Introduction
Viral hepatitis is a necroinflammatory liver disease
of variable severity. Persistent infection of hepatitis B virus
(HBV) is often associated with chronic liver disease that leads to
the development of cirrhosis and hepatocellular carcinoma. The
persistence of HBV infection is commonly considered as a result of
an inadequate host immune response. It is generally acknowledged
that the cellular immune response contributes to viral clearance,
particularly T-cell immunity for HBV (1). The correlation between viral spread
and CD4+ T cell priming determines the outcome of HBV
infection (2). CD4+ T
cells are classified into two types of T helper cells on the
activation of a certain antigen: Th1 and Th2. These cells differ in
their pattern of secreted cytokines. Th1 cells secrete interferon-γ
(IFN-γ), interleukin-2 (IL-2) and tumor necrosis factor-β (TNF-β),
which aid in the clearance of intracellular pathogens, while Th2
cells secrete IL-4, IL-5 and IL-10 which help alleviate
extracellular infections.
Some discoveries have led to the identification of
two major transcription factors, T-bet and GATA-3, as the master
regulators of the Th1 and Th2 differentiation programs,
respectively. T-bet is required for differentiation of Th1 cells
defined by the expression of signature Th1 genes, including the
cytokine IFN-γ and the cell surface receptor (3,4). Th2
cell differentiation is usually associated with GATA-3
upregulation, while deletion of the GATA-3 gene blocks Th2
commitment (5). The H2.0-like
homeobox (HLX1) is a member of the homeobox gene family which
contains a helix-turn-helix DNA-combination domain consisting of 61
amino acids, whose expression closely correlates with the immune
system. It has synergistic effects on Th1 cell development with
T-bet. Activation of the transcription factor STAT-6 promotes Th2
cell differentiation mediated by inducing expression of GATA-3 and
may directly transactivate the IL-4 gene (6).
However, the link between T-bet and the pathogenesis
of CHB remains unclear. In order to demonstrate the role of T-bet
in patients with chronic HBV infection, we analyzed the expression
of T-bet in CD4+ T cells and the effect of
overexpression of T-bet on Th cell differentiation in CHB
patients.
Patients and methods
Patients
Between December 2010 and June 2011, 45 CHB patients
(31 males and 14 females; average age, 38.0±12.1 years; 34 with
positive HBeAg, 11 with negative HBeAg; average ALT, 227.1±163.3
IU/l), and 8 AHB patients (6 males and 2 females; average age,
45.0±14.5 years; average ALT, 1775.4±835.7 IU/l) admitted to The
Sixth People’s Hospital Affiliated to Shanghai Jiaotong University,
Shanghai, China, and 10 healthy controls were enrolled in the
study. Of all subjects, 23 CHB patients, 8 AHB patients and 10
healthy controls were included in the T-bet mRNA expression assay,
while 22 CHB patients were included in the lentiviral vector
transduction study. All CHB patients were seropositive for HBsAg
and HBcAb for more than 6 months, with HBV-DNA >1×103
copies/ml. Patients with hepatitis C virus, hepatitis D virus or
human immunodeficiency virus (HIV) infection, autoimmune disease,
or with a history of receiving antiviral or immunosuppressive
therapy within 6 months or alcohol abuse were excluded.
This study was approved by the local ethics
committee, and all patients provided written informed consent prior
to study enrollment.
Enrichment of CD4+ T
cells
A RosetteSep human CD4+ T cell enrichment
cocktail (StemCell Technologies, Vancouver, BC, Canada) was used
for enrichment of CD4+ T cells from whole blood. A total
of 1 ml RosetteSep cocktail was added to 20 ml peripheral sodium
heparin-anticoagulant blood samples, which cross-linked unwanted
cells to red blood cells. Whole blood was diluted with an equal
volume of phosphate-buffered saline (PBS), layered over 20 ml
Ficoll-Paque (StemCell Technologies), and centrifuged for 20 min at
1200 × g at room temperature. CD4+ T cells were enriched
at the sample/medium interface. The purity of the sorted population
was detected by flow cytometry and was consistently greater than
80%.
Real time-PCR for T-bet mRNA
Total cellular RNA was extracted using RNAiso
reagent (Takara Bio Inc., Otsu, Japan). Using the
PrimeScript® RT reagent kit (Perfect Real Time) (Takara
Bio Inc.) and following the manufacturer’s instructions, reverse
transcription was performed using 1 μg of total RNA mixed with 5 μl
of 5X PrimeScript buffer, 1 μl of PrimeScript® RT Enzyme
mix I primed with 1 μl of Oligo dT and 1 μl of Random 6 mers at
37°C for 15 min and 85°C for 5 sec. A total of 0.5 μl of cDNA was
used for real time-PCR using SYBR Premix Ex Taq (Takara Bio Inc.)
with primers specific for genes encoding T-bet as follows: forward,
5′-GGTTGCGGAGACATGCTGA-3′; reverse, 5′-GTAGGCGTAGGCTCCAAGG-3′. The
amplification conditions were as follows: inactivation for 5 min at
95°C followed by 40 cycles of amplification of 10 sec at 95°C, 20
sec at 60°C and 20 sec at 72°C. T-bet mRNA expression was
normalized to the transcript levels of the internal control, the
housekeeping gene GAPDH, with primers as follows: forward,
5′-ATGGGGAAGGTGAAGGTCG-3′; reverse, 5′-GGGGTCATTGATGGCAACAATA-3′.
The amount of target was calculated by 2-ΔΔCt. Three
parallel reactions of each sample and internal control were
run.
Lentiviral vector transduction
Two recombinant lentiviral vectors were constructed;
one vector expressing T-bet and GFP (pGC-FU-T-bet) and the other
containing GFP only (pGC-FU). There were two groups for infection,
pGC-FU-T-bet and pGC-FU. CD4+ T cells were seeded at a
density of 1×106 cells/well in a 24-well plate, then
activated with 25 μl Dynabeads® Human T-Activator
CD3/CD28 and expanded with 30 U/ml rIL-2 (PeproTech Inc., Rocky
Hill, NJ, USA). After 24 h of culture, CD4+ T cells were
infected with 200 μl/well of either lentiviral vector
(1×108/ml) with 100 μl Polybrene (50 μg/ml; Sigma, St.
Louis, MO, USA). Infected cells were examined daily until GFP
expression was evident and the supernatants were collected for the
cytokine assays, while the cells were collected for the HLX1,
GATA-3 and STAT-6 mRNA and protein assays every other day.
Enzyme-linked immunosorbent assay (ELISA)
for cytokine assay
Cultured supernatants of infected CD4+ T
cells were collected on days 3, 5 and 7, and ELISA kits (R&D
Systems Inc., Minneapolis, MN, USA) were used to quantify the
concentrations of IFN-γ, IL-2, IL-4 and IL-10.
Real time-PCR for HLX1, GATA-3 and STAT-6
mRNA
HLX1, GATA-3 and STAT-6 mRNA were assayed by real
time-PCR using the method previously described. The sequences of
the primers were as follows: HLX1 forward, 5′-GCAGCAATC
ACCAACGCAG-3′; reverse, 5′-GGGTCAAATTCCGCA GACAAA-3′; GATA-3
forward, 5′-GTGCTTTTTAACATC GACGGTC-3′; reverse,
5′-AGGGGCTGAGATTCCAGGG-3′; and STAT-6 forward,
5′-GCCAAAGCCCTAGTGCTGAA-3′; reverse,
5′-GACGAGGGTTCTCAGGACTTC-3′.
Western blot analysis for HLX1, GATA-3
and STAT-6
Total proteins were extracted using RIPA Lysis
Buffer (Beyotime Institute of Biotechnology, Jiangsu, China), and
determined using bicinchoninic acid (BCA) protein assay kits
(Beyotime Institute of Biotechnology), loaded onto 10%
SDS-polyacrylamide gels and transferred to a polyvinylidene
difluoride membrane (PVDF) via electroblotting. The membranes were
incubated with rabbit anti-HLX1 (1:1,200; Abbiotec, San Diego, CA,
USA) and horseradish-peroxidase (HRP)-conjugated anti-GAPDH
(1:10,000; Abbiotec), respectively, followed by incubation with
HRP-conjugated sheep anti-rabbit antibody (1:10,000; Abbiotec).
Specific protein bands on the membranes were visualized using the
enhanced chemiluminescence method (Amersham, Piscataway, NJ, USA),
and analyzed using Image J software (version 1.6 NIH) to determine
the relative levels of HLX1 defined as the optical density ratio of
HLX1 over GAPDH. Expression of GATA-3 and STAT-6 was also detected
in this way using rabbit anti-GATA-3 (1:3,000; Epitomics, Inc.,
Burlingame, CA, USA) and rabbit anti-STAT-6 (1:500; Epitomics,
Inc.), respectively.
Statistical analysis
All data are expressed as mean ± standard deviation
(SD), and were analyzed with SPSS 13.0 for Windows. Correlations
were evaluated using the Pearson’s correlation test. Comparisons
between groups were performed using the independent-samples t-test
or by one-way ANOVA for experiments with more than two subgroups.
Two-tailed P<0.05 was considered to indicate a statistically
significant difference.
Results
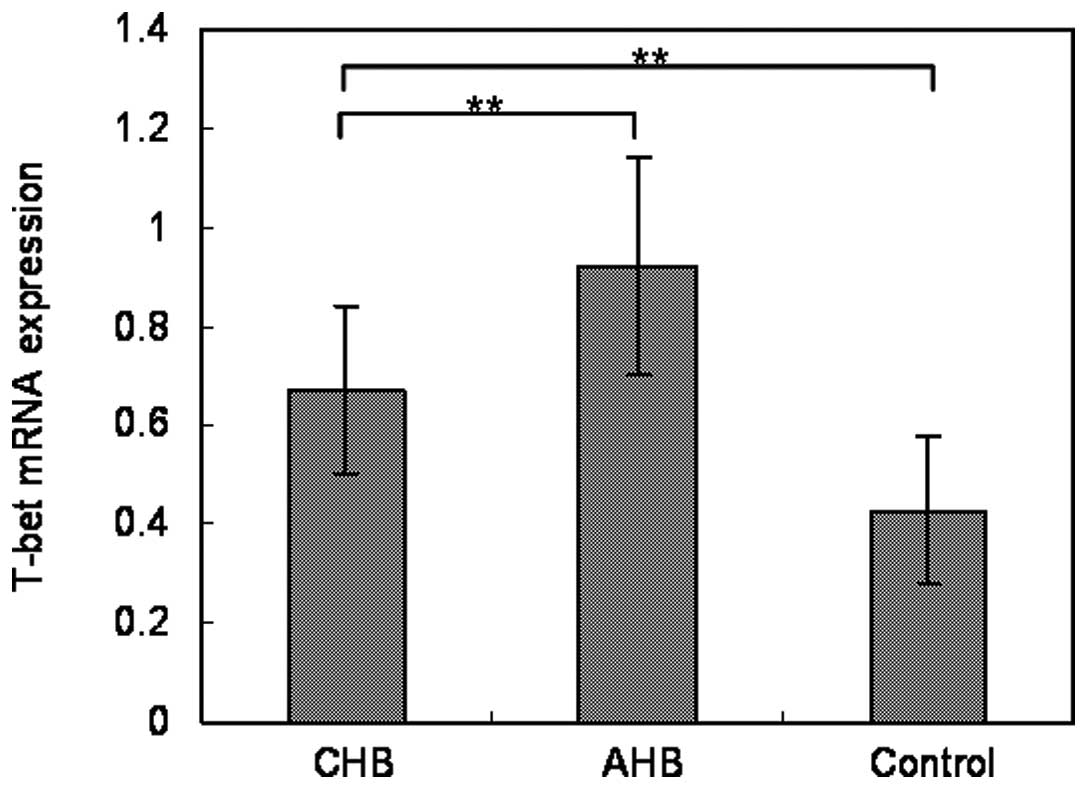
T-bet mRNA expression was significantly
repressed in CHB patients compared with AHB patients
To investigate the correlation between T-bet and
chronic HBV infection, T-bet mRNA from CHB patients (n=23), AHB
patients (n=8) and healthy controls (n=10) were detected by RT-PCR.
The expression of T-bet mRNA in CD4+ T cells from the
peripheral blood of CHB patients was demonstrated to be lower than
that in the AHB patients (P< 0.01), but higher than that in the
healthy controls (P<0.01) (Fig.
1).
Lentivirally overexpressed T-bet
regulates Th1/Th2 cytokine expression in CHB patients
Cultured supernatants of CD4+ T cells on
days 3, 5 and 7 after infection were measured by ELISA. Compared
with pGC-FU transduction, IFN-γ expression following pGC-FU-T-bet
transduction was significantly upregulated after 3 days
(P<0.05), 5 days (P<0.01) and 7 days (P<0.01) (Fig. 2). There was no difference in IL-2
expression at day 3, but expression was significantly enhanced at
days 5 (P<0.05) and 7 (P<0.05). Production of IL-4
(P<0.05) and IL-10 (P<0.01) was significantly downregulated
at day 5, but was not statistically different at days 3 or 7. These
results demonstrated that the Th1-type cytokines, IFN-γ and IL-2,
were increased, while Th2-type cytokines, IL-4 and IL-10, were
decreased from CD4+ T cells of CHB patients after
pGC-FU-T-bet transduction. This indicated that lentivirally
overexpressed T-bet may regulate Th1/Th2 differentiation and
function.
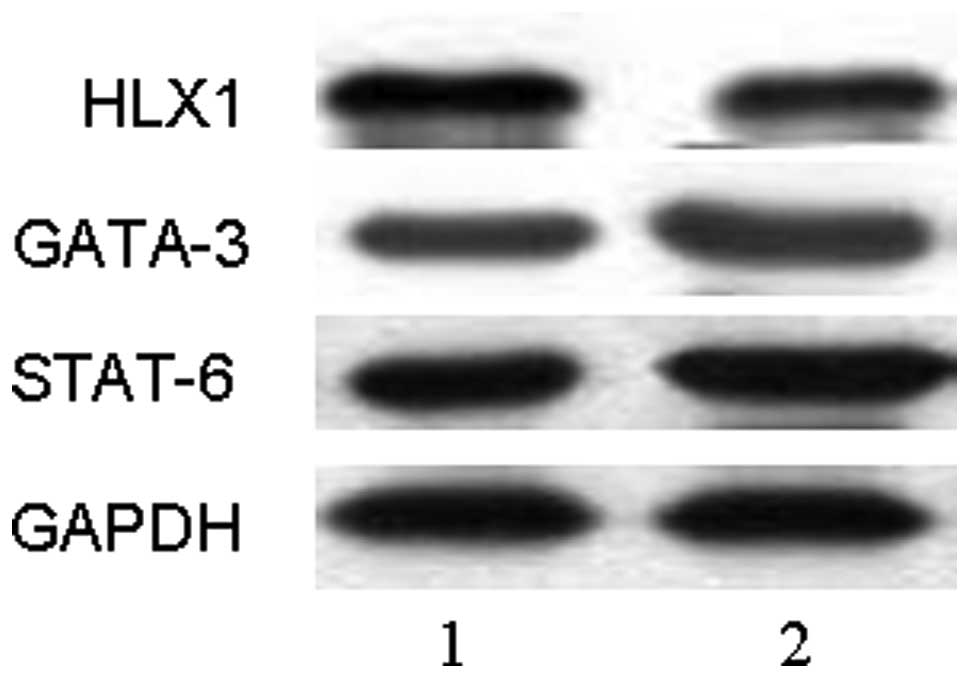
Lentivirally overexpressed T-bet enhances
HLX1 expression and represses GATA-3 and STAT-6 expression
To investigate the mechanism of T-bet overexpression
regulating Th1/Th2 differentiation, the expression of HLX1, GATA-3
and STAT-6 mRNA were detected by RT-PCR and the proteins were
detected by western blot analysis at day 5 after transfection. The
results revealed that expression of HLX1 was significantly
upregulated after pGC-FU-T-bet transduction (P<0.01), while
expression of GATA-3 and STAT-6 were downregulated (P<0.01)
(Figs. 3 and 4). This indicated that HLX1, GATA-3 and
STAT-6 may be involved in the process of lentivirally overexpressed
T-bet regulating Th1/Th2 differentiation.
Discussion
Persistent HBV infection is characterized by a weak
adaptive immune response, considered to be due to inefficient
CD4+ T cell priming early in the infection and
subsequent development of a quantitatively and qualitatively
ineffective CD8 cytotoxic T-lymphocyte (CTL) response. The
HBV-specific CTL response plays a fundamental role in viral
clearance and pathogenesis of liver disease. The peripheral blood
CTL response in chronically infected patients is weak and narrowly
focused (7). During HBV infection
a balance between the virus and the host’s immune system is
established, and, apart from CTL, the Th1-type cytokine IFN-γ, may
also exert a control on HBV replication (8,9). Th1
cells may predominantly contribute to recovery from disease by
promoting a cell immune response, which is mediated by producing
protective antibodies and enhancing the activity of natural killer
(NK) and macrophage cells, particularly CTL in CHB patients
(10,11). Host immune reactions biased towards
Th2 with production of IL-4 in HBV-infected individuals have been
attributed to persistent HBV infection (12). Finally, an imbalance between
Th1/Th2 responses may be responsible for the dysregulated immune
status in the progression of HBV infection. However, the molecular
mechanism involved in this process remains unclear.
T-bet is the master regulator of Th1 development. A
link between T-bet expression and immune responsiveness causing HBV
infection has been demonstrated by the identification that T-bet
expression in CHB patients was decreased when compared with that of
AHB patients. In accordance with this finding, it has been reported
that T-bet was downregulated during chronic LCMV compared to
expression in acute LCMV infection (13). This indicated that deficiency of
T-bet during chronic viral infection may contribute to the
stimulative effect of T-bet on protective responses in chronic HBV
infection.
Lentiviral introduction of T-bet into peripheral
blood CD4+ T cells is a likely candidate for regulating
the Th1/Th2 immune response. Lentiviral vectors are uniquely suited
for gene delivery applications due to their capacity for
integrating large transgenes into genomes, maintaining persistent
gene expression, efficient transduction of dividing and
non-dividing cells, low genotoxicity due to insertional
mutagenesis, and weak anti-vector host immunity (14–17).
To further study the function of T-bet overexpression in CHB
patients, cytokine production was assessed after CD4+ T
cells were transfected with T-bet-expressing lentivirus. When T-bet
was overexpressed, there was increased expression of Th1-type
cytokines, IFN-γ and IL-2, and decreased expression of Th2-type
cytokines, IL-4 and IL-10. These results established that
overexpression of T-bet stimulated a Th1-dependent immune response
in CHB patients. T cell proliferation was found to be indirectly
promoted by activation of IL-2 transcription (18), and Th1 differentiation required
partial revision to include the major, recently observed,
contribution of IL-2 (19).
Further studies are required to identify whether IL-2 contributed
to a slight Th1 skewing induced by overexpression of T-bet.
In addressing the mechanism by which the
polarization of Th cells towards Th1 type occurs when T-bet is
overexpressed, the results highlighted the significance of HLX1,
given the elevation of HLX1 in CD4+ T cells transfected
with T-bet-expressing lentivirus. HLX1 was specifically expressed
in Th1 cells at high levels. Kinetic studies have demonstrated that
HLX1 was expressed at a steady level in naïve CD4+ T
cells, and upregulated upon commitment to Th1 lineage (20). Mullen et al demonstrated
that HLX1 induced by interacting with T-bet and enhanced
T-bet-mediated IFN-γ production (21). HLX1 also induced IFN-γ expression
when overexpressed at an early time matching the natural time
course of HLX1 upregulation during Th1 cell differentiation. When
expressed as a transgene in CD4+ T cells, HLX1 prevented
the silencing of the IFN-γ gene in CD4+ T cells
undergoing Th2 cell differentiation, which resulted in additional
cells producing IFN-γ (22).
However, it remains unclear whether HLX1 is involved in
T-bet-induced bias towards developing a Th1 cytokine response in
patients with CHB. This study elucidated these observations by
examining the expression of HLX1. The results demonstrated that
HLX1 was elevated in CD4+ T cells with increasing Th1
cytokine production after lentiviral introduction of T-bet.
Evidence supporting the involvement of HLX1 in Th1 cytokine
augmentation induced by lentiviral expression of T-bet has been
observed, suggesting a HLX1-dependent contribution to the shift of
Th cells in favor of Th1 induced by T-bet in patients with CHB.
GATA-3 is considered a ‘master regulator’ of Th2
development based on its ability to promote Th2 commitment when
ectopically expressed in cells under various regulatory situations
(23,24). Inhibition of GATA-3 using either
small interfering RNA or conditional deletion significantly limited
Th2 differentiation and cytokines (25,26).
T-bet inhibited GATA-3 binding to target genes, thus suppressing
Th2 differentiation (27). Based
on these observations, it is plausible to consider that GATA-3 is
involved in the inhibition of Th2 cytokines induced by T-bet
overexpression in patients with CHB. In this study, a modest
reduction in GATA-3 transcription and mRNA in CD4+ T
cells infected with T-bet-expressing lentivirus was observed,
suggesting that repressed GATA-3 contributed to T-bet-mediated
limited differentiation into a Th2 lineage in patients with CHB.
Th2 cells are also generated by activating naïve T cells through
TCR crosslinking in the presence of exogenous IL-4 whose receptor
signals are transduced by STAT-6. STAT-6 which is involved in
upstream signaling of GATA-3, has physiological importance for the
Th2 program, which can be largely attributed to its ability to
synergize with TCR to upregulate GATA-3 (28). The expression of Th2 cytokines,
including IL-4, IL-5 and IL-13, was diminished in
STAT-6-/- mice (29).
The regulatory effect of STAT-6 on GATA-3 function and Th2 cytokine
production raised the possibility that Th2 cytokine suppression
induced by lentivirally overexpressed T-bet was partly mediated by
STAT-6. CD4+ T cells infected with T-bet-expressing
lentivirus demonstrated significantly reduced STAT-6 transcript and
mRNA levels, suggesting that T-bet overexpression destabilized Th2
cell development by interfering with STAT-6 expression. This
observation established a link between T-bet overexpression and
STAT-6, and subsequently Th2 cell development inhibition. However,
it remains uncertain whether this STAT-6 effect was or was not
GATA-3-independent. Detailed future studies are required to
experimentally address this possibility.
Based on the present findings, it is possible to
conclude that T-bet was suppressively expressed in CHB patients.
Lentivirally overexpressed T-bet induced regulation of Th1/Th2
immune responses in CHB patients. T-bet overexpression may be
immunostimulatory for Th1 type responses mediated by HLX1, which
have important implications for enhancing weak immunity in CHB
patients. In contrast, lentivirally overexpressed T-bet suppressed
the development of Th2 type responses via interacting with GATA-3
and STAT-6. T-bet-expressing lentivirus warrants further
investigation as a possible target and as a candidate therapeutic
measure for stimulating the immune response and establishing
control of infection in CHB patients.
References
|
1
|
Guidotti LG and Chisari FV: Immunobiology
and pathogenesis of viral hepatitis. Annu Rev Pathol. 1:23–61.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asabe S, Wieland SF, Chattopadhyay PK, et
al: The size of the viral inoculum contributes to the outcome of
hepatitis B virus infection. J Virol. 83:9652–9662. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lewis MD, Miller SA, Miazgowicz MM, Beima
KM and Weinmann AS: T-bet’s ability to regulate individual target
genes requires the conserved T-box domain to recruit histone
methyltransferase activity and a separate family member-specific
transactivation domain. Mol Cell Biol. 27:8510–8521. 2007.
|
|
4
|
Beima KM, Miazgowicz MM, Lewis MD, Yan PS,
Huang TH and Weinmann AS: T-bet binding to newly identified target
gene promoters is cell type-independent but results in variable
context-dependent functional effects. J Biol Chem. 281:11992–12000.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pai SY, Truitt ML and Ho IC: GATA-3
deficiency abrogates the development and maintenance of T helper
type 2 cells. Proc Natl Acad Sci USA. 101:1993–1998. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ansel KM, Djuretic I, Tanasa B and Rao A:
Regulation of Th2 differentiation and Il4 locus accessibility. Annu
Rev Immunol. 24:607–656. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gutiérrez-García ML, Fernandez-Rodriguez
CM, Lledo-Navarro JL and Buhigas-Garcia I: Prevalence of occult
hepatitis B virus infection. World J Gastroenterol. 17:1538–1542.
2011.
|
|
8
|
Raimondo G, Pollicino T, Cacciola I and
Squadrito G: Occult hepatitis B virus infection. J Hepatol.
46:160–170. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mizukoshi E, Sidney J, Livingston B, et
al: Cellular immune responses to the hepatitis B virus polymerase.
J Immunol. 173:5863–5871. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brooks DG, Teyton L, Oldstone MB and
McGavern DB: Intrinsic functional dysregulation of CD4 T cells
occurs rapidly following persistent viral infection. J Virol.
79:10514–10527. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jiang R, Feng X, Guo Y, et al: T helper
cells in patients with chronic hepatitis B virus infection. Chin
Med J (Engl). 115:422–424. 2002.PubMed/NCBI
|
|
12
|
Akpolat N, Yahsi S, Godekmerdan A,
Demirbag K and Yalniz M: Relationship between serum cytokine levels
and histopathological changes of liver in patients with hepatitis
B. World J Gastroenterol. 11:3260–3263. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kao C, Oestreich KJ, Paley MA, et al:
Transcription factor T-bet represses expression of the inhibitory
receptor PD-1 and sustains virus-specific CD8+ T cell
responses during chronic infection. Nat Immunol. 12:663–671. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kootstra NA and Verma IM: Gene therapy
with viral vectors. World J Gastroenterol. 43:413–439.
2003.PubMed/NCBI
|
|
15
|
Montini E, Cesana D, Schmidt M, et al: The
genotoxic potential of retroviral vectors is strongly modulated by
vector design and integration site selection in a mouse model of
HSC gene therapy. Clin Invest. 119:964–975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abordo-Adesida E, Follenzi A, Barcia C, et
al: Stability of lentiviral vector-mediated transgene expression in
the brain in the presence of systemic antivector immune responses.
Hum Gene Ther. 16:741–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mátrai J, Chuah MK and VandenDriessche T:
Recent advances in lentiviral vector development and applications.
Mol Ther. 18:477–490. 2010.
|
|
18
|
Meuer SC, Hussey RE, Cantrell DA, et al:
Triggering of the T3-Ti antigen-receptor complex results in clonal
T-cell proliferation through an interleukin 2-dependent autocrine
pathway. Proc Natl Acad Sci USA. 81:1509–1513. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao W, Lin JX, Wang L, Li P and Leonard
WJ: Modulation of cytokine receptors by IL-2 broadly regulates
differentiation into helper T cell lineages. Nat Immunol.
12:551–559. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng WP, Zhao Q, Zhao X, et al:
Up-regulation of Hlx in immature Th cells induces IFN-gamma
expression. J Immunol. 172:114–122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mullen AC, Hutchins AS, High FA, et al:
Hlx is induced by and genetically interacts with T-bet to promote
heritable T(H)1 gene induction. Nat Immunol. 3:652–658.
2002.PubMed/NCBI
|
|
22
|
Hamalainen-Laanaya HK, Kobie JJ, Chang C
and Zeng WP: Temporal and spatial changes of histone 3 K4
dimethylation at the IFN-gamma gene during Th1 and Th2 cell
differentiation. J Immunol. 179:6410–6415. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farrar JD, Ouyang W, Löhning M, et al: An
instructive component in T helper cell type 2 (Th2) development
mediated by GATA-3. J Exp Med. 193:643–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nawijn MC, Dingjan GM, Ferreira R, et al:
Enforced expression of GATA-3 in transgenic mice inhibits Th1
differentiation and induces the formation of a T1/ST2-expressing
Th2-committed T cell compartment in vivo. J Immunol. 167:724–732.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skapenko A, Leipe J, Niesner U, et al:
GATA-3 in human T cell helper type 2 development. J Exp Med.
199:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu J, Min B, Hu-Li J, et al: Conditional
deletion of Gata3 shows its essential function in T(H)1-T(H)2
responses. Nat Immunol. 5:1157–1165. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang ES, Hong JH and Glimcher LH: IL-2
production in developing Th1 cells is regulated by
heterodimerization of RelA and T-bet and requires T-bet serine
residue 508. J Exp Med. 202:1289–1300. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu J, Guo L, Watson CJ, Hu-Li J and Paul
WE: Stat6 is necessary and sufficient for IL-4’s role in Th2
differentiation and cell expansion. J Immunol. 166:7276–7281.
2001.
|
|
29
|
Goenka S and Kaplan MH: Transcriptional
regulation by STAT6. Immunol Res. 50:87–96. 2011. View Article : Google Scholar
|