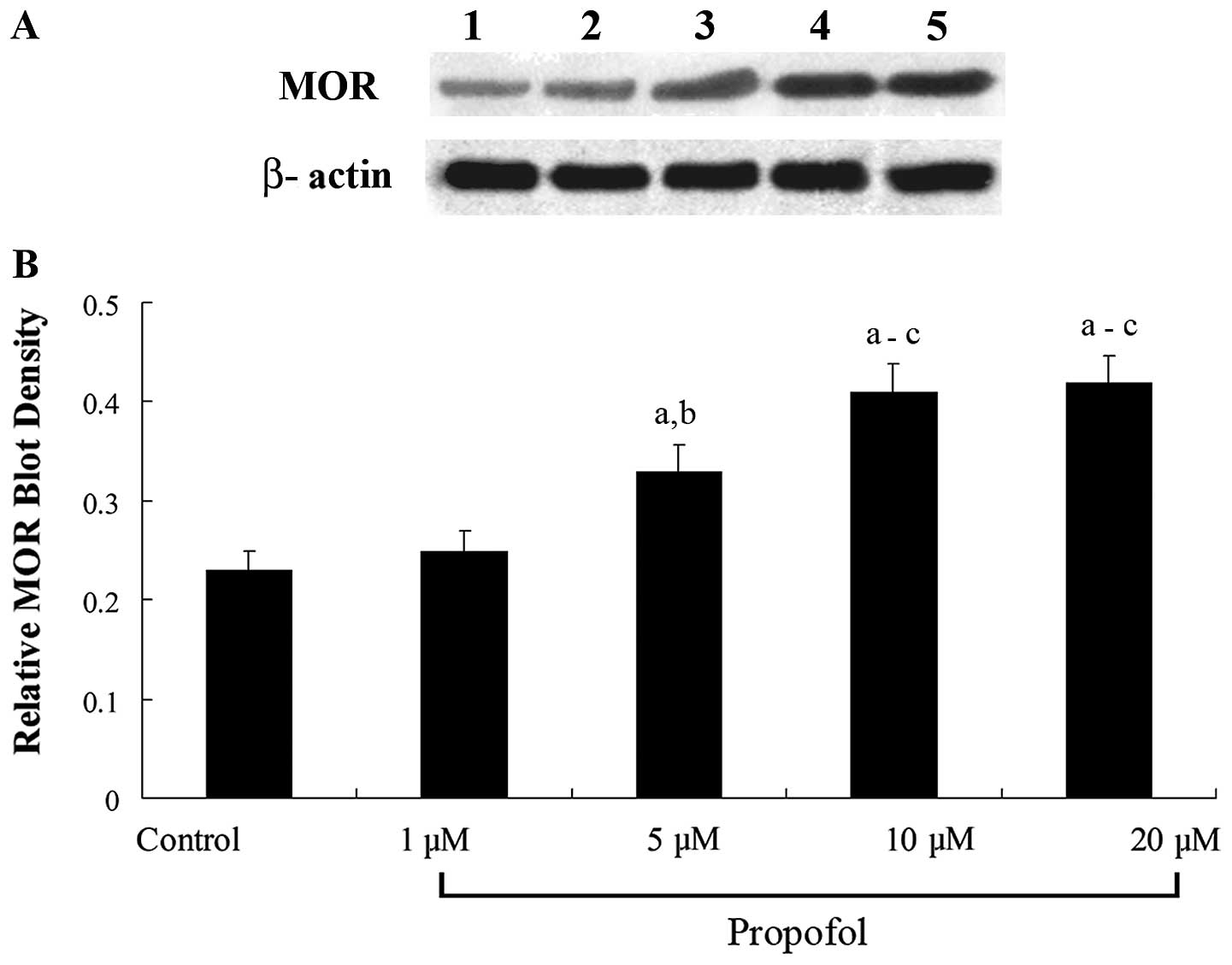
Western blot analyses revealed that propofol
treatment for 12 h dose-dependently increased the MOR protein
levels in the SH-SY5Y cells (Fig.
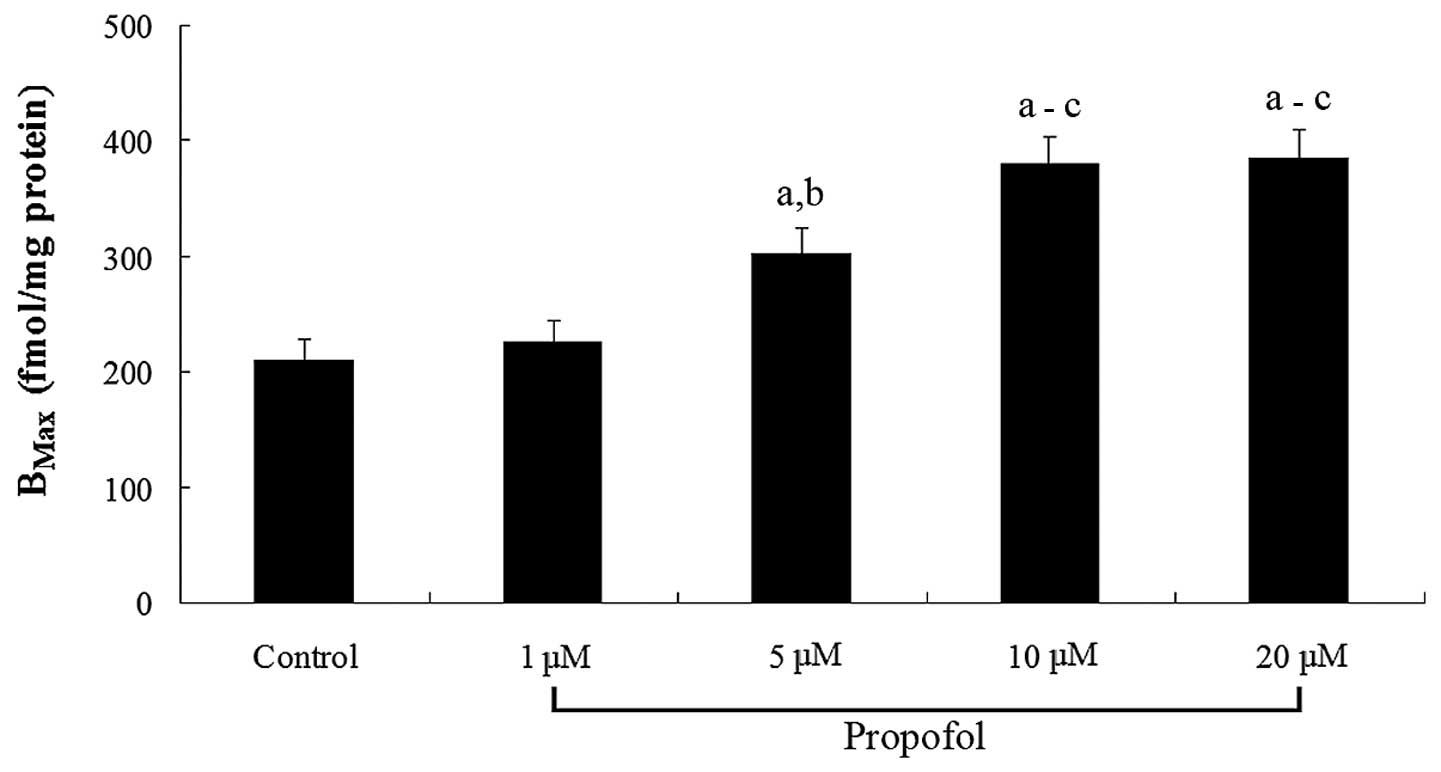
1). In cells treated for 12 h, propofol dose-dependently
increased the MOR density (Bmax) in the cell membranes
(Fig. 2). The results suggest that
propofol significantly increases the density of ligand-binding MORs
in the SH-SY5Y cell membranes by increasing the MOR mRNA
levels.
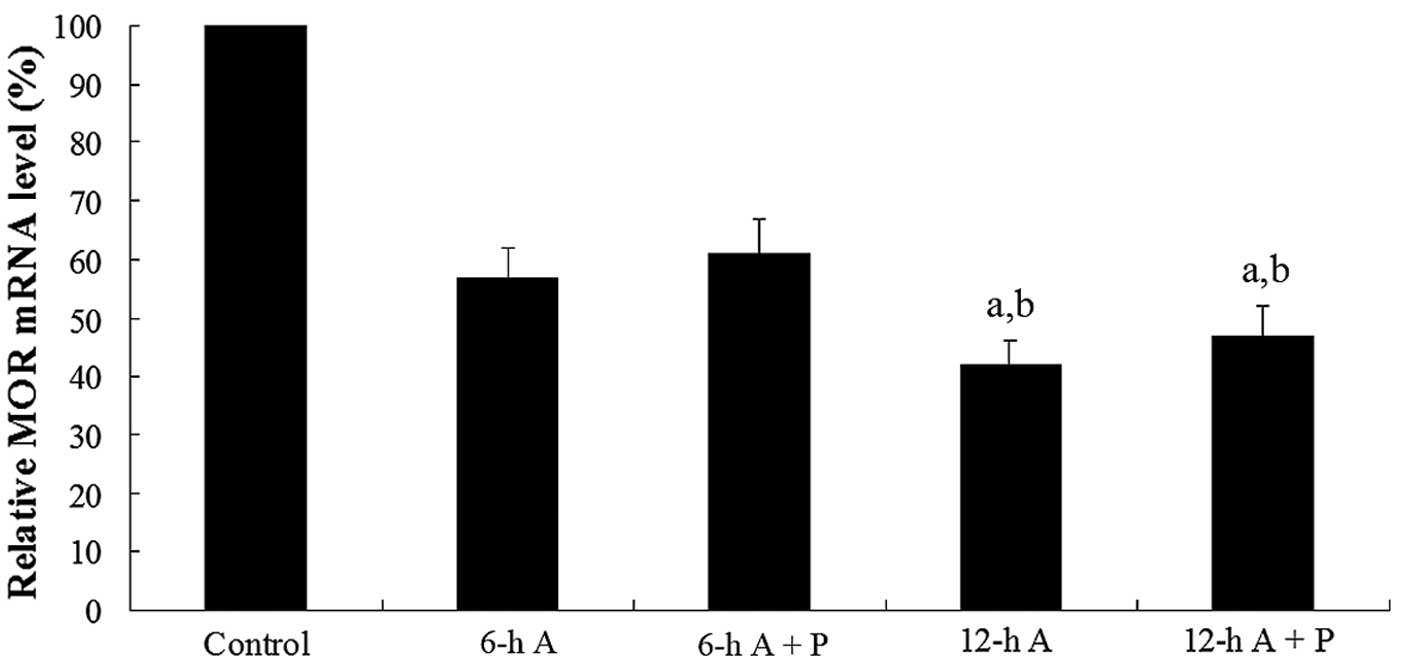
To evaluate the effects of propofol on MOR mRNA
stability, the SH-SY5Y cells were treated with the transcription
inhibitor actinomycin D (14). By
blocking transcription, we were able to determine whether propofol
affects the stability of MOR mRNA. The cells were pretreated with
actinomycin D (1 mg/ml) for 30 min and then cultured for 6 or 12 h
in medium containing actinomycin D (1 mg/ml) with or without
propofol (10 μM). Real-time quantitative RT-PCR assays showed that
the MOR mRNA levels significantly decreased with time after
actinomycin D treatment. In the presence of actinomycin D, propofol
had no significant effect on the MOR mRNA levels over time
(Fig. 3). The results suggest that
propofol increases MOR expression at the transcriptional level,
rather than enhancing the stability of MOR mRNA at the
post-transcriptional level.
Discussion
In the present study, we have demonstrated that
propofol is able to significantly increase MOR expression in
SH-SY5Y human neuroblastoma cells at the transcriptional level,
leading to an increased density of ligand-binding MORs in the cell
membranes. This study provides the first evidence suggesting a
functional link between propofol and the opioid system.
The actions of propofol and opioids are functionally
linked with GABA and NMDA receptors in neurons (5–8). In
this study, we used SH-SY5Y cells since they are human neuronal
cells that endogenously express MORs as well as GABA and NMDA
receptors (14–16), making them an excellent tool for an
in vitro study concerning propofol’s effects on MOR
expression and the underlying molecular mechanisms. Based on our
findings, it would be of note to identify signaling pathways
involved in the propofol-induced transcriptional regulation of the
MOR gene in future studies.
Propofol dose- and time-dependently increases MOR
expression (Table I) in neuronal
cells, which results in an enhanced density of ligand-binding MORs
in the cell membranes (Fig. 2).
Thus, it is reasonable to suggest that long-term or frequent
propofol sedation may potentiate the effects of endogenous μ-opioid
peptides (e.g., endorphins and endomorphins) or μ-opioid analgesics
(e.g., morphine and fentanyl), including side effects such as
sedation, respiratory depression, euphoria and dependence.
Significant experimental evidence suggests the endogenous opioid
system (opioid peptides and receptors) with the development of
dependence on a variety of drugs of abuse (17). Stimulation of the activity of
distinct components of the endogenous opioid system by opioids or
by other drugs of abuse, may mediate some of their reinforcing
effects (17). Recent studies
suggest that MOR and μ-opioid peptides are involved in the
addictive processes induced by cannabinoids, nicotine and alcohol
(18–20). Our findings suggest that by
increasing MOR expression in neuronal cells, propofol has the
potential to enhance the activity of the endogenous μ-opioid system
and thus has the potential for abuse. A growing body of literature
has documented propofol abuse in humans (4). The possibility of dependence and
withdrawal is significant in determining propofol’s potential for
abuse. Previous studies have suggested that propofol dependence and
withdrawal is a phenomenon that is primarily observed in prolonged
propofol use (21–25). Evidence suggests that chronic
second-hand exposure to the aerosolized intravenous anesthetics
propofol and fentanyl may cause sensitization and subsequent opiate
addiction among anesthesiologists and surgeons (26). The molecular mechanism underlying
propofol abuse is unknown. Based on our findings, we propose that
propofol abuse following long-term or chronic propofol exposure may
be partially attributable to propofol-induced MOR expression and
that the combined use of propofol and opioid analgesics may enhance
the development of opioid dependence and addiction.
In addition to being a widely used sedative-hypnotic
agent, propofol is also known to be a potent, dose-dependent
depressant of respiration (27).
The mechanism by which propofol modulates the central respiratory
network is not fully understood. According to our findings, the
sedative and respiratory depression effects of propofol may be
partially attributable to propofol-induced MOR expression during
long-term or chronic use, which may enhance the activity of the
endogenous μ-opioid system. Long-term or chronic use of propofol
may also exacerbate the depression of respiration by μ-opioid
analgesics. However, further studies are required to demonstrate
the interaction between propofol and the opioid system at the
systems biology level.
In conclusion, propofol dose- and time-dependently
enhances MOR expression in SH-SY5Y human neuroblastoma cells at the
transcriptional level, leading to an increased density of
ligand-binding MORs in the cell membranes. This study demonstrates
for the first time a link between propofol and the opioid system,
thereby providing new insights into the propofol mechanism of
action and potential for abuse.
References
|
1
|
Marik PE: Propofol: an immunomodulating
agent. Pharmacotherapy. 25:S28–S33. 2005. View Article : Google Scholar
|
|
2
|
Tramèr M, Moore A and McQuay H: Propofol
anaesthesia and postoperative nausea and vomiting: quantitative
systematic review of randomized controlled studies. Br J Anaesth.
78:247–255. 1997.PubMed/NCBI
|
|
3
|
Schaer H: Propofol infusion for the
maintenance of short-term anesthesia. Anaesthesist. 37:187–192.
1988.(In German).
|
|
4
|
Wilson C, Canning P and Caravati EM: The
abuse potential of propofol. Clin Toxicol (Phila). 48:165–170.
2010. View Article : Google Scholar
|
|
5
|
Kotani Y, Shimazawa M, Yoshimura S, Iwama
T and Hara H: The experimental and clinical pharmacology of
propofol, an anesthetic agent with neuroprotective properties. CNS
Neurosci Ther. 14:95–106. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalyuzhny AE, Dooyema J and Wessendorf MW:
Opioid- and GABA(A)-receptors are co-expressed by neurons in rat
brain. Neuroreport. 11:2625–2628. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang G, Chen W and Marvizón JC: Src
family kinases mediate the inhibition of substance P release in the
rat spinal cord by μ-opioid receptors and GABA(B) receptors, but
not α2 adrenergic receptors. Eur J Neurosci. 32:963–973.
2010.PubMed/NCBI
|
|
8
|
Mao J: NMDA and opioid receptors: their
interactions in antinociception, tolerance and neuroplasticity.
Brain Res Brain Res Rev. 30:289–304. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Hasani R and Bruchas MR: Molecular
mechanisms of opioid receptor-dependent signaling and behavior.
Anesthesiology. 115:1363–1381. 2011.PubMed/NCBI
|
|
10
|
Mayer P and Höllt V: Pharmacogenetics of
opioid receptors and addiction. Pharmacogenet Genomics. 16:1–7.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Toro R, Baiula M and Spampinato S:
Expression of the repressor element-1 silencing transcription
factor (REST) is influenced by insulin-like growth factor-I in
differentiating human neuroblastoma cells. Eur J Neurosci.
21:46–58. 2005.PubMed/NCBI
|
|
12
|
Zhang Y, Wang D, Johnson AD, Papp AC and
Sadée W: Allelic expression imbalance of human mu opioid receptor
(OPRM1) caused by variant A118G. J Biol Chem. 280:32618–32624.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munson PJ and Rodbard D: Ligand: a
versatile computerized approach for characterization of
ligand-binding systems. Anal Biochem. 107:220–239. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guarino G and Spampinato S: Nandrolone
decreases mu opioid receptor expression in SH-SY5Y human
neuroblastoma cells. Neuroreport. 19:1131–1135. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petroni D, Tsai J, Mondal D and George W:
Attenuation of low dose methylmercury and glutamate
induced-cytotoxicity and tau phosphorylation by an
N-methyl-D-aspartate antagonist in human neuroblastoma (SHSY5Y)
cells. Environ Toxicol. Oct 5–2011.(Epub ahead of print).
View Article : Google Scholar
|
|
16
|
Massone S, Vassallo I, Fiorino G, et al:
17A, a novel non-coding RNA, regulates GABA B alternative splicing
and signaling in response to inflammatory stimuli and in Alzheimer
disease. Neurobiol Dis. 41:308–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gianoulakis C: Endogenous opioids and
addiction to alcohol and other drugs of abuse. Curr Top Med Chem.
4:39–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
López-Moreno JA, López-Jiménez A, Gorriti
MA and de Fonseca FR: Functional interactions between endogenous
cannabinoid and opioid systems: focus on alcohol, genetics and
drug-addicted behaviors. Curr Drug Targets. 11:406–428.
2010.PubMed/NCBI
|
|
19
|
Berrendero F, Robledo P, Trigo JM,
Martín-García E and Maldonado R: Neurobiological mechanisms
involved in nicotine dependence and reward: participation of the
endogenous opioid system. Neurosci Biobehav Rev. 35:220–231. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ray LA, Barr CS, Blendy JA, Oslin D,
Goldman D and Anton RF: The role of the Asn40Asp polymorphism of
the mu opioid receptor gene (OPRM1) on alcoholism etiology and
treatment: a critical review. Alcohol Clin Exp Res. 36:385–394.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carrasco G, Molina R, Costa J, Soler JM
and Cabré L: Propofol vs midazolam in short-, medium-, and
long-term sedation of critically ill patients: a cost-benefit
analysis. Chest. 103:557–564. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller LJ and Wiles-Pfeifler R: Propofol
for the long-term sedation of a critically ill patient. Am J Crit
Care. 7:73–76. 1998.PubMed/NCBI
|
|
23
|
Boyle WA, Shear JM, White PF and Schuller
D: Long-term sedative infusion in the intensive care unit: propofol
vs midazolam. J Drug Dev. 4(Suppl): S43–S45. 1991.
|
|
24
|
Cammarano WB, Pittet JF, Weitz S,
Schlobohm RM and Marks JD: Acute withdrawal syndrome related to the
administration of analgesic and sedative medications in adult
intensive care unit patients. Crit Care Med. 26:676–684. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Au J, Walker S and Scott DHT: Withdrawal
syndrome after propofol infusion. Anaesthesia. 45:741–742. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McAuliffe PF, Gold MS, Bajpai L, et al:
Second-hand exposure to aerosolized intravenous anesthetics
propofol and fentanyl may cause sensitization and subsequent opiate
addiction among anesthesiologists and surgeons. Med Hypotheses.
66:874–882. 2006. View Article : Google Scholar
|
|
27
|
Kashiwagi M, Osaka Y, Onimaru H and Takeda
J: Optical imaging of propofol-induced central respiratory
depression in medulla-spinal cord preparations from newborn rats.
Clin Exp Pharmacol Physiol. Jan 19–2011.(Epub ahead of print).
View Article : Google Scholar
|