Introduction
Schwann cells (SCs) are the principal cells of the
peripheral nervous system. They often serve as the seed cells for
the tissue engineering of artificial neurons and contribute
significantly to the recovery from peripheral nerve injury
(1,2). SCs ensure favorable conditions for
the regeneration of axons and guide the distal growth of axons by
secreting the corresponding nerve growth factors and extracellular
matrix (3). However, as end-stage
cells, directly derived SC lines do not attach well and have low
proliferative potential; therefore, clinical applications that
require pure SCs in large quantities are limited. It has been shown
that SCs express various markers during different stages of
development and injury repair processes, indicating that SCs have
proliferative potential that varies according to context and are
not functionally and structurally homogeneous. In this regard, to
further expand the scope of the clinical applications of SCs, we
introduce in this study a new approach for the culturing of SCs
from the adult mouse in vitro.
Materials and methods
Animals
A total of 15 clean 6- to 8-week-old c57 mice were
provided by the Shanghai Silaike Experimental Animal Center. The
study was approved by the ethics committee of Shanghai Jiaotong
University
Reagents and equipment
DMEM and FBS were purchased from Hyclone (Shanghai,
China). Forskolin was obtained from Sigma (St. Louis, MO, USA).
Heregulin-β-1 and basic-FGF (b-FGF) were from Peprotech, Inc.
(Rocky Hill, NJ, USA). Collagenase NB4 (neutral protease, grade II)
was from Roche Diagnostics GmbH (Mannheim, Germany). Dispase was
purchased from Serva (Heidelberg, Germany). The multiclonal s100β
rabbit antibody and fluorescent mounting medium were from Dako
(Ely, UK). The multiclonal p75NTR rabbit antibody was from Abcam
(Cambridge, UK). The multiclonal goat anti-rabbit IgG-rhodamine was
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Medium containing growth factors
Schwann cell culture medium (SCCM) was prepared by
supplementing DMEM with 10% FBS, 2 μM forskolin, 10 ng/ml
heregulin-β-1 and 50 ng/ml b-FGF.
Poly-lysine coating
Culture dishes were covered with 0.1 mg/ml
poly-lysine working solution, incubated at 37°C for 1 h, washed
with double-distilled water and refrigerated at 4°C until use.
Animal surgery and in vitro tissue
culture
Adult c57 mice were sacrificed by cervical
dislocation and surface sterilized in 75% ethanol for 10 min. Both
sides of the sciatic nerves were surgically exposed on a clean
bench and a 1.5-cm fragment was removed for use. The skin was
stripped under a dissection scope (Motic, Xiameng, China) and the
sciatic nerves were rinsed with PBS and placed in DMEM supplemented
with 10% FBS or SCCM in a 6-well plate. The plate was incubated at
37°C under 5% CO2 in a CO2 incubator (Forma
Scientific, Inc., Marietta, OH, USA) for one week. The media were
changed once every two days.
Cell culture and purification
Fresh sciatic nerves and the sciatic nerves that had
been incubated in DMEM with 10% FBS or SCCM were rinsed with PBS,
cut into 2-mm pieces and digested with a mixture of 0.2%
collagenase NB4 and 0.2% dispase at 37°C. The digested materials
were then centrifuged at 1,500 rpm for 5 min and the supernatants
were discarded. The pellets were resuspended in SCCM and replated
in poly-lysine coated dishes following cell counting. The dishes
were incubated at 37°C under 5% CO2. The cells obtained
from the SCCM cultured nerves were purified following 48 h of
incubation (4).
Immunostaining with s100β and p75NTR
S100β and p75NTR immunostaining was performed for p0
and p1 cells as follows. The cells were fixed with 4%
paraformaldehyde and membranes were permeabilized with 0.3% Triton
X-100. The samples were then blocked with goat serum at 37°C for 30
min, incubated with primary antibody at 37°C for 1 h, washed for 5
min with PBS three times and incubated with secondary antibody at
37°C for 30 min. Finally, the cell nuclei were stained with DAPI.
Images were captured under a fluorescent microscope (Nikon, Tokyo,
Japan). Cells in three randomly selected fields were counted for SC
purity calculations.
Frozen section and s100β
immunostaining
Fresh sciatic nerves and the sciatic nerves that had
been incubated in DMEM with 10% FBS or SCCM were fixed, dehydrated
and frozen-sliced into 8-μm sections. Immunostaining and DAPI
staining were performed as described in the previous section.
Samples were observed and images captured under a confocal
microscope.
Statistical analysis
All data are expressed as the average ± standard
deviation (x±s). Statistical analyses were performed using the SPSS
13.0 software. Comparisons between the averages of the groups were
evaluated using the Student’s t-test. P<0.05 was considered to
indicate a statistically significant result.
Results
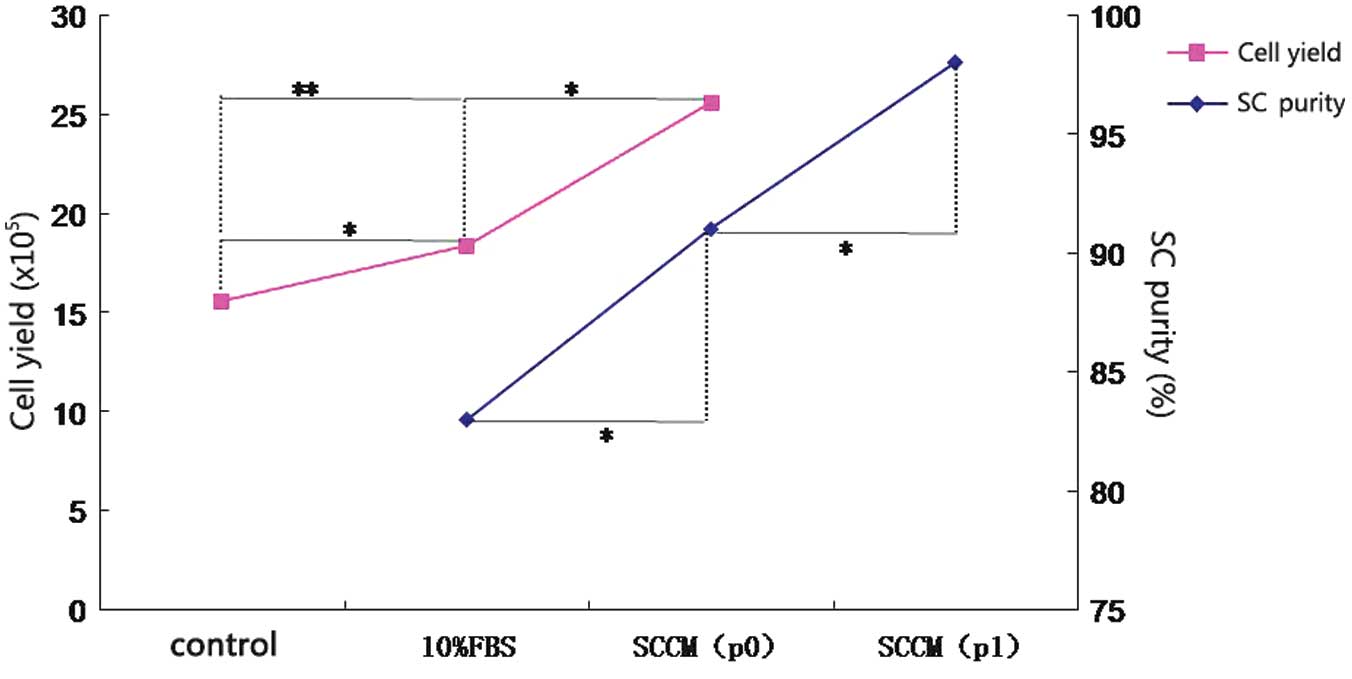
Cell counting
Cell suspensions (20 μl) for the various groups were
counted using a hemacytometer prior to inoculation. The cell count
for the SCCM group (2.56×106/3N) was significantly
higher than the counts for the control (1.56×106/3N) and
the DMEM with 10% FBS groups (1.84×106/3N) (Fig. 1).
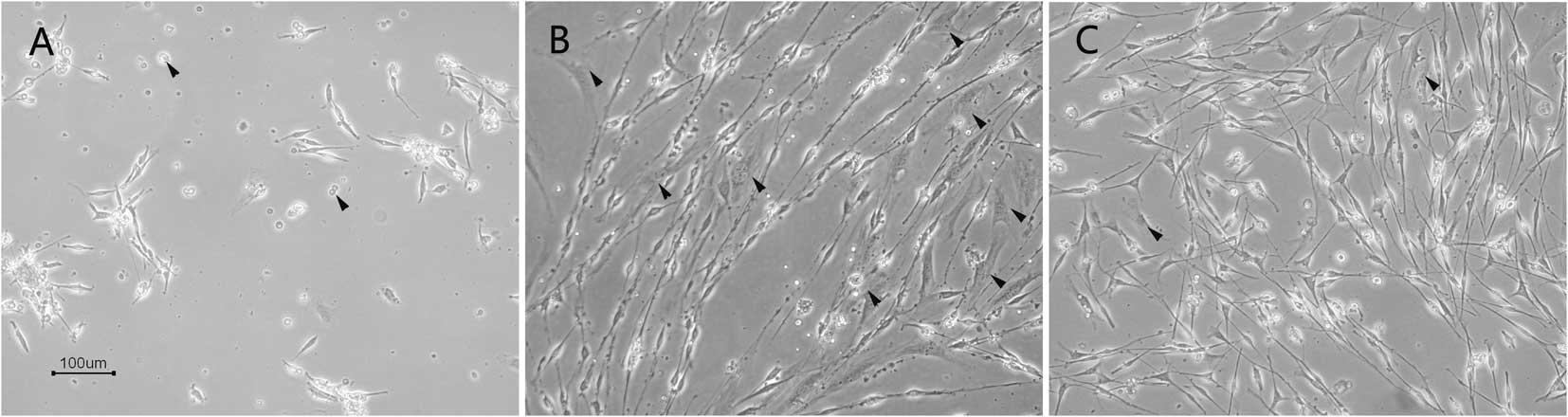
Observation of cell growth
At 48 h after inoculation, the attachment of the
cells was observed under an inverse microscope. Two types of cells
with different morphologies were obtained. SCs were small and
appeared in long fusiform, bipolar or tripolar shapes with strong
refraction while fibroblasts were large and appeared in flat
multilateral or irregular shapes with weak refraction. The
uncultured cells which remained suspended were dead. Only a small
fraction of the cells attached and proliferated. The majority of
the cells derived from the sciatic nerves cultured in DMEM with 10%
FBS were observed to be attached, however a high percentage of the
cells were fibroblasts. The cells derived from the sciatic nerves
cultured in SCCM attached and only a few of them were fibroblasts
(Fig. 2).
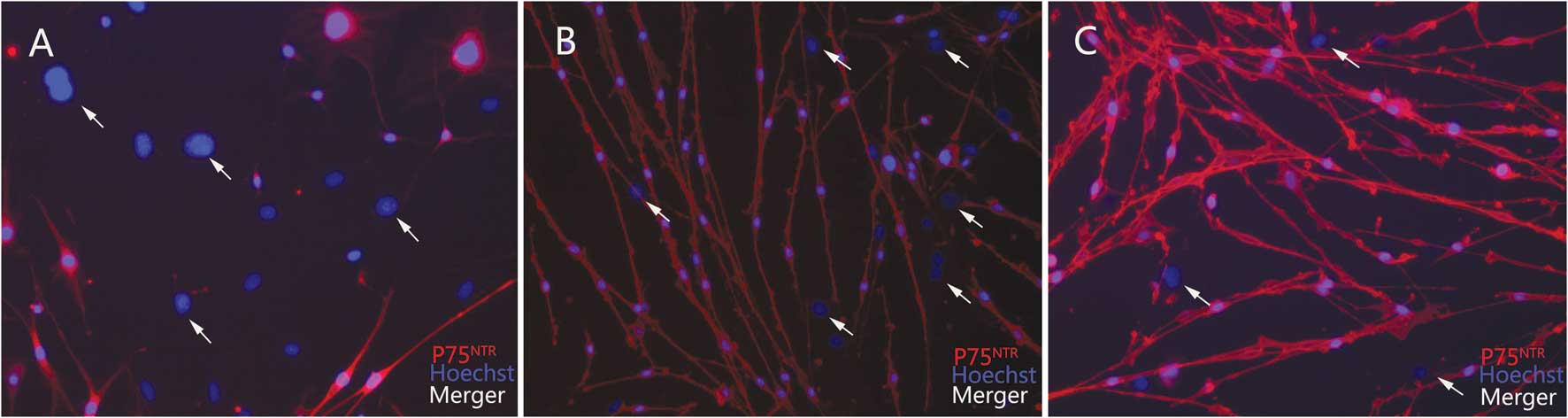
Immunostaining and cell purity
determination
After 48 h of incubation, cells from the various
groups were stained with anti-p75NTR antibody. Almost all bipolar
or tripolar cells stained positive, while the fibroblasts stained
negative (Fig. 3). The purities of
the SCs from the DMEM with 10% FBS group and the SCCM group were 83
and 91%, respectively (Fig. 1).
The purity of the SCs was not determined for cells derived from
fresh sciatic nerves since the cells were not attached, even
following 72 h of incubation.
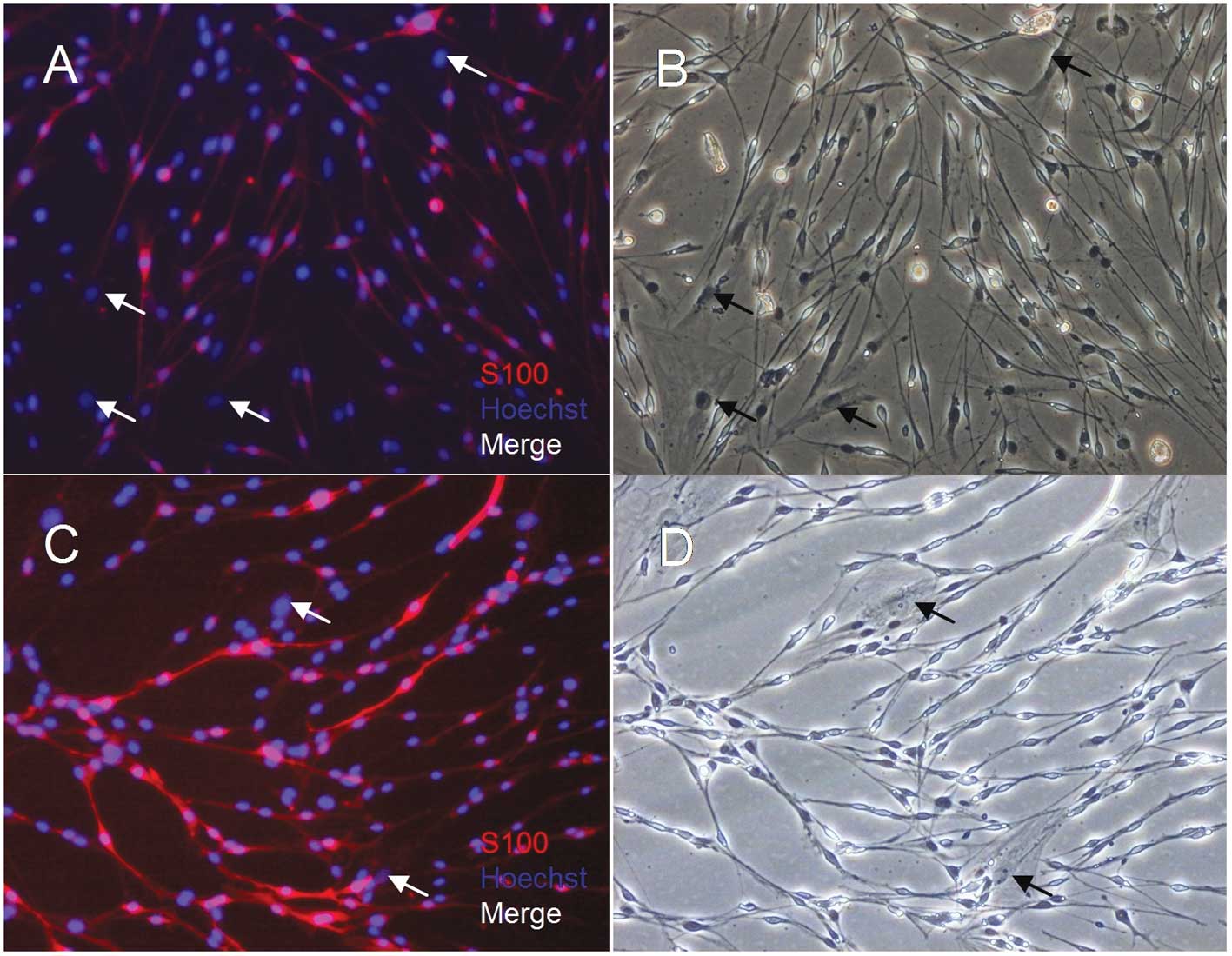
Following one simple purification (p1) step for the
cells derived from the sciatic nerves cultured in SCCM, almost all
bipolar and tripolar cells were stained positive for s100β
(Fig. 4). The purity of the SCs
was 98% (Fig. 1).
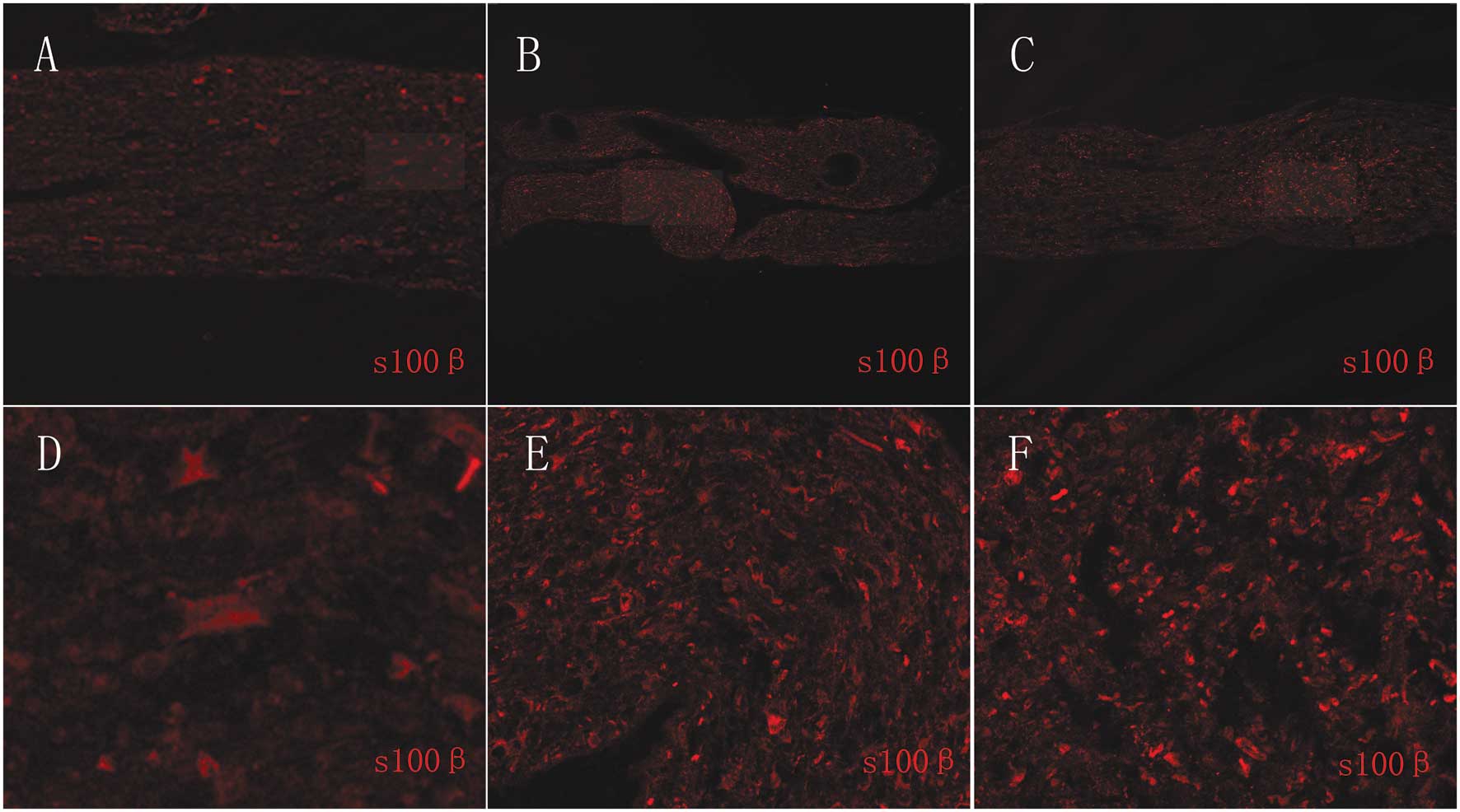
Histological section of s100β
immunostaining samples
Cells derived from the sciatic nerves cultured in
SCCM attached well and resulted in high yields of highly pure SCs.
Therefore, we investigated the changes in the SCs at the tissue
level. The SCs from the sciatic nerves cultured in SCCM
proliferated and formed bands of Büngner, which is similar to the
Wallerian degeneration process following nerve injury. The SCs from
sciatic nerves cultured in DMEM with 10% FBS proliferated but did
not form bands of Büngner. The SCs from fresh sciatic nerves did
not proliferate (Fig. 5).
Discussion
The development of neural tissue engineering
provides a new route for peripheral nerve repair. SCs are seed
cells for neural tissue engineering and contribute significantly to
the repair of injured peripheral nerves. However, as end-stage
cells, SCs are difficult to culture and are usually obtained in
poor yields and low purities. In addition, despite the efforts of
many scientists to induce Schwann-like cells from stem cells
(5–7), further applications of these methods
are limited due to low induction rates, dedifferentiation following
the removal of the inducing agents and the tumor-prone properties
of the induced cells (8).
To obtain high quantities of high quality SCs, the
in vitro culturing and proliferation of SCs has been
extensively used. Previous studies have shown that SCs have
different proliferative potentials at different developmental
stages and are not functionally and structurally homogeneous.
During the Wallerian degeneration of nerve injury, SCs
dedifferentiate, express neurotrophic factors, cell adhesion
molecules (9,10) and the immature SC marker glial
fibrillary acidic protein (GFAP) and upregulate p75NTR (11–13).
These may facilitate SC proliferation and attachment and promote
nerve regeneration. In 1999, Keilhoff et al(14) obtained large quantities of SCs with
high proliferative potential following in vivo
pre-degeneration (Wallerian degeneration) for a week and in
vitro recultivation. However, in vivo pre-degeneration
requires two surgical procedures, is time-consuming and causes a
delay in treatment. The clinical applications of this approach are
also limited due to individual differences and the resulting
difficulties in estimating the timing of the desired
pre-degeneration effect. In our study, the sciatic nerves of adult
mice were pre-degenerated in vitro in media that mimicked
in vivo conditions. In 2010, Kraus et al(15) reported an in vitro
pre-degeneration process in which sciatic nerves were incubated in
DMEM with 10% FBS for one week and large quantities of pure SCs
were obtained. Based on these advances, we included in our medium
forskolin, heregulin-β-1 and b-FGF (SCCM) to promote the growth of
the SCs. The experimental conditions used by Kraus et al
were used for our positive control. After one week, the SC count in
the SCCM group was 2.56×106/3N, which was significantly
higher than the counts in the DMEM with 10% FBS group and the
uncultured group (Fig. 1). When
viewed 48 h post-inoculation, many cells attached and these cells
were confirmed to be SCs by immunostaining. Only small amounts of
fibroblasts were present. The purity of the SCs in p0 was 91%,
compared with 83% purity in the DMEM with 10% FBS group. Following
one simple purification step, the purity reached 98%. In 1995, Dong
et al(16) described the
concentration-dependent death of in vitro cultured cells -
the cells died more readily at low concentrations. Cells cultured
in vitro secrete mitosis-promoting growth factors (17) and cell-cell contacts not only
further enhance the release of these factors to induce cell
division and proliferation (18)
but also suppress the growth of fibroblasts.
We also investigated the proliferation of SCs
induced by forskolin, heregulin-β-1 and b-FGF at the tissue level.
Forskolin, heregulin-β-1 and b-FGF stimulated the proliferation of
SCs, which formed bands of Büngner and promoted the
dedifferentiation of SCs. These effects were not significant in the
uncultured and the DMEM with 10% FBS groups.
We have developed, in this study, a method for
cultivating a large number of pure SCs in vitro. This
finding helps to fulfill the need to develop artificial neurons and
further enriches SC research and the possible clinical applications
of SCs.
References
|
1
|
Meek MF and Coert JH: Clinical use of
nerve conduits in peripheral nerve repair:reiew of literature. J
Reconstr Microsurg. 18:97–109. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen ZL, Berger A, Hierner R, et al: A
Schwann cell-seeded intrinsic framework and its satisfactory
biocompatibility for a bioartificial nerve graft. Microsurgery.
21:6–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frostick SP, Yin Q and Kemp GJ: Schwann
cells, neurotrophic factors, and peripheral nerve regeneration.
Microsurgery. 18:397–405. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jin YQ, Liu W, Hong TH and Cao Y:
Efficient Schwann cell purification by differential cell detachment
using multiplex collagenase treatment. J Neurosci Methods.
170:140–148. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dezawa M, Takahashi I, Esaki M, et al:
Sciatic nerve regeneration in rats induced by transplantation of in
vitro differentiated bone-marrow stromal cells. Eur J Neurosci.
14:1771–1776. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kingham PJ, Kalbermatten DF, Mahay D, et
al: Adipose-derived stem cells differentiate into a Schwann cell
phenotype and promote neurite outgrowth in vitro. Exp Neurol.
207:267–274. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang L, Zhu JK, Liu XL, et al: Phenotypic
and functional characteristics of rat adipose tissue-derived
stromal cells differentiated into Schwann-like cells in vitro. Chin
J Microsurgery. 30:430–432. 2007.(In Chinese).
|
|
8
|
Cogle CR, Theise ND, Fu D, et al: Bone
marrow contributes to epithelial cancers in mice and humans as
developmental mimicry. Stem Cells. 25:1881–1887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bolin LM, Iismaa TP and Shooter EM:
Isolation of activated adult Schwann cells and a spontaneously
immortal Schwann cell clone. J Neurosci Res. 33:231–238. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fannon AM, Sherman DL, Ilyina-Gragerova G,
et al: Novel E-cadherin mediated adhesion in peripheral nerve:
Schwann cell architecture is stabilized by autotypic adherens
junctions. J Cell Biol. 129:189–202. 1995.PubMed/NCBI
|
|
11
|
Jessen KR and Mirsky R: Negative
regulation of myelination: Relevance for development, injury, and
demyelinating disease. Glia. 56:1552–1565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee HK, Seo IA, Suh DJ, et al:
Interleukin-6 is required for the early induction of glial
fibrillary acidic protein in Schwann cells during Wallerian
degeneration. J Neurochem. 108:776–786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lemke G and Chao M: Axons regulate Schwann
cell expression of the major myelin and NGF receptor genes.
Development. 102:499–504. 1988.PubMed/NCBI
|
|
14
|
Keilhoff G, Fansa H, Schneider W, et al:
In vivo predegeneration of peripheral nerves: an effective
technique to obtain activated Schwann cells for nerve conduits. J
Neurosci Methods. 89:17–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kraus A, Täger J, Kohler K, et al:
Efficacy of various durations of in vitro predegeneration on the
cell count and purity of rat Schwann-cell cultures. J Neurotrauma.
27:197–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dong Z, Brennan A, Liu N, et al: Neu
differentiation factor is a neuron-glia signal and regulates
survival, proliferation, and maturation of rat Schwann cell
precursors. Neuron. 15:585–596. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eccleston PA, Collarini EJ, Jessen KR, et
al: Schwann cells secrete a PDGF-like factor: evidence for an
autocrine growth mechanism involving PDGF. Eur J Neurosci.
2:985–992. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sasagasako N, Toda K, Hollis M and Quarles
RH: Myelin gene expression in immortalized Schwann cells:
relationship to cell density and proliferation. J Neurochem.
66:1432–1439. 1996. View Article : Google Scholar : PubMed/NCBI
|