Introduction
Hypertensive disorders are a leading cause of
perinatal morbidity and mortality in pregnancy. Preeclampsia (PE)
is a common pregnancy-specific syndrome that affects at least 5% of
all pregnancies worldwide (1,2). It
is characterized by hypertension (RR ≥140/90 mmHg) and proteinuria
of ≥300 mg/24 h, developing after midgestation in previously
normotensive pregnant females. Although the exact etiology of PE
remains unknown, pathological studies have demonstrated the
abnormal development of an ischemic placenta (2). Shallow endovascular trophoblast
invasion in the spiral arteries and generalized endothelial cell
dysfunction are key factors, while placental ischemia, oxidative
stress and maternal-fetal immune maladaptation affect the
development of PE. The only treatment is delivery of the placenta,
after which the symptoms regress rapidly. Evidence has demonstrated
that hypertensive disorders in non-pregnant and pregnant patients
are associated with alterations in different miRNA expressions of
specific tissues (3–5).
miRNAs are non-protein coding RNAs, and functionally
negative regulators of gene expression by antisense complementarity
to specific messenger RNAs (6,7).
They act by targeting the RNA-induced silencing complex to
complementary sites within the 3′-untranslated region (UTR) of
their target mRNAs. Depending on the degree of base pairing between
the miRNA and the 3′-UTR, either degradation or translational
repression of the targeted mRNA occurs. Although they account for
less than 1% of all human genes, miRNAs have been estimated to
regulate up to 30% of all protein-encoding genes (8). Their best-known representatives are
the 18–24 nucleotide long single-stranded miRNA. The mean number of
copies per cell is between 10 and 50000, depending on the tissue
and the miRNA (9,10).
Few studies have presented data regarding a
comprehensive list of the human miRNAs expressed in the placenta
and the possible roles of the different miRNAs in the
pathophysiology of pregnancy-related disorders (11–13).
Taking into consideration the high number of miRNAs, further
studies regarding the expression of different miRNA in
pregnancy-related hypertensive disorders may be important in
understanding the pathophysiology of this disease.
In the present study, we performed a real-time
polymerase chain reaction PCR analysis of hsa-miR-325. hsa-miR-325
is located at Xq21.1. Different databases (http://www.mirdb.org; http://www.nextprot.org) were evaluated, and
hsa-miR-325 was selected as it is a non-studied miRNA that targets
genes and candidate protein regulatory pathways affecting different
etiological factors, including body mass index (BMI), blood
pressure regulation, oxidative stress, endometrial function and
heat-shock protein regulation, thus playing a key role in the
development of hypertensive disorders in pregnancy.
Materials and methods
Samples
A total of 31 placenta samples were collected at
delivery from females with PE and 28 from normotensive pregnant
females in this prospective study. The clinical characteristics of
the patients are shown in Table I.
All the females were pregnant with a single fetus. The tissue
samples were collected 2–3 cm in radial distance from the margin of
the placenta. Placenta samples were stored at −80°C. Small RNAs
were obtained using the High Pure miRNA Isolation kit (Roche,
Mannheim, Germany) according to the manufacturer’s instructions.
Strict anti-contamination procedures were used throughout. Double
isolations were conducted from each placental sample. Each sample
measurement was performed in triplicate.
 | Table IClinical characteristics of
normotensive and preeclamptic patients, and correlation with
hsa-miR-325 expression in PE cases. |
Table I
Clinical characteristics of
normotensive and preeclamptic patients, and correlation with
hsa-miR-325 expression in PE cases.
| Variable | Controls (n=28) | Preeclampsia
(n=32) | P-value | r-value |
|---|
| Age (years) | 28 (20–41) | 29 (18–39) | NS | 0.003 |
| Systolic blood
pressure (mmHg) | 110 (90–135) | 160 (138–210) | <0.05 | −0.13 |
| Diastolic blood
pressure (mmHg) | 70 (57–86) | 98 (94–130) | <0.05 | −0.23 |
| Gestational age at
sampling (weeks) | 36 (35–40) | 37 (30–41) | NS | 0.1 |
| Number of cesarean
sections (% of total deliveries in group) | 42% | 46% | NS | - |
| BMI at delivery | 22.3 (21.6–31.2) | 28.4 (26.2–36.1) | <0.05 | 0.26 |
| Fetal birth weight
(grams) | 3600 (2700–4200) | 3100 (1800–3900) | NS | −0.01 |
miRNA detection
The miRNA profile of a certain tissue type is
determined by miRNA microarray and sequencing. The small size of
miRNAs makes the application of conventional quantitative PCR
(QPCR) techniques difficult. However, this difficulty is relieved
by using stem-loop or hairpin oligonucleotide QPCR. This method
uses a primer with a hairpin structure during the reverse
transcription which has a 5–8 base pair long overhang at the 3′ end
specific to the miRNA, thus the size of cDNA transcribed from miRNA
is increased during the transcription with the stem-loop region of
the primer. The product generated through this method is suitable
for quantitative examination with QPCR in the subsequent step. The
benefits of the method are specificity (the reverse transcription
and the quantitative PCR are miRNA specific) and sensitivity (the
starting quantity of RNA is between 1 and 10 ng).
Specific detection and quantitative examination of
miRNAs by stem-loop quantitative PCR was conducted with the use of
Universal ProbeLibrary (UPL) technology (miRNA design software;
UD-GenoMed Ltd.; Astrid Research Ltd.).
Protocol
Reverse transcription
The reverse transcription assay (10 μl) comprised 10
ng RNA, 1X reverse transcriptase buffer, 20 units RNase inhibitor,
1 mM dNTP, 10 units reverse transcriptase and 50 nM stem-loop
primer. The components used for the reverse transcription were
components of the Transcriptor First Strand cDNA Synthesis kit
(Roche).
QPCR
The composition of the QPCR assay (10 μl) was 2 μl
RT product, 1X LightCycler 480 Probes Master mix (Roche), 375 nM
forward and universal reverse primers (Table II) and 125 nM UPL probe. The
quantitative PCR assay was conducted as follows: denaturation for
10 min at 95°C, 45 cycles of 95°C, annealing for 10 sec at 58°C and
30 sec at 72°C, and extension for 10 min at 40°C, which was
conducted using the Applied LightCycler 480 Real-Time PCR system
(Roche). The results were normalized using snoRNA202 RNA and the
ΔCt method.
 | Table IIhsa-miR-325 primers. |
Table II
hsa-miR-325 primers.
| Primer | Sequence |
|---|
| Stem-loop RT |
5′-GTTGGCTCTGGTGCAGGGTCCGAGGTATTCGCACCAGAGCCAACACACTT-3′ |
| Forward |
5′-GTCCTAGTAGGTGTCCAGT-3′ |
| Universal
reverse |
5′-GTGCAGGGTCCGAGGT-3′ |
The threshold cycle of fluorescence (Ct) for each
sample was determined by real-time PCR in order to evaluate the
association between the PE and the normotensive groups using the
ΔΔCt method. ΔΔCt was the difference in the ΔCt value of PE and the
mean ΔCt value of the normotensive cases (ΔΔCt = ΔCt PE - mean ΔCt
normotensive). ΔCt was the difference in the Ct value between the
hsa-miR-325 and snoRNA202 (ΔCt = Ct miR-325 - Ct snoRNA202).
Differences in the expression levels of miRNA were measured by
comparing the ΔCt values of the PE and normotensive groups.
The study protocol was approved by the Regional
Institutional Committee of Medical Ethics at Semmelweis University,
and written informed consent was obtained from each patient. The
study was conducted in accordance with the Declaration of
Helsinki.
Statistical analysis
Statistical analysis was determined using a paired
t-test of the PE tissue samples compared to the normotensive cases.
The association between miRNA expression levels and
clinicopathological parameters was analyzed using a non-parametric
test (Mann-Whitney U test between the two groups). P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using the Statistical Program
for Social Sciences (SPSS) software 16.0 (SPSS Incorporated,
Chicago, IL, USA).
Results
Expression of hsa-miR-325
Expression levels of hsa-miR-325 were detected in 31
PE and 28 normotensive placental tissue samples by real-time PCR.
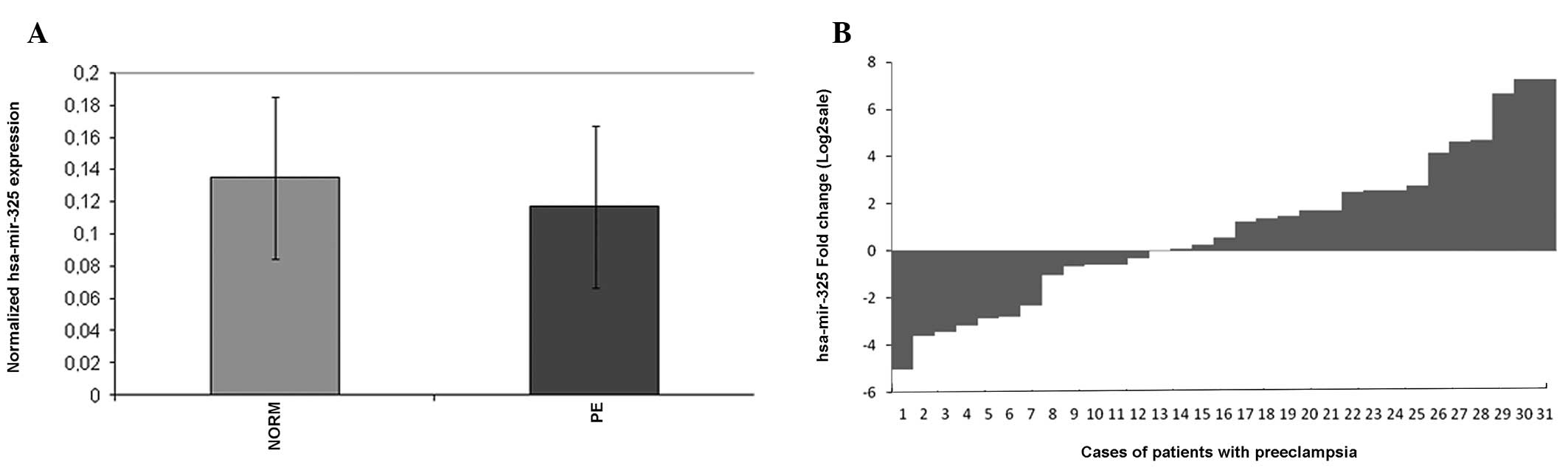
The values of ΔCt (mean ± SD) were 0.117±0.07 in the PE tissues and
0.135±0.051 in the normotensive cases (Fig. 1). Statistically significant
differences were found in hsa-miR-325 and expression levels between
the PE and normotensive placental tissues (p<0.05). A total of
70.9% (22/31) of the cases demonstrated a >50% reduction in
hsa-miR-325 expression levels in the PE tissues compared with the
normotensive cases.
Association between the hsa-miR-325
expression levels and clinicopathological characteristics in
PE
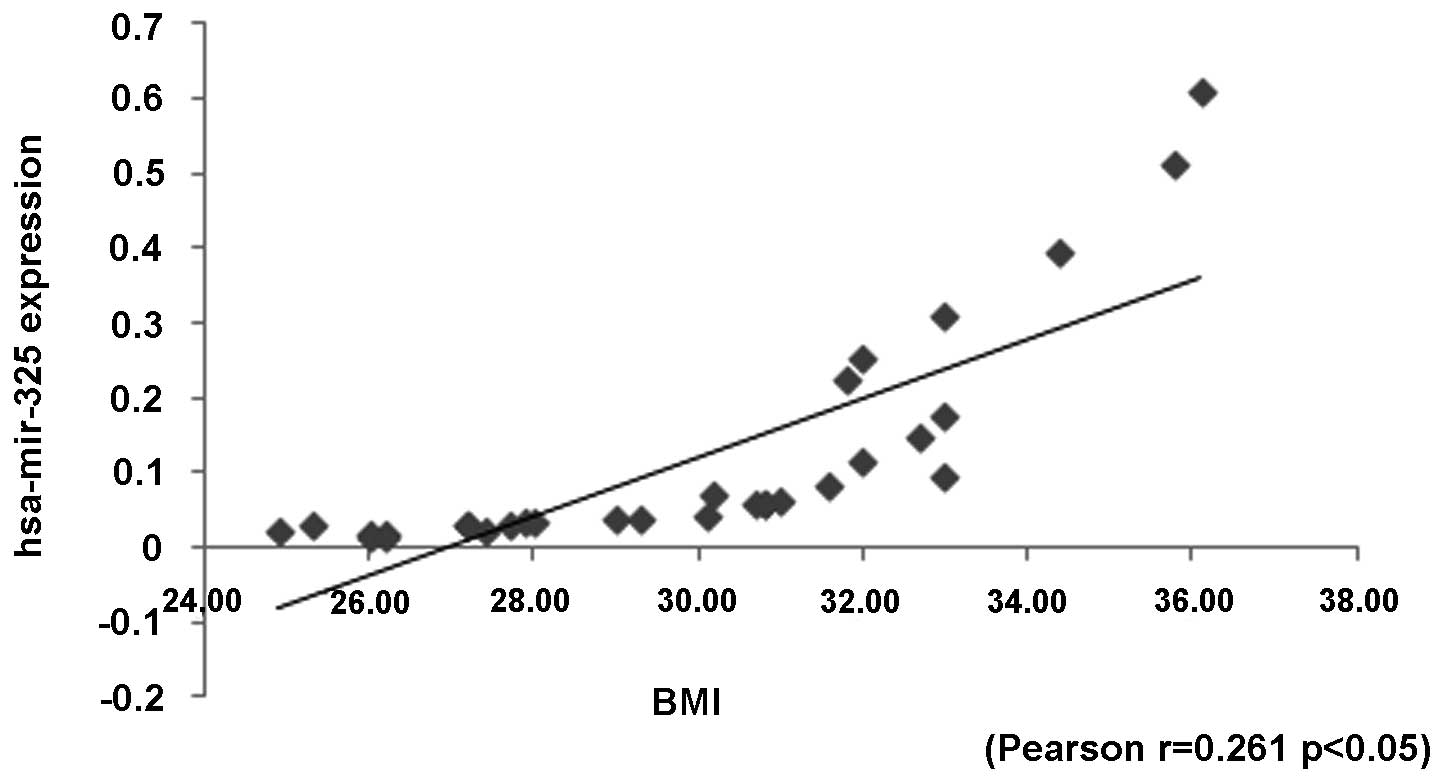
The downregulated hsa-miR-325 significantly
correlated with diastolic blood pressure and BMI, respectively
(p=0.015, p=0.065; Pearson correlation index: −0.23, 0.261,
respectively). Moreover, the expression levels of hsa-miR-325 were
significantly lower in the patients with blood pressure >160/110
mmHg (p=0.016). Therefore, the preeclamptic patients with severe PE
had significantly lower expression levels of hsa-miR-325 (Fig. 2). However, there was no significant
difference between hsa-miR-325 expression levels and other
clinicopathological characteristics, including age, pregnancy age
at delivery, method of delivery and fetal birth weight.
Discussion
PE is characterized by vasospasm, reduced placental
perfusion and abnormal placentation. The main cause of fetal
compromise is disturbance in uteroplacental perfusion. There are a
number of hypotheses regarding the main cause of this disorder,
including abnormal placentation, immunological background, and
abnormal inflamatory response. We reviewed the literature with a
focus on the etiological factors of PE and found that molecular
pathways affecting etiological factors are important in the
pathophysiology of PE. miRNAs have strong effects on the expression
of different target genes and protein transcription.
Several studies have reported that specific miRNAs
are overexpressed and underexpressed in PE (14). Pineles et al found that the
expression of two miRNAs (miR-210 and miR-182) was significantly
higher in PE compared to the control group (15). The absence or altered expression of
these miRNAs may result in the re-programming of a number of their
target genes in preeclamptic patients. Alteration of the miRNA
expression in PE suggests the down- or upregulation of potential
target genes which may contribute to the pathology of PE. The
association between PE and the altered miRNA expression suggests
the possibility of a functional role for miRNA in this disease.
Our study presents an analysis of hsa-miR-325
expression in preeclamptic placental samples with the use of miRNA
PCR technology. hsa-miR-325 is a less studied miRNA with possible
target genes and proteins involved in the regulatory pathways of
oxidative stress and heat shock protein regulation, including
MAP4K4 (mediates TNF-α signaling and adipogenesis) serine/threonine
kinase (plays a role in the response to environmental stress),
CUGBP (Hsp70 regulation), UPF0554 [catalyzes the hydrolysis of ATP
coupled with the exchange of Na(+) and K(+) ions across the plasma
membrane, expressed in renal tubules and placental trophoblasts)
and SLC25A13 (catalyzes the calcium-dependent exchange of
cytoplasmic glutamate with mitochondrial aspartate across the
mitochondrial inner membrane).
Our results indicate that hsa-miR-325 expression is
associated with PE. Furthermore, we found a strong correlation
between hsa-miR-325 and the blood presure of preeclamptic patients.
miRNA expression also correlates with maternal BMI at delivery.
Changes in hsa-miR-325 expression, in the case of pregnancy-related
hypertensive disorders, may affect the oxidative stress pathways
and heat-shock protein production. These factors have a strong
correlation with the developement of PE (16–18).
Our findings therefore may provide novel targets for further
investigation of the pathogenesis of PE and these differential
miRNAs may be potential markers for the diagnosis of PE. However,
follow-up studies are required to confirm the significance of
hsa-miR-325.
Acknowledgements
The study was designed by L.L., and performed by
L.L. and B.N. Data were analyzed by L.L. and A.M. The manuscript
was written by L.L. and B.N, with discussion suggestions by A.M.,
A.S. and J.R. This work was supported by the János Bolyai Research
Scholarship of the Hungarian Academy of Sciences.
References
|
1
|
Witlin AG and Sibai BM: Hypertension in
pregnancy: current concepts of preeclampsia. Annu Rev Med.
48:115–127. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dekker GA and Sibai BM: Etiology and
pathogenesis of preeclampsia: current concepts. Am J Obstet
Gynecol. 179:1359–1375. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pineles BL, Romero R, Montenegro D, et al:
Distinct subsets of microRNAs are expressed differentially in the
human placentas of patients with preeclampsia. Am J Obstet Gynecol.
196:2612007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu Y, Li P, Hao S, Liu L, et al:
Differential expression of microRNAs in the placentae of Chinese
patients with severe pre-eclampsia. Clin Chem Lab Med. 47:923–929.
2009.PubMed/NCBI
|
|
5
|
Bátkai S and Thum T: MicroRNAs in
hypertension: mechanisms and therapeutic targets. Curr Hypertens
Rep. 14:79–87. 2012.PubMed/NCBI
|
|
6
|
Bushati N and Cohen SM: MicroRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar
|
|
7
|
Griffiths-Jones S, Grocock RJ, Van Dongen
S, et al: MiRBase: microRNA sequences, targets and gene
nomenclature. Nucleic Acids Res. 34:140–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao S and Liu MF: Mechanisms of
microRNA-mediated gene regulation. Sci China C Life Sci.
52:1111–1116. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim LP, Lau NC, Garrett-Engele P, et al:
Microarray analysis shows that some microRNAs downregulate large
numbers of target mRNAs. Nature. 433:769–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Draghici S, Khatri P, Martins RP, et al:
Global functional profiling of gene expression. Genomics.
81:98–104. 2003.PubMed/NCBI
|
|
11
|
Prieto DM and Markert ÚR: MicroRNAs in
pregnancy. J Reprod Immunol. 88:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maccani MA, Padbury JF and Marsit CJ:
miR-16 and miR-21 expression in the placenta is associated with
fetal growth. PLoS One. 6:June 15–2011.(Epub ahead of print).
|
|
13
|
Kotlabova K, Doucha J and Hromadnikova I:
Placental-specific microRNA in maternal circulation -
identification of appropriate pregnancy-associated microRNAs with
diagnostic potential. J Reprod Immunol. 89:185–191. 2011.
View Article : Google Scholar
|
|
14
|
Zhu X-M, Han T, Sargent IL, et al:
Differential expression profile of microRNAs in human placentas
from preeclamptic pregnancies vs normal pregnancies. Am J Obstet
Gynecol. 200:6612009.PubMed/NCBI
|
|
15
|
Pineles BL, Romero R, Montenegro D, et al:
Distinct subsets of microRNAs are expressed differentially in the
human placentas of patients with preeclampsia. Am J Obstet Gynecol.
3:261–266. 2007.PubMed/NCBI
|
|
16
|
Ekambaram P: HSP70 expression and its role
in preeclamptic stress. Indian J Biochem Biophys. 48:243–255.
2011.PubMed/NCBI
|
|
17
|
Asmathulla S, Koner BC and Papa D: Does
oxidative stress play a role in altered plasma protein homeostasis
in pregnancy-induced hypertension? Acta Physiol Hung. 98:339–346.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valenzuela FJ, Pérez-Sepúlveda A, Torres
MJ, et al: Pathogenesis of preeclampsia: the genetic component. J
Pregnancy. 2012:1–December;2011.(E-pub ahead of print).
|