Introduction
As one of the most lethal gynecological cancers,
ovarian cancer is the fifth leading cause of cancer-related
mortality among women in Western countries. Most ovarian cancer
cells originate from the ovarian surface epithelium and display a
range of histological subtypes (1). Platinum is the first-line drug for
the chemotherapy of ovarian cancer (2).
The TNF-related apoptosis-inducing ligand (TRAIL) is
a TNF family member capable of inducing apoptosis through
caspase-dependent mechanisms (3–6).
TRAIL binds four major different receptors, two of which, death
receptor (DR)4 and DR5, induce apoptosis; however, the other two,
decoy receptor (DcR)1 and DcR2, do not have the intracytoplasmic
death domain to transduce apoptotic death signals and, thus, they
protect cells from TRAIL-mediated cell death by interfering with
signaling through DR4 and DR5 (7,8).
Another receptor, osteoprotegerin (OPG), is a soluble receptor that
may play a more prominent role in bone and myeloid cell development
(9). In tumor cells, the
expression of DR5 which is rare or even absent in healthy cells
predominates over that of DR4; therefore, DR5 plays a major role in
TRAIL-induced apoptosis (4,10).
In order to find an alternative for TRAIL for clinical treatment,
monoclonal antibodies inducing the apoptosis of tumor cells via
targeting DR5 have been developed, such as TRA-8, Apomab and
CS-1008 (2,12–14).
The DR5 monoclonal antibody (D-6) is a type of
murine monoclonal antibody directed againts DR5, produced by Santa
Cruz Biotechnology Co. In this study, we explored the
apoptosis-inducing effects of the DR5 monoclonal antibody (D-6),
alone or in combination with the platinum-based drug, cisplatin, on
the A2780 ovarian cancer cell line.
Materials and methods
Cell culture and reagents
All animal procedures were approved by the Animal
Care and Scientific Committee of Sichuan University, Chengdu China.
The A2780 cells were kindly provided by Kanghong Co. (Chengdu,
China). The DR5 monoclonal antibody (D-6) was purchased from Santa
Cruz Biotechnology Co. (Santa Cruz, CA, USA). Cisplatin was
purchased from the West China Second Hospital, Sichuan University.
The RPMI-1640 culture medium, dimethylsulfoxide (DMSO) and fetal
bovine serum were purchased from Gibco (Carslbad, CA, USA). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cell proliferation assay kit and trypsin were purchased from Sigma
(Sigma, St. Louis, USA). The Annexin V-FITC apoptosis detection kit
and protein extraction kit were purchased from KeyGen Biotech Co.
(Nanjing, China). The BCA protein assay kit, SDS-PAGE protein
sample buffer (5X), SDS-PAGE gel preparation kit and the BeyECL
Plus chemiluminescence kit were purchased from Beyotime Co.
(Jiangsu, China). The caspase 3, 8 and 9 precursor antibodies were
purchased from Santa Cruz Biotechnology Co. Polyvinylidene
difluoride (PVDF) membranes were purchased from Millipore
(Billerica, MA, USA). The enhanced chemiluminescence (ECL)
detection kit and X-ray films were purchased from Roche (Basel,
Switzerland).
Apoptosis observation under a
microscope
The A2780 cells were counted and cultured in 6-well
plates at a concentration of 2×105 cells/well. they were
then divided into four groups: the control group, where 2 ml
culture medium was added to the wells; the cisplatin group, where 2
ml culture medium containing 2.5 μg/ml cisplatin was added; the DR5
(D-6) group, where 2 ml culture medium containing 2 μg/ml DR5 (D-6)
was added; and the combination group, where 2 ml culture medium
containing 2.5 μg/ml cisplatin and 2 μg/ml DR5 (D-6) was added.
Twenty-four hours after the culture, the morphological changes of
each group were observed under an inverted microscope.
MTT analysis
The A2780 ovarian cancer cells were first counted
and incubated in two 96-well plates at a concentration of
5×103 cells/well and a volume of 0.1 ml/well in 30 wells
of each plate. After culture in the RPMI-1640 culture medium
containing 10% fetal bovine serum, the 30 wells of one plate were
randomized into six groups (five wells/group) and 0.1 ml culture
medium containing 0, 0.125, 0.25, 0.5, 1 and 2 μg/ml DR5 (D-6) was
added, after drying out the supernatant. The other plate was also
divided into six groups (five wells/group) and 0.1 ml culture
medium containing 2.5 μg/ml cisplatin and 0.125, 0.25, 0.5, 1 and 2
μg/ml DR5 (D-6) was added, after drying out the supernatant, except
for one group (the contol) in which 0.1 ml culture medium was
added. After 48 h of culture in the incubator, 10 μl MTT was added
to each well. The plates were then incubated at 37°C for an
additional 4 h to allow MTT to form formazan crystals by reacting
with metabolically active cells. The formazan crystals were
solubilized in 0.1 ml DMSO per well at 37°C for 10 min. The
absorbance values of the solution in each well were measured at 450
nm using a multiscanner autoreader. The cell growth inhibition rate
was calculated as follows: [1 - (OD test - OD blank)/(OD control -
OD blank) ×100% (11).
Flow cytometry
The A2780 ovarian cancer cells were counted and
cultured in four wells of a six-well plate at a concentration of
2×105 cells/well and a volume of 2 ml/well. After
culture in the incubator for 24 h, the supernatant was dried out
and the four wells were randomized into four groups: the control
group, where 2 ml culture medium was added; the DR5 (D-6) group,
where 2 ml culture medium containing 1 μg/ml DR5 (D-6) was added;
the cisplatin group, where 2 ml culture medium containing 2.5 μg/ml
cisplatin was added; and the combination group, where 2 ml culture
medium containing 1 μg/ml DR5 (D-6) and 2.5 μg/ml cisplatin was
added. After 24 h in the incubator, the cells were digested with
EDTA-free trypsin and washed twice with PBS. For each group,
1–5×105 cells were collected. The cells were then
suspended in binding buffer (500 μl), and then 5 μl Annexin V-FITC
and 5 μl PI were added. After 10 min of reaction in a dark
environment, the cell apoptotic rate was evaluated by flow
cytometry.
Western blot analysis
The A2780 ovarian cancer cells were counted and
cultured in the four culture dishes at a concentration of
106 cells/well and a volume of 5 ml/well. After culture
in the incubator for 24 h, the supernatant was dried out and the
four wells were randomized into four groups: the control group,
where 5 ml culture medium was added; the DR5 (D-6) group, where 5
ml culture medium containing 2 μg/ml DR5 (D-6) was added; the
cisplatin group, where 5 ml culture medium containing 2.5 μg/ml
cisplatin was added; and the combination group, where 5 ml culture
medium containing 2 μg/ml DR5 (D-6) and 2.5 μg/ml cisplatin was
added. After 48 h in the incubator, the cells from each group were
collected and protein extraction was carried out using the protein
extraction kit. Protein (35 μg) extracted from the cells of each
group was subjected to SDS-PAGE electrophoresis and then the gel
was electroblotted on a PVDF membrane for 60 min. The membrane was
incubated with 5% non-fat dry milk in TBS for 1 h to block
non-specific binding sites, and then incubated with the appropriate
primary antibody concentration (1:200 dilution for caspase 3, 8 and
9 precursors) overnight. The membrane was subsequently rinsed in
TBS-T and incubated for 1 h at 37°C with secondary antibody. After
incubation, the membrane was finally rinsed and visualized with ECL
detection reagents.
Statistical analysis
The cell growth inhibition rate and apoptotic rate
were analyzed by one-way ANOVA and the Student's t-test. All
statistical analyses were performed using the SPSS 17.0 software
package. All P-values were two-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Morphological changes observed under a
microscope
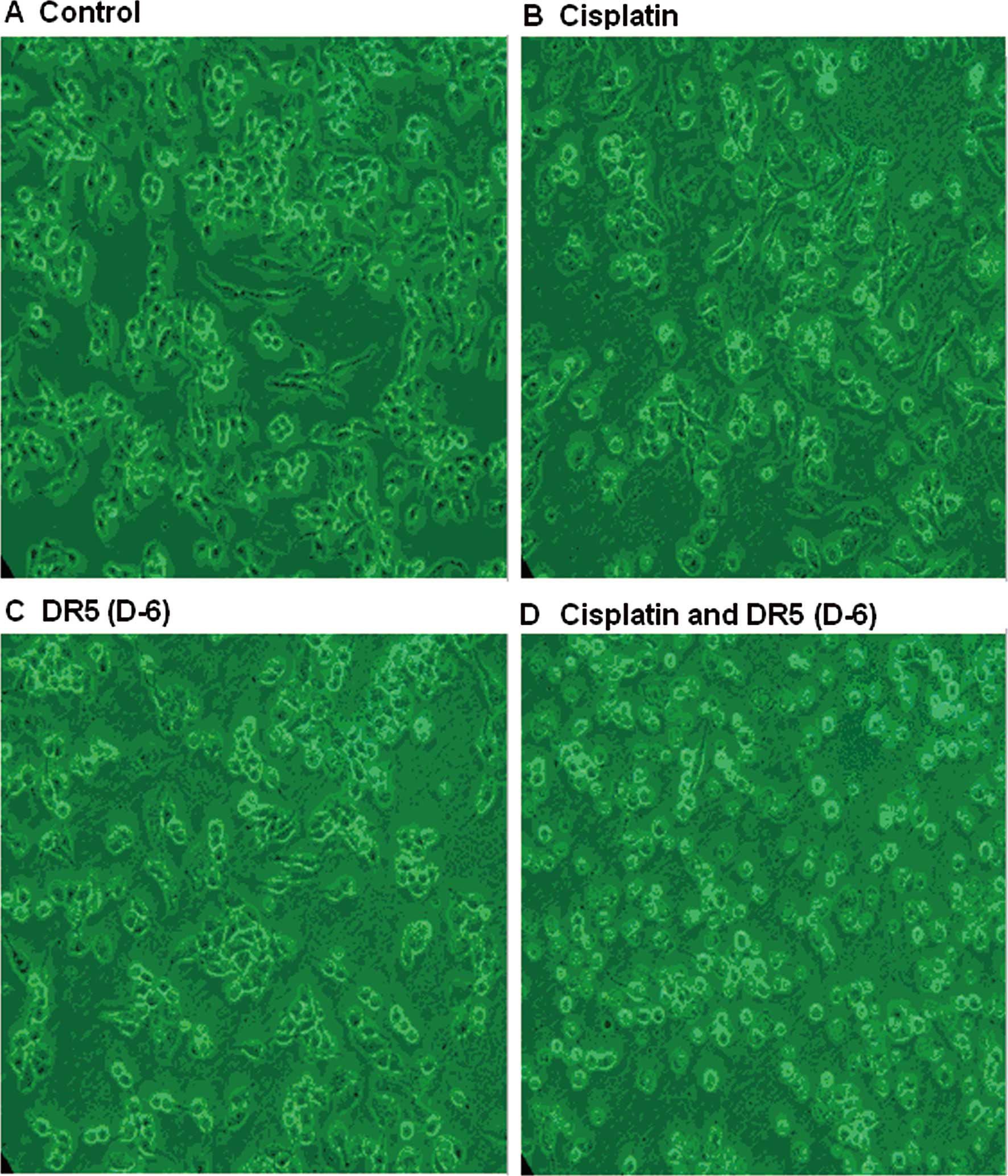
As shown in Fig. 1,
24 h after the treatment, the cells of both the DR5 (D-6) and
cisplatin groups showed evident apoptosis; these effects were more
evident in the DR5 (D-6) and cisplatin combination group. In the
combination group, there were many floating cells and only a few
cells grew along the wall. The cells underwent significant changes
in morphology; their original shape was completely altered, the
cytoplasm became rougher, the nucleus became pycnotic and the
refractive index in the cells decreased, demonstrating significant
cellular damages. By contrast, A2780 cells in the control group did
not demonstrate significant morphological changes.
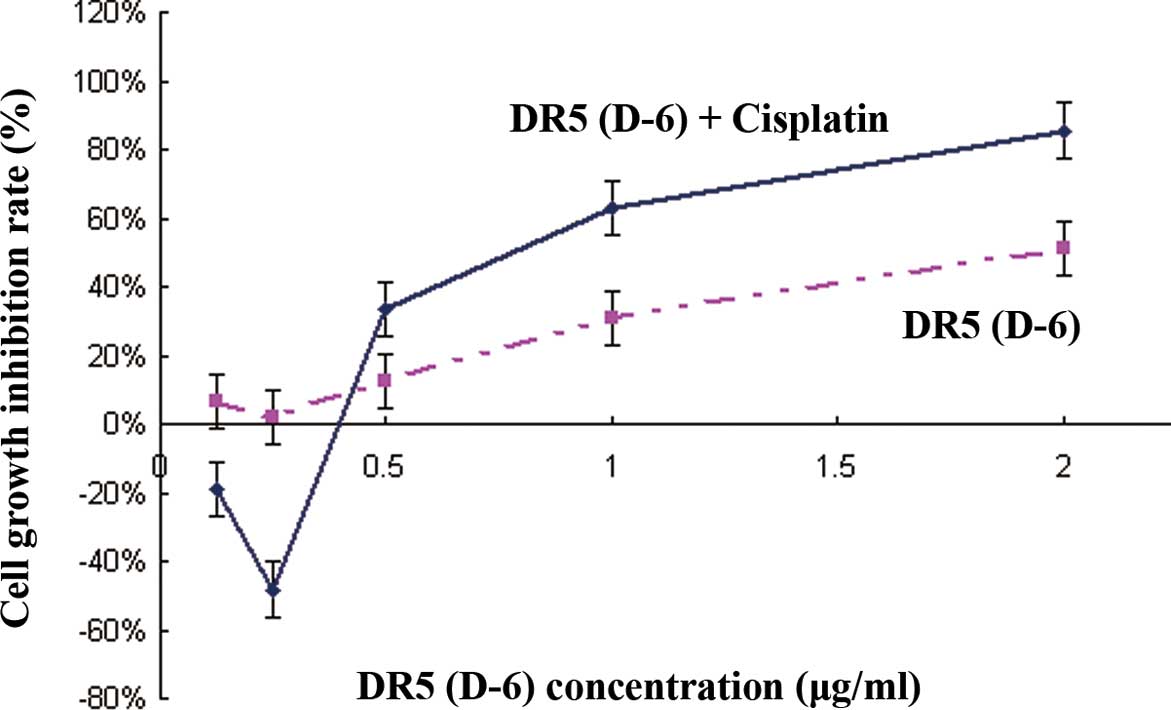
Cell growth inhibition rate
The cell growth inhibition rates were analyzed by
MTT assays. As shown in Fig. 2,
the cell growth inhibition rates increased following the increasing
DR5 (D-6) concentrations >0.25 μg/ml (P<0.05) in both groups,
and in the DR5 (D-6) and cisplatin combination group these rates
were more significant than in the DR5 (D-6) group (P<0.05).
However, the cell inhibition rates decreased following treatment
with DR5 (D-6) concentrations of <0.25 μg/ml in both groups, and
there were evident cell growth-promoting effects in the DR5 (D-6)
and cisplatin combination group.
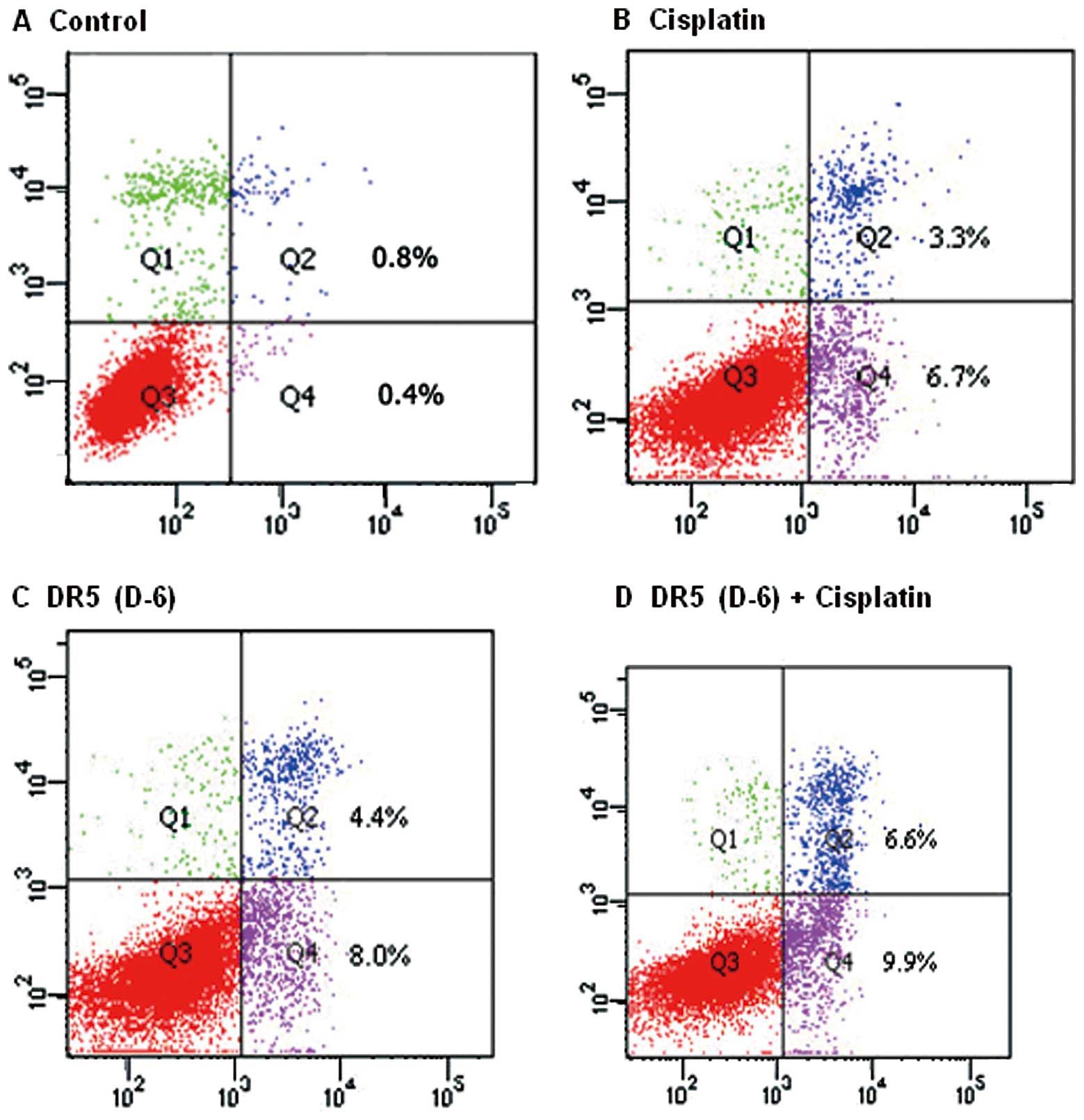
Apoptosis inducing effects of cisplatin
and DR5 (D-6) on the A2780 human ovarian cancer cell line
The apoptotic rates were measured by flow cytometry.
The apoptotic rates were 1.1±0.1, 12.90±0.70, 12.70±1.35 and
16.77±0.37%, in the control, DR5 (D-6), cisplatin and combination
groups, respectively (Fig. 3). The
apoptotic rate of the DR5 (D-6) and cisplatin combination group was
significantly higher than that of the other three groups
(P<0.05); it was higher in the DR5 (D-6) and cisplatin groups
than in the control group (P<0.05). However, there was no
significant difference between the DR5 (D-6) group and the
cisplatin group (P>0.05).
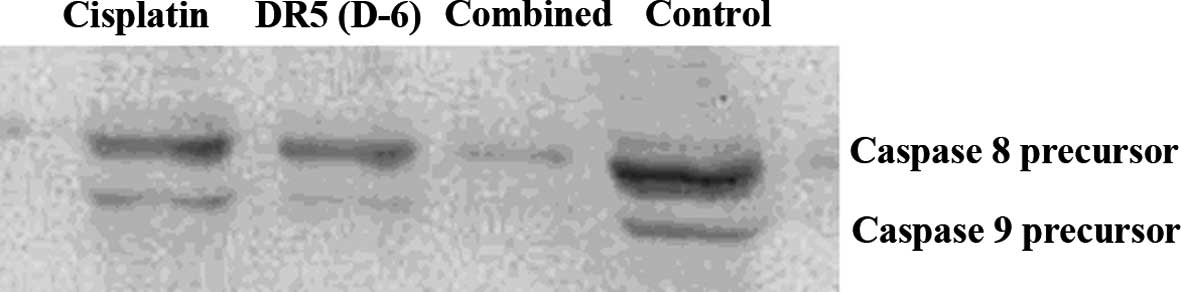
Western blot analysis of intracellular
protein changes
As shown in Figs. 4
and 5, 48 h after treatment, the
caspase 3, 8 and 9 precursors decreased in the DR5 (D-6), cisplatin
and DR5 (D-6) and cisplatin combination groups compared to the
control group; this decrease was more significant in the
combination group.
Discussion
Various cancer therapies using TRAIL-induce
apoptosis have been developed over the past few years, and many DR5
monoclonal antibodies, including murine or humanized, have been
produced, such as TRA-8, Apomab and CS-1008 (2,12–14).
The DR5 monoclonal antibodies have been used to a greater extent
than TRAIL, since they have a longer half-life in the circulatory
system and have been shown to have significant anti-tumor effects
(7,15,16).
A number of studies have focused on the mechanism of
TRAIL-induced apoptosis, and it is generally accepted that the
caspase-dependent pathway is involved (5,6,10).
Upon the binding of TRAIL to either DR4 or DR5 on the cell
membrane, DR4 and DR5 promote the formation of the death-inducing
signaling complex (DISC) via the intracellular death domain (DD),
recruiting certain adaptor proteins (5). After the formation of DISC, caspase 8
and 10 are recruited to DISC, and are activated automatically by
protein hydration and, thereafter, downstream effectors, such as
caspase 3, 6 and 7 are activated, through a series of cascade
reactions to trigger biological reactions of apoptosis (13). Exposure to chemotherapy may
stimulate the intrinsic pathway which causes the destabilization of
the mitochondrial membrane with the release of cytochrome c,
leading to caspase 3 activation. During the cascade reaction,
caspase 9 has also been shown to be highly activated (6,17).
In this study, we show that DR5 (D-6), a novel DR5
monoclonal antibody, is effective in inducing apoptosis of the
A2780 ovarian cancer cell line. The combination of DR5 (D-6) and
cisplatin was even more effective. In the experiment under the
inverted microscope, although both the DR5 (D-6) and cisplatin
groups showed apoptosis-inducing effects, the effects in the
combination group were more significant. In the combination group,
most of the cells were changed into round-shaped and floated on the
surface. Therefore, we hypthesize that they had a synergistic
function in inducing apoptosis.
The results of MTT revealed that the A2780 ovarian
cancer cells were sensitive to DR5 (D-6) in a dose-dependent
manner, and this effect was enhanced by cisplatin. A number of
studies have investigated the effect of other DR5 monoclonal
antibodies on other tumor cells in combination with chemotherapy or
radiotherapy (18–21). It is thought that chemotherapy
sensitizes the apoptosis-inducing effect of the DR5 monoclonal
antibody (20,22). A number of studies have provided
evidence of a correlation between cisplatin and DR5 monoclonal
antibodies. Chemotherapy not only sensitizes the target cells to
the DR5 monoclonal antibody, but DR5 monoclonal antibody may also
enhance the effects of chemotherapy (14,22).
This is very important for the platinum-resistant types of cancer,
such as ovarian cancer. Most ovarian cancers are sensitive to
platinum-based chemotherapy at the time of diagnosis; however,
recurrence of the disease is frequent and, ultimately,
platinum-resistant disease develops in all patients (1). Thus, the DR5 monoclonal antibody may
play a role in the treatment of ovarian caner. In this study, we
found that the A2780 ovarian cancer cell line did not undergo
apoptosis under the concentration of 0.25 μg/ml. This phenomenon
occurred after many repeated examinations. The reason is unknown
and requires further study.
The results of flow cytometry showed that the
treatment of the A2780 ovarian cancer cells with DR5 (D-6),
cisplatin or the combination of both produced apoptosis-inducing
effects; these effects were more significant in the combination
group. In order to observe the internal reaction of the ovarian
cells after treatment, we examined the expression of caspase 3, 8
and 9 precursors, which are usually used as reaction proteins of
apoptotic pathways (8). Western
blot analysis showed that the caspase 3, 8 and 9 precursors
decreased evidently in the treatment group. Therefore, we believe
that the apoptosis of the ovarian cancer cells in this study
occured in a caspase-dependent manner.
In conclusion, the monoclonal antibody (D-6)
directed against DR5 was capable of inducing the apoptosis of
ovarian cancer cells in a dose-dependent manner, and this effect
was enhanced by cisplatin. The combined treatment of cells with the
DR5 monoclonal antibody and cisplatin may be a promising treatment
for ovarian cancer.
Acknowledgements
This study was supported by grants from the National
Natural Scientific Foundation of China (no. 30973192).
Abbreviations:
|
DR5 (D-6)
|
monoclonal antibody (D-6) against
death receptor 5
|
|
OD
|
optical density
|
|
DD
|
death domain
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
EDTA
|
ethylenediaminetetra-acetic acid
|
|
DISC
|
death-inducing signaling complex
|
References
|
1
|
Bevis KS, McNally LR, Sellers JC, Della
Manna D, Londono Joshi A, Amm H, Straughn JM Jr and Buchsbaum DJ:
Anti-tumor activity of an anti-DR5 monoclonal antibody, TRA-8, in
combination with taxane/platinum-based chemotherapy in an ovarian
cancer model. Gynecol Oncol. 121:193–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Estes JM, Oliver PG, Straughn JM Jr, Zhou
T, Wang W, Grizzle WE, Alvarez RD, Stockard CR, LoBuglio AF and
Buchsbaum DJ: Efficacy of anti-death receptor 5 (DR5) antibody
(TRA-8) against primary human ovarian carcinoma using a novel ex
vivo tissue slice model. Gynecol Oncol. 105:291–298. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buchsbaum DJ, Forero-Torres A and LoBuglio
AF: TRAIL-receptor antibodies as a potential cancer treatment.
Future Oncol. 3:405–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Du YW, Chen JG, Bai HL, Huang HY, Wang J,
Li SL, Liu GC, Jiang Q, Chai J, Zhao YP and Ma YF: A novel
agonistic anti-human death receptor 5 monoclonal antibody with
tumoricidal activity induces caspase- and mitochondrial-dependent
apoptosis in human leukemia Jurkat cells. Cancer Biother
Radiopharm. 26:143–152. 2011. View Article : Google Scholar
|
|
5
|
Du YW, Liu GC, Wang J, Zhao YP, Li SL,
Chen JG, Jiang Q, Cai J and Ma YF: Caspase-dependent molecular
mechanisms of anti-human DR5 monoclonal antibody mDRA-6 inducing
apoptosis of human leukemia Jurkat cells. Ai Zheng. 28:112–116.
2009.PubMed/NCBI
|
|
6
|
Wu XX and Kakehi Y: Enhancement of
lexatumumab-induced apoptosis in human solid cancer cells by
Cisplatin in caspase-dependent manner. Clin Cancer Res.
15:2039–2047. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J, Lin Z, Qiao CX, Lv M, Yu M, Xiao
H, Wang Q, Wang L, Feng J, Shen B, Ma Y and Li Y: Characterization
of a novel anti-DR5 monoclonal antibody WD1 with the potential to
induce tumor cell apoptosis. Cell Mol Immunol. 5:55–60. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang YG, Zhao KP, Chen JG, Zhang JY, Yu M,
Li Y and Shen BF: The characteristic of an anti-human DR5 antibody
A6. Cell Mol Immunol. 5:183–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yagita H, Takeda K, Hayakawa Y, Smyth MJ
and Okumura K: TRAIL and its receptors as targets for cancer
therapy. Cancer Sci. 95:777–783. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo Y, Chen C, Zheng Y, Zhang J, Tao X,
Liu S, Zheng D and Liu Y: A novel anti-human DR5 monoclonal
antibody with tumoricidal activity induces caspase-dependent and
caspase-independent cell death. J Biol Chem. 280:41940–41952. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Wang S, Chen C and Zhuang G:
Induction of tumor cell apoptosis via Fas/DR5. Cell Mol Immunol.
3:467–471. 2006.PubMed/NCBI
|
|
12
|
Kang Z, Chen JJ, Yu Y, Li B, Sun SY, Zhang
B and Cao L: Drozitumab, a human antibody to death receptor 5, has
potent antitumor activity against rhabdomyosarcoma with the
expression of caspase-8 predictive of response. Clin Cancer Res.
17:3181–3192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yada A, Yazawa M, Ishida S, Yoshida H,
Ichikawa K, Kurakata S and Fujiwara K: A novel humanized anti-human
death receptor 5 antibody CS-1008 induces apoptosis in tumor cells
without toxicity in hepatocytes. Ann Oncol. 19:1060–1067. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin H, Yang R, Ross J, Fong S, Carano R,
Totpal K, Lawrence D, Zheng Z, Koeppen H, Stern H, Schwall R and
Ashkenazi A: Cooperation of the agonistic DR5 antibody apomab with
chemotherapy to inhibit orthotopic lung tumor growth and improve
survival. Clin Cancer Res. 14:7733–7740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duiker EW, Mom CH, de Jong S, Willemse PH,
Gietema JA, van der Zee AG and de Vries EG: The clinical trail of
TRAIL. Eur J Cancer. 42:2233–2240. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Camidge DR: Apomab: an agonist monoclonal
antibody directed against Death Receptor 5/TRAIL-Receptor 2 for use
in the treatment of solid tumors. Expert Opin Biol Ther.
8:1167–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu YJ, Ma YF, Zhang J, Zhao YP, Bai HL
and Li SL: Synergistic lethal effect of mDRA-6 and nimesulide on
human hepatocellular cancer cell line SMMC-7721. Ai Zheng.
27:374–378. 2008.PubMed/NCBI
|
|
18
|
Li SL, Ma YF, Liu GC, Zhang J, Bai HL, Liu
YJ and Lu F: Adriamycin enhances anti-human DR5 monoclonal antibody
(mDRA-6) induced HL-60 cells apoptosis. Zhonghua Xue Ye Xue Za Zhi.
27:461–464. 2006.PubMed/NCBI
|
|
19
|
Ding B, Wu X, Fan W, Wu Z, Gao J, Zhang W,
Ma L, Xiang W, Zhu Q, Liu J, Ding X and Gao S: Anti-DR5 monoclonal
antibody-mediated DTIC-loaded nanoparticles combining chemotherapy
and immunotherapy for malignant melanoma: target formulation
development and in vitro anticancer activity. Int J Nanomedicine.
6:1991–2005. 2011.
|
|
20
|
Kendrick JE, Straughn JM Jr, Oliver PG,
Wang W, Nan L, Grizzle WE, Stockard CR, Alvarez RD and Buchsbaum
DJ: Anti-tumor activity of the TRA-8 anti-DR5 antibody in
combination with cisplatin in an ex vivo human cervical cancer
model. Gynecol Oncol. 108:591–597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Straughn JM Jr, Oliver PG, Zhou T, Wang W,
Alvarez RD, Grizzle WE and Buchsbaum DJ: Anti-tumor activity of
TRA-8 anti-death receptor 5 (DR5) monoclonal antibody in
combination with chemotherapy and radiation therapy in a cervical
cancer model. Gynecol Oncol. 101:46–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rajeshkumar NV, Rasheed ZA, Garcia-Garcia
E, Lopez-Rios F, Fujiwara K, Matsui WH and Hidalgo M: A combination
of DR5 agonistic monoclonal antibody with gemcitabine targets
pancreatic cancer stem cells and results in long-term disease
control in human pancreatic cancer model. Mol Cancer Ther.
9:2582–2592. 2010. View Article : Google Scholar
|