Introduction
Acute ischemic stroke (AIS) is a common cause of
morbidity and mortality in industrialized countries. According to
the World Health Organization, stroke affects approximately 5.8
million individuals each year worldwide (1). Stroke may trigger an inflammatory
reaction that lasts several months. It has been reported that the
suppression of inflammation with a variety of drugs reduces infarct
volume and improves clinical outcomes in animal models of stroke
(2). Extracellular matrix (ECM)
remodeling, effected by a group of significant inflammatory
molecules, including matrix metalloproteinases (MMPs), may be a key
factor in the development of inflammation in the central nervous
system. Proteoglycans are thought to cause matrix remodeling and
modulate the activity of growth factors and cytokines (3).
Decorin (DCN) is a small proteoglycan that consists
of a single glycosaminoglycan side chain and is linked to a core
protein containing leucine-rich repeats of 24 amino acids. It is
present in the ECM of a variety of tissues and cell types (4). During the past decade, accumulating
evidence has suggested that DCN interacts with a variety of
biological molecules, including type I collagen, fibronectin,
thrombospondin, epidermal growth factor receptors (EGF-Rs),
insulin-like growth factor-1 receptors (IGF-1Rs), platelet-derived
growth factor (PDGF), complement C1q and MMPs, particularly MMP-2
and MMP-9 (5–8). These factors are involved in matrix
assembly and may be involved in the regulation of fundamental
biological functions, including cell attachment, migration and
proliferation (9–12). It has also been reported that DCN
is involved in the regulation of collagen fibrillogenesis, ECM
remolding or deposition and cancer cell growth. However, no studies
have investigated the expression of DCN in patients with AIS and in
particular the correlation between DCN and MMP-2 has not been
explored.
The present study aimed to investigate the levels of
DCN and MMP-2 in normal volunteers as well as in subjects with AIS.
Furthermore, the correlations between plasma DCN and MMP-2 and
other varying clinical factors in the subjects were analyzed and
the role of DCN as a biomarker of risk for AIS was explored.
Subjects and methods
Subjects and assessment
Patients with AIS were recruited from the Department
of Neurology, Changhai Hospital, Second Military Medical
University, Shanghai, between April 2010 and May 2011. A total of
120 volunteers matched for age, gender and cardiovascular risk
factors, including hypertension, diabetes mellitus (DM),
hypercholesterolemia and heart disease, were included as controls.
Diagnosis of AIS was defined by focal neurological signs or
symptoms of potential vascular origin that persisted for >24 h
and confirmed using brain computed tomography (CT) and/or magnetic
resonance imaging (MRI) within 7 days following the onset of stroke
according to The International Classification of Diseases (9th
Revision) (13). Exclusion
criteria for the two groups were: (a) infectious disease or
traumatic injury in the previous month; (b) myocardial infarction
in the previous year; (c) severe renal and liver failure; (d)
history of any chronic inflammatory disease; (e) history of cancer.
Smoking history and history of hypertension, DM,
hypercholesterolemia, or any heart disease were recorded.
In this study, stroke subtype was classified
according to the Trial of Org 10172 in Acute Stroke Treatment
(TOAST) criteria (14): (a)
large-artery atherosclerosis (LAAS); (b) cardioembolic infarct
(CEI); (c) lacunar infarct (LAC); (d) stroke of other determined
etiology (ODE); (e) stroke of undetermined etiology (UDE).
Stroke severity was scored using the National
Institutes of Health Stroke Scale (NIHSS) on admission and at 72 h
(15).
This project was performed according to the
principles of the Declaration of Helsinki and approved by the local
ethics commission. All patients gave informed consent. All data
were evaluated by a trained neurologist and a researcher who were
blinded to each other’s assessment.
Laboratory tests and DCN
measurements
The blood samples from all patients were collected
by laboratory specialists on the first day of admission. Routine
laboratory tests, including red blood cell (RBC), white blood cell
(WBC), platelet (PLT), plasma glucose, fibrinogen degradation
products (FDP), fibrinogen (FIB), prothrombin time (PT), activated
partial thromboplastin time (APTT), triglyceride (TG), total
cholesterol (TC), low-density lipoprotein (LDL) and high-density
lipoprotein (HDL) were determined using an automatic analyzer
(Hitachi 7060, Tokyo, Japan).
Blood samples for DCN and MMP-2 were collected into
chilled tubes containing EDTA-Na2 (1 mg/ml blood) and
centrifuged at 4,000 rpm at 4°C for 10 min. The supernatants were
decanted and frozen at −80°C until assayed. DCN was measured using
enzyme-linked immunosorbent assay (ELISA) with Raybio human DCN
ELISA kits (Raybiotech, Norcross, GA, USA) according to the
manufacturer’s instructions. MMP-2 was treated using R&D Human
MMP-2 Immunoassay kit (R&D Systems, Minneapolis, MN, USA)
following the manual procedure. Intra- and inter-assay coefficients
of variation for the two were <10%.
Statistical analyses
All statistical analyses were performed using SPSS
17.0 software. Values are expressed as mean ± SD or, in the case of
non-normally distributed data, as median and interquartile range.
Data that were normally distributed were analyzed using a Student’s
t-test (for two groups) or one-way analysis of variance (ANOVA; for
three groups). Non-normally distributed data were analyzed using
the nonparametric Mann-Whitney U test (for two groups) or
Kruskal-Wallis test (for three groups). Pearson’s correlation
coefficient was calculated to evaluate a possible correlation in
continuous variables. Multiple stepwise linear regression analyses
were performed to identify independent determinants of plasma DCN
concentration. Logistic regression analyses were used to assess
whether stroke was related to traditional atherosclerotic risk
factors and DCN. For the assessment of the accuracy of the
parameter of DCN in discriminating between stroke patients and
controls, receiver operating characteristic curve (ROC) analyses
were performed. Data were considered to be statistically
significant when P<0.05.
Results
Plasma levels of DCN and MMP-2 are
decreased in AIS patients
The study population consisted of 102 AIS patients
(71 men; 31 women) and 120 controls. Demographic and clinical
characteristics are shown in Table
I. The vascular risk factors, including ischemic heart disease
(IHD), hypertension, atrial fibrillation (AF), DM, hyperlipidemia,
smoking and alcohol consumption did not differ significantly
between the groups. No significant differences in PLT, PT, APTT,
D-dimer, serum triglycerides (TGs), HDL and total cholesterol (TC)
were observed between the two groups (Table I).
 | Table IClinical characteristics and
laboratory parameters of patients with and without stroke. |
Table I
Clinical characteristics and
laboratory parameters of patients with and without stroke.
| Demographic | AIS (n=102) | Control
(n=120) | P-value |
|---|
| Male, n (%) | 71 (69.6) | 76 (63.3) | 0.393 |
| Age, years | 61.3±14.4 | 61.6±12.2 | 0.168 |
| Stroke subtype of
etiology (TOAST), n (%) |
| Cardioembolic
infarcts | 8 | | |
| Large-artery
atherosclerosis | 59 | | |
| Lacunar
infarct | 8 | | |
| Stroke of other
determined etiology | 7 | | |
| Stroke of
undetermined etiology | 20 | | |
| Risk factor, n
(%) |
| History of
ischemic heart disease | 9 (8.8) | 15 (12.5) | 0.379 |
| Hypertension | 64 (62.7) | 77 (64.2) | 0.274 |
| Diabetes
mellitus | 23 (22.5) | 27 (22.5) | 0.993 |
| Atrial
fibrillation | 10 (9.8) | 13 (10.8) | 0.802 |
|
Hyperlipidemia | 7 (6.9) | 10 (8.3) | 0.681 |
| Cigarette smoking,
n (%) | 56 (54.9) | 51 (42.5) | 0.193 |
| Never | 46 (45.1) | 69 (57.5) | |
| Former | 23 (22.5) | 21 (17.5) | |
| Current | 33 (32.4) | 30 (25) | |
| Alcohol intake, n
(%) | 55 (53.9) | 60 (50) | 0.801 |
| Non-drinker,
n | 47 | 60 | |
| Former-drinker,
n | 34 | 42 | |
| Drinker, n | 21 | 18 | |
| Laboratory
results |
| RBC
(x1012/l) | 4.6±0.6 | 4.3±0.4 | 0.002 |
| WBC
(x109/l) | 7.4±2.3 | 6.7±1.3 | 0.006 |
| PLT
(x109/l) | 206.5±47.8 | 202.2±37.0 | 0.462 |
| PT (s) | 13.9±5.0 | 13.1±1.6 | 0.147 |
| APTT (s) | 37.9±9.4 | 37.7±3.8 | 0.865 |
| FIB (g/l) | 3.56±1.29 | 3.16±0.44 | 0.004 |
| D-dimer
(μg/ml) | 1.09±2.36 | 0.67±0.50 | 0.60 |
| FDP (μg/ml) | 5.89±7.41 | 2.97±1.31 | <0.001 |
| LDL (mmol/l) | 3.08±1.20 | 2.56±0.51 | <0.001 |
| HDL (mmol/l) | 1.01±0.22 | 1.00±0.19 | 0.767 |
| TG (mmol/l) | 1.58±0.66 | 1.65±0.64 | 0.47 |
| TC (mmol/l) | 4.70±1.17 | 4.45±0.74 | 0.063 |
| Plasma glucose
(mmol/l) | 6.2±2.1 | 5.1±1.4 | <0.001 |
| Decorin
(pg/ml) | 7094.8±2150.67 |
8950.04±1267.65 | <0.001 |
| MMP-2 (ng/ml) | 132.37±37.2 | 185.92±33.94 | <0.001 |
| Vital signs at
admission |
| Body temperature
(°C) | 36.5±0.5 | 36.6±0.5 | 0.078 |
| SBP (mmHg) | 139.8±20.0 | 129.6±13.4 | <0.001 |
| DBP (mmHg) | 84.8±11.0 | 75.4±9.5 | 0.099 |
| Heart rate
(/min) | 77.6±6.5 | 74.2±8.9 | <0.001 |
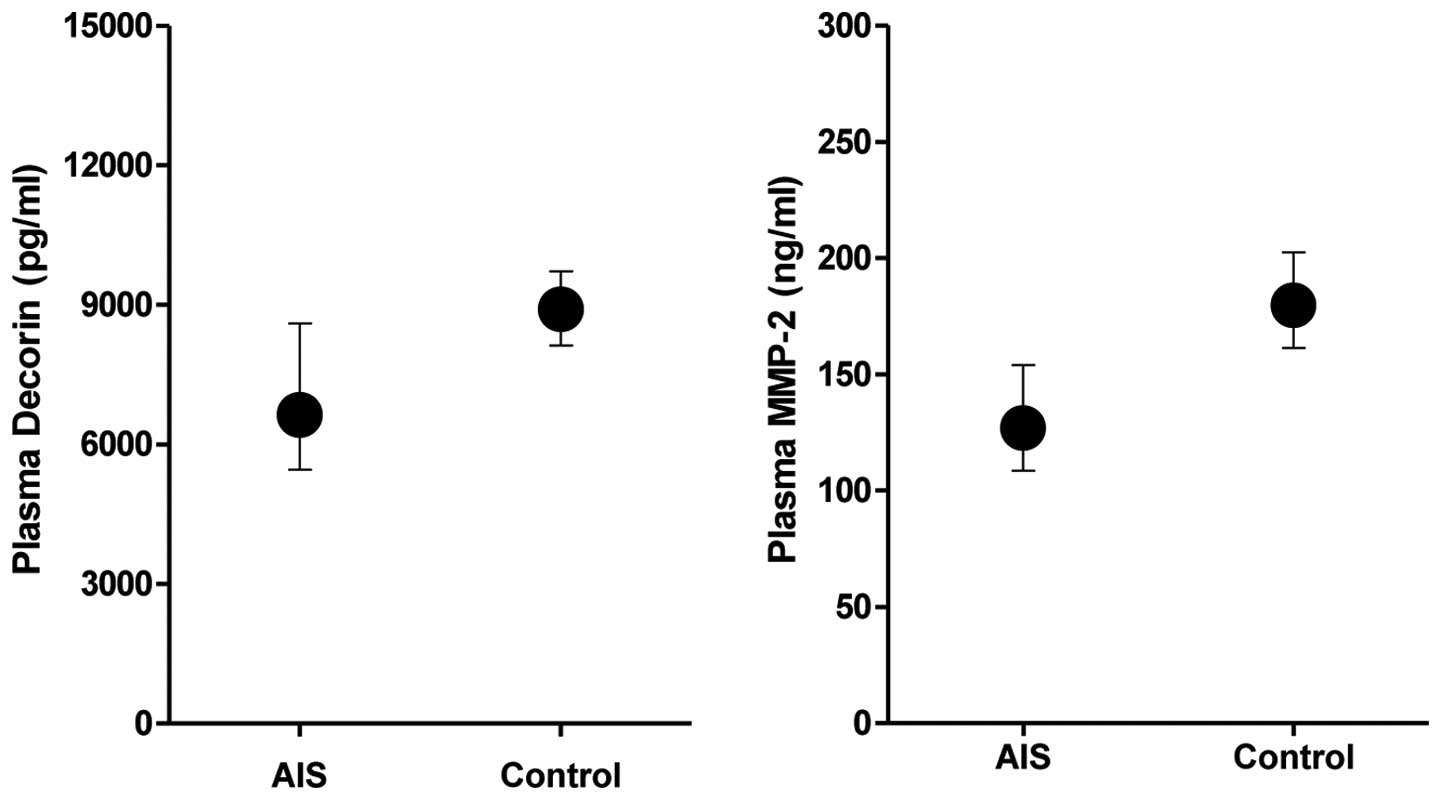
Plasma levels of DCN were significantly lower in AIS
patients compared with individuals in the control group
(P<0.001, Table I, Fig. 1). Similarly, plasma levels of MMP-2
were significantly lower in patients than in the controls
(P<0.001, Table I, Fig. 1). The plasma DCN levels were
positively correlated with MMP-2 (R=0.332; P<0.001).
Plasma DCN levels are lowest in patients
with LAAS
Patients were divided into five subgroups according
to the criteria used in TOAST. The five stroke subgroups were as
follows: CEI, n=8; LAAS, n=59; LAC, n=8; ODE, n=7; UDE, n=20. The
DCN levels were 9183.2±1892.65, 6640.63±2050.75, 7877.87±2022.54,
6966.03±2104.68 and 7331.09±2150.67 in groups CEI, LAAS, LAC, ODE
and UDE, respectively. The lowest DCN levels were identified in
patients with LAAS. Patients with CEI demonstrated the highest DCN
levels among the five groups. Fig.
3 shows that DCN levels were significantly lower in patients
with LAAS, LAC, ODE and UDE than those in controls (P<0.001,
P=0.028, P<0.001, P<0.001, respectively). However, no
difference was noted in DCN levels in patients with CEI as compared
with controls.
DCN levels <8,500 pg/ml are associated
with AIS
Correlation analyses (Table II) revealed a positive correlation
between DCN levels and MMP-2 (R=0.332; P<0.001) and AF (R=0.181;
P=0.007). A negative correlation was revealed between DCN levels
and systolic blood pressure (R=−0.188; P<0.001), diastolic blood
pressure (R=−0.180; P=0.005), plasma glucose (R=−0.159; P=0.017),
FDP (R=−0.199; P=0.003) and LDL (R=−0.154; P=0.022). However there
was no correlation between DCN levels and NIHSS (R=0.12; P=0.906).
Multiple stepwise linear regression analyses revealed that MMP-2
and AF were significant independent determinants of DCN (β=0.341,
P<0.001; β=0.197, P=0.002, respectively), when the vascular risk
factors, including age, gender, hypertension, DM, smoking, alcohol,
TC, LDL and HDL were taken into account as independent variables.
Following adjustment for smoking, alcohol, DM and hypertension,
logistic regression analyses revealed that DCN levels <8,500
pg/ml were associated with AIS (OR=4.8; 95% CI: 2.1–11.1;
P<0.001).
 | Table IIPearson correlation between DCN and
clinical laboratory parameters. |
Table II
Pearson correlation between DCN and
clinical laboratory parameters.
| DCN |
|---|
|
|
|---|
| Patient
characteristics | R | P-value |
|---|
| Age | −0.14 | 0.830 (NS) |
| Systolic blood
pressure | −0.188 | 0.005b |
| Diastolic blood
pressure | −0.180 | 0.007b |
| Ischemic heart
disease | −0.026 | 0.705 (NS) |
| Atril
fibrillation | 0.181 | 0.007b |
| Plasma glucose | −0.159 | 0.017a |
| Fibrinogen | −0.045 | 0.509 (NS) |
| FDP | −0.199 | 0.003b |
| D-dimer | −0.122 | 0.069 (NS) |
| Total
cholesterol | −0.084 | 0.213 (NS) |
| Triglyceride | 0.059 | 0.382 (NS) |
| High-density
lipoprotein | −0.058 | 0.391 (NS) |
| Low-density
lipoprotein | −0.154 | 0.022a |
| NIHSS | 0.12 | 0.906 (NS) |
| MMP-2 | 0.332 | <0.001b |
Plasma DCN (8,500 pg/ml) has a
sensitivity and specificity for AIS of 79.4 and 62.8%,
respectively
To define the optimal cut-off value for DCN levels,
an ROC curve was constructed (Fig.
2). The controls (n=120) were divided into the patients with
known vascular risk factors (n=77) and those without (n=43) and
three cut-off values were calculated (8,000, 8,500 and 9,000 pg/ml)
which provided various accuracies (Table III). The cut-off value used for
DCN levels was 8,500 pg/ml and the area under the curve (AUC) for
DCN was 0.798 (95% CI: 0.73–0.87; P<0.001) in the patients with
AIS and controls without known vascular risk factors (n=43). The
sensitivity and specificity of plasma DCN (8,500 pg/ml) for AIS
were 79.4 and 62.8%, respectively.
 | Table IIICut-off values of DCN in AIS and
controls. |
Table III
Cut-off values of DCN in AIS and
controls.
| Cut-off
(pg/ml) | Sensitivity
(%) | Specificity
(%) |
|---|
| 9,000 | 79.4 | 47.5 |
| 8,500 | 73.5 | 60.0 |
| 8,000 | 69.6 | 78.3 |
| 8,500a | 79.4 | 62.8 |
| 8,500b | 79.4 | 39.0 |
Discussion
DCN is a ubiquitous small extracellular
proteoglycan. It is a composite molecule, 100 kDa in size, with a
protein core and attached glycosaminoglycans (GAGs) (16). It was cloned from a human embryonic
fibroblast line and named PG40 due to its protein core (40 kDa)
(4). Previous studies have
suggested that DCN is involved in the regulation of collagen
fibrillogenesis, ECM remolding, tumor growth and metastasis,
angiogenesis, renal and pulmonary fibrosis, muscular dystrophy,
wound healing and myocardial infarction (17–20).
Although previous studies have furthered our understanding of DCN
in various pathological processes, the role of DCN in patients with
AIS has not been investigated. The present study examined plasma
DCN and MMP-2 in subjects with AIS. For the first time, this study
revealed that there are lower plasma levels of DCN in subjects with
AIS compared with those in controls. Decreased DCN levels
(<8,500 pg/ml) had 79.4% sensitivity and 62.8% specificity for
AIS, therefore this cut-off point might be a useful indicator for
ischemic stroke.
MMPs underlie a tight regulatory process resulting
in the equilibrium between the synthesis and degradation of ECM
components (21–23). This equilibrium may be disturbed
following stroke, leading to the decreased synthesis of MMP-2.
However, conflicting data have reported an increase (24) or decrease (25) in plasma levels following ischemic
stroke. The observed decrease in MMP-2 levels following AIS in the
current study may be explained pathophysiologically as a failure to
detect circulating MMP-2 due to an accelerated MMP turnover,
binding of MMP-2 to damaged tissue or increased utilization of
MMP-2 for local matrix remodelling (26). In the current study, plasma DCN
levels were positively correlated with MMP-2. Therefore, these
results are in agreement with those of a previous study, in which a
recombinant adenovirus vector containing a DCN gene transfection to
rat mesangial and tubular cells led to the repressed expression of
transforming growth factor-β (TGF-β) and collagen type IV, whilst
it promoted the expression of MMP-2 and -9 (27). Similarly, Al Haj Zen et
al(28) demonstrated that DCN
may affect the production of metalloproteinases and cytokines
through the adenovirus-mediated overexpression of DCN in the human
gingival fibroblasts. In this study, the authors also demonstrated
that DCN infection resulted in the decreased expression of MMP-1,
MMP-3, TGF-β and IL-1β. However, the levels of MMP-2, tissue
inhibitor of metalloproteinase -2 (TIMP-2) and IL-4 were markedly
increased. Consistent with previous studies, the results of the
current study also suggest that plasma DCN levels are involved in
the development of ischemic stroke by interacting with MMP-2.
Atherosclerosis, an underlying cause of a large
proportion of strokes, is a multifactorial process which
synergistically induces oxidative stress and a chronic inflammatory
state (29). Lipids, particularly
cholesterol transported in circulating LDL particles, are key
factors that are associated with the formation of the
atherosclerotic plaque. In a previous study, the authors reported a
reduction of atherosclerosis development in the animal model of
ApoE−/− mice treated using adenoviruses containing the human DCN
gene (3). The secreted DCN formed
complexes with 40% of plasma TGF-β1 of ApoE−/− mice, leading to a
reduction in plasma TGF-β1. The overexpression of DCN was
accompanied by a reduction in plaque macrophage content.
Atherosclerosis protection is achieved since transient DCN
overexpression changes the plaque phenotype to that of lower
inflammation. Similarly, in the current study, the level of DCN was
the lowest in the LAAS group and it was negatively correlated with
LDL-C. This result is consistent with previous studies and suggests
that plasma DCN levels are involved in the development of
atherosclerosis. The correlation between DCN and NIHSS was also
evaluated. NIHSS is a reliable and commonly used score for clinical
assessment of the severity of stroke. However, no correlation was
identified between DCN levels and NIHSS in the present study. This
might be due to the small sample size. Therefore, further studies
are required to clarify the correlation between the DCN and
NIHSS.
During AIS, the brain initiates a complex cascade of
ischemic events at various levels. Excitotoxicity, oxidative
stress, blood-brain barrier dysfunction, inflammation and ECM
remolding are the most significant pathophysiological processes
involved in this cascade (30–32).
Cytokines, including IL-1, IL-6, tumor necrosis factor-α (TNF-α)
and TGF-β are produced by a variety of activated cell types and are
significant mediators of stroke induced inflammation that may
contribute to the progression of cerebral infarction (33–35).
Yamaguchi et al(36)
demonstrated that DCN is a natural inhibitor of transforming growth
factor-β1 (TGF-β1). Al Haj Zen et al(3) reported that DCN reduces inflammation
by downregulating TGF-β and IL-1β expression through
adenovirus-mediated overexpression of DCN in the human gingival
fibroblasts. These studies indicate that inflammatory cytokines
explain the change of DCN expression during stroke.
There are several limitations to the present study.
First, the sample size was relatively small. Second, there were no
long-term follow-up results. Thus, further studies are required to
confirm these preliminary results.
In conclusion, the present study is the first to
report that plasma DCN levels are decreased in patients with AIS,
particularly in the LAAS group, and that the level of DCN is
positively correlated with MMP-2 levels. Of note, decreased plasma
DCN concentrations (<8,500 pg/ml) are associated with increased
risk for ischemic stroke. Our findings indicate that DCN is
involved in the pathogenesis of ischemic stroke and that a
reduction of plasma DCN may be a useful indicator for ischemic
stroke.
Acknowledgements
This study was supported by grants from the Chinese
National Science Foundation (no. 30973102). The authors are
particularly grateful to the patients who volunteered to
participate in this study.
Abbreviations:
|
AF
|
atrial fibrillation
|
|
AIS
|
acute ischemic stroke
|
|
ANOVA
|
analysis of variance
|
|
APTT
|
activated partial thromboplastin
time
|
|
AUC
|
area under the curve
|
|
CEI
|
cardioembolic infarcts
|
|
CT
|
computed tomography
|
|
DBP
|
diastolic blood pressure
|
|
DM
|
diabetes mellitus
|
|
ECM
|
extracellular matrix
|
|
EGF-R
|
epidermal growth factor
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
FDP
|
fibrinogen degradation products
|
|
FIB
|
fibrinogen
|
|
GAG
|
glycosaminoglycan
|
|
HDL
|
high-density lipoprotein
|
|
IGF-1R
|
insulin-like growth factor-1
receptor
|
|
IHD
|
ischemic heart disease
|
|
IL
|
interleukin
|
|
LAAS
|
large-artery atherosclerosis
|
|
LAC
|
lacunar infarct
|
|
LDL
|
low-density lipoprotein
|
|
MMP
|
matrix metalloproteinase
|
|
MRI
|
magnetic resonance imaging
|
|
NIHSS
|
National Institutes of Health Stroke
Scale
|
|
ODE
|
stroke of other determined
etiology
|
|
PDGF
|
platelet-derived growth factor
|
|
PLT
|
platelet
|
|
PT
|
prothrombin time
|
|
RBC
|
red blood cell
|
|
SBP
|
systolic blood pressure
|
|
SD
|
standard deviation
|
|
TC
|
total cholesterol
|
|
TG
|
triglyceride
|
|
TGF-β
|
transforming growth factor-β
|
|
TIMP
|
tissue inhibitor of
metalloproteinase
|
|
TNF-α
|
tumor necrosis factor-α
|
|
TOAST
|
Trial of ORG10172 in Acute Stroke
Treatment
|
|
UDE
|
stroke of undetermined etiology
|
|
WBC
|
white blood cell
|
References
|
1
|
Truelsen T, Heuschmann PU, Bonita R, et
al: Standard method for developing stroke registers in low-income
and middle income countries: experiences from a feasibility study
of a stepwise approach to stroke surveillance (STEPS Stroke).
Lancet Neurol. 6:134–139. 2007. View Article : Google Scholar
|
|
2
|
Saenger AK and Christenson RH: Stroke
biomarkers: progress and challenges for diagnosis, prognosis,
differentiation and treatment. Clin Chem. 56:21–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al Haj Zen A, Caligiuri G, Sainz J, et al:
Decorin overexpression reduces atherosclerosis development in
apolipoprotein E-deficient mice. Atherosclerosis. 187:31–39.
2006.PubMed/NCBI
|
|
4
|
Chen S and Birk DE: Focus on molecules:
decorin. Exp Eye Res. 92:444–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Sun H, Owens RT, et al: Decorin
suppresses prostate tumor growth through inhibition of epidermal
growth factor and androgen receptor pathways. Neoplasia.
11:1042–1053. 2009.PubMed/NCBI
|
|
6
|
Johnson PY, Potter-Perigo S, Gooden MD, et
al: Decorin synthesized by arterial smooth muscle cells is retained
in fibrin gels and modulates fibrin contraction. J Cell Biochem.
101:281–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldoni S, Humphries A, Nyström A, et al:
Decorin is a novel antagonistic ligand of the Met receptor. J Cell
Biol. 185:743–754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Reed CC, Waterhouse A, Kirby S, et al:
Decorin prevents metastatic spreading of breast cancer. Oncogene.
24:1104–1110. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iozzo RV, Moscatello DK, McQuillan DJ and
Eichstetter I: Decorin is a biological ligand for the epidermal
growth factor receptor. J Biol Chem. 274:4489–4492. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schönherr E, Sunderkotter C, Iozzo RV and
Schaefer L: Decorin, a novel player in the insulin-like growth
factor system. J Biol Chem. 280:15767–15772. 2005.PubMed/NCBI
|
|
11
|
Kresse H and Schönherr E: Proteoglycans of
the extracellular matrix and growth control. J Cell Physiol.
189:266–274. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Merle B, Durussel L, Delmas PD and
Clezardin P: Decorin inhibits cell migration through a process
requiring its glycosaminoglycan side chain. J Cell Biochem.
75:538–546. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Yu H, Sun K, et al: Promoter
variant of angiopoietin-2 and plasma angiopoietin-2 are associated
with risk of stroke recurrence in lacunar infarct patients. Biochem
Biophys Res Commun. 398:212–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Adams HP Jr, Bendixen BH, Kappelle LJ, et
al: Classification of subtype of acute ischemic stroke. Definitions
for use in a multicenter clinical trial TOAST Trial of Org 10172 in
Acute Stroke Treatment. Stroke. 24:35–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brott T, Adams HP Jr, Olinger CP, et al:
Measurements of acute cerebral infarction: a clinical examination
scale. Stroke. 20:864–870. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan W, Wang P, Zhao CX, et al: Decorin
gene delivery inhibits cardiac fibrosis in spontaneously
hypertensive rats by modulation of transforming growth
factor-β/Smad and p38 mitogen-activated protein kinase signaling
pathways. Hum Gene Ther. 20:1190–1200. 2009.PubMed/NCBI
|
|
17
|
Zhang Z, Garron TM, Li XJ, et al:
Recombinant human decorin inhibits TGF-β1-induced contraction of
collagen lattice by hypertrophic scar fibroblasts. Burns.
35:527–537. 2009.
|
|
18
|
Zheng F, Lu W, Wu F, et al: Recombinant
decorin ameliorates the pulmonary structure alterations by
down-regulating transforming growth factor-β1/SMADS signaling in
the diabetic rats. Endocr Res. 35:35–49. 2010.PubMed/NCBI
|
|
19
|
Sanches JC, Jones CJ, Aplin JD, et al:
Collagen fibril organization in the pregnant endometrium of
decorin-deficient mice. J Anat. 216:144–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schaefer L and Iozzo RV: Biological
functions of the small leucine-rich proteoglycans: from genetics to
signal transduction. J Biol Chem. 283:21305–21309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Murphy G: Matrix metalloproteinases and
their inhibitors. Acta Orthop Scand Suppl. 266:55–60.
1995.PubMed/NCBI
|
|
22
|
de Nooijer R, Verkleij CJ, von der Thüsen
JH, et al: Lesional overexpression of matrix metallopmteinase-9
promotes intraplaque hemorrhage in advanced lesions but not at
earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol.
26:340–346. 2006.PubMed/NCBI
|
|
23
|
Bäck M, Ketelhuth DF and Agewall S: Matrix
metalloproteinases in atherothrombosis. Prog Cardiovasc Dis.
52:410–428. 2010.
|
|
24
|
Horstmann S, Kalb P, Koziol J, et al:
Profiles of matrix metalloproteinases, their inhibitors and laminin
in stroke patients: influence of different therapies. Stroke.
34:2165–2170. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vukasovic I, Tesija-Kuna A, Topic E, et
al: Matrix metalloproteinases and their inhibitors in different
acute stroke subtypes. Clin Chem Lab Med. 44:428–434. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Horstmann S, Su Y, Koziol J, et al: MMP-2
and MMP-9 levels in peripheral blood after subarachnoid hemorrhage.
J Neurol Sci. 251:82–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong FQ, Li H, Wu F, et al: Effects of
overexpression of decorin on matrix metalloproteinases 2 and 9 in
rat mesangial and tubular cells. Zhonghua Yi Xue Za Zhi.
88:3444–3447. 2008.(In Chinese).
|
|
28
|
Al Haj Zen A, Lafont A, Durand E, et al:
Effect of adenovirus-mediated overexpression of decorin on
metalloproteinases, tissue inhibitors of metalloproteinases and
cytokines secretion by human gingival fibroblasts. Matrix Biol.
22:251–258. 2003.PubMed/NCBI
|
|
29
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:419–420. 1999. View Article : Google Scholar
|
|
30
|
Nilupul Perera M, Ma HK, Arakawa S, et al:
Inflammation following stroke. J Clin Neurosci. 13:1–8.
2006.PubMed/NCBI
|
|
31
|
Elkind MS: Inflammatory mechanisms of
stroke. Stroke. 41(Suppl 10): S3–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tuttolomondo A, Di Sciacca R, Di Raimondo
D, et al: Inflammation as a therapeutic target in acute ischemic
stroke treatment. Curr Top Med Chem. 9:1240–1260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tuttolomondo A, Di Raimondo D, Di Sciacca
R, et al: Fetuin-A and CD40 L plasma levels in acute ischemic
stroke: Differences in relation to TOAST subtype and correlation
with clinical and laboratory variables. Atherosclerosis.
208:290–296. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kiewert C, Mdzinarishvili A, Hartmann J,
et al: Metabolic and transmitter changes in core and penumbra after
middle cerebral artery occlusion in mice. Brain Res. 1312:101–107.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang J, Upadhyay UM and Tamargo RJ:
Inflammation in stroke and focal cerebral ischemia. Surg Neurol.
66:232–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yamaguchi Y, Mann DM and Ruoslahti E:
Negative regulation of transforming growth factor-β by the
proteoglycan decorin. Nature. 346:281–4. 1990.
|