Introduction
Neutrophil gelatinase-associated lipocalin (NGAL), a
member of the lipocalin family of small secreted proteins, was
originally identified as a protein stored in specific granules of
human neutrophils (1). Besides
being expressed in neutrophils, NGAL is expressed in the majority
of tissues normally induced in epithelial cells during inflammation
(2,3), delivering iron to cells during
formation of the tubular epithelial cells of the primordial kidney
(4), and protecting against acute
ischemic renal injury, thus it is involved in apoptosis as a
survival factor (5,6). NGAL has been identified in a variety
of normal and pathological human tissues. A cell type-specific
pattern of expression has been observed in the bronchus, stomach,
small intestine, pancreas, kidney, prostate gland and thymus
(3). Expression of NGAL has also
been detected in human cancers, including colorectal (2), breast (7), pancreatic (8), bladder (9), ovarian (10), esophageal squamous cell carcinoma
(11) and gastric carcinoma
(12), and its homologue is also
expressed during chicken embryo development in hypertrophic
cartilage (13), and in the
hypertrophic region of growth plate cartilage in developing rat
embryos (14).
The receptor of the homologue of NGAL in mice 24 p3
(24 p3R) was identified and the corresponding human receptor
(NGALR) was also found (15,16).
NGALR was reported to have a function dependent on the status of
NGAL. NGAL and NGALR constitute a novel iron delivery pathway that
is crucial to the survival, growth and maturation of cells
(17). This observation led
investigators to explore the co-expression of NGAL and NGALR. NGALR
expression in esophageal squamous cell carcinoma was found to be
significantly higher compared to normal esophageal epithelium
(18). More recently, we found
that NGAL and NGALR might be involved in the progression of
esophageal squamous cell carcinoma and gliomas, and their
expression can be considered as independent prognostic factors in
these tumors (19,20). However, little is known regarding
the systematic demonstration of NGAL and NGALR expression in
various human organs at different developmental stages.
To improve the understanding of the function of
these two proteins in human development, we aimed to demonstrate
the expression of NGAL and NGALR in human embryo, fetus and normal
adult tissues by employing tissue microarray technology and
immunohistochemical staining.
Materials and methods
Samples
Forty human samples consisting of 3 embryos, 11
fetuses and 26 postnatal specimens were examined in the study
(Table I). Intact embryos and
fetuses were obtained from the Department of Gynaecology and
Obstetrics, Central Hospital of Shantou, Shantou, China. Samples
were collected from 14 healthy pregnant females undergoing elective
termination of pregnancy at 4–22 weeks of gestation. Specimens were
fixed immediately in 4% buffered-formalin solution, and visible
organs were embedded in paraffin blocks. Normal human tissue
sections (from autopsy specimens) were obtianed from the Department
of Forensic Medicine, Shantou University Medical College, Shantou,
China. The specimens were collected between 2002 and 2005. Tissues
from the cerebellum, cerebrum, thymus, lung, trachea, heart,
esophagus, stomach, large intestine, small intestine, liver,
pancreas, kidney, spleen, thymus, lymph-node, pituitary, adrenal
gland and thyroid gland were collected. The study was conducted
with permission from the local ethics committees.
 | Table IDescription of specimens. |
Table I
Description of specimens.
| Human stage | Number of cases |
|---|
| Embryo | 3 |
| 4 weeks’
gestation | 1 |
| 5–8 weeks’
gestation | 2 |
| Fetus | 11 |
| 9–12 weeks’
gestation | 5 |
| 13–16 weeks’
gestation | 3 |
| 17–22 weeks’
gestation | 3 |
| Postnatal stages | 26 |
| Neonate (<28
days) | 5 |
| 1–19 years | 6 |
| 20–39 years | 11 |
| >40 years | 4 |
Construction of tissue microarrays
Representative regions from each tissue were
selected from hematoxylin- and eosin-stained sections and marked on
individual paraffin blocks. Samples were selected from the
specimens with a large quantity of tissue available for correlative
studies. Two tissue cores, measuring 1.8 mm in diameter and ranging
in length from 1.0 to 3.0 mm depending on the depth of the tissue
in the donor block, were obtained from each specimen. Each core was
precisely arrayed into a new paraffin block. The microarrays were
serially sectioned (4 μm), and stained with hematoxylin and eosin
to verify tissue sampling and completeness. Unstained sections were
baked overnight at 56°C prior to immunohistochemistry.
Immunohistochemical staining
Slides were dried in an oven (55–60°C) and the
paraffin was removed through several changes of xylene. The slides
were hydrated through a series of graded alcohol to water followed
by incubation with 3% hydrogen peroxide for 10 min. For antigen
retrieval, the slides were autoclaved in 0.01 M citrate buffer (pH
6.0) at 120°C for 3 min. Sections were then incubated with 10%
normal goat serum in phosphate-buffered saline (PBS) for 15 min at
room temperature to block non-specific binding. After rinsing with
PBS, the slides were incubated at 4°C overnight with mouse
anti-human NGAL monoclonal antibody (1:50 dilution; R&D
Systems, Minneapolis, MN, USA) or rabbit anti-human NGALR
polyclonal antibody (1:10 dilution, Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China). After further rinsing
with PBS, the tissue sections were incubated for 15 min at room
temperature with polymer helper solution (Polymer Detection System
kit, Golden Bridge International, Inc., Mukilteo, WA, USA), and
then rinsed with PBS. The slides were then incubated for 20 min at
room temperature with streptavidin peroxidase-conjugated goat
anti-mouse IgG (Polymer Detection System kit). Subsequently, the
slides were stained with 0.003% 3,3-diaminobenzide
tetrahydrochloride and 0.005% hydrogen peroxide in 0.05 M Tris-HCl
(pH 7.2), and counterstained using Mayer’s hematoxylin prior to
being dehydrated and mounted. A metastatic esophageal carcinoma
previously demonstrated to have immunoreactivity was used as a
positive control in each series of experiments (11). Negative controls were prepared by
substituting PBS for primary antibody.
NGAL-positive samples were defined as those
demonstrating brown signals in the cell membrane and cytosol.
Immunoreactivity was measured semiquantitatively using a scale from
(−) to (+++), where (−) represented no detectable immunostaining;
(+) that <25% of the cells were reactive; (++) that >50% of
the cells were reactive; and (+++) that >50% of the cells were
reactive. Finally, scales of (−), (+), (++) and (+++) were defined
as negative, weakly positive, moderately positive and strongly
positive staining, respectively.
Results
The distribution of NGAL and NGALR in
normal human tissues as determined by immunostaining
Expression of NGAL and NGALR was homogeneous in the
postnatal stages; however, differential expression was observed
during embryonic and fetal development (Table II).
 | Table IIDistribution of NGAL and NGALR in
normal human tissues determined by immunostaining. |
Table II
Distribution of NGAL and NGALR in
normal human tissues determined by immunostaining.
| | NGAL/NGALR |
|---|
| |
|
|---|
| | Embryo | Fetus | Postnatal stages |
|---|
| |
|
|
|
|---|
| Organ/system | Tissue | 4–8 weeks’
gestation | 8–16 weeks’
gestation | 17–22 weeks’
gestation | Neonate (<28
days) | 1–19 years | 20–39 years | >40 years |
|---|
| Nervous system |
| Nerve cell | | −/+ | −/+++ | +/+++ | +/+++ | +/+++ | +/+++ | +/+++ |
| Nerve fiber | | −/+ | −/++ | −/++ | −/++ | −/++ | −/++ | −/++ |
| Cardiovascular
system |
| Heart | | NA | −/− | −/− | −/− | −/− | −/− | −/− |
| Artery | | NA | −/− | −/− | −/− | −/− | −/− | −/− |
| Respiratory
system |
| Lung | | NA | ++/++ | ++/++ | −/++ | −/++ | −/++ | −/++ |
| Trachea | | NA | ++/++ | ++/++ | −/− | −/− | −/− | −/− |
| Alimentary
system |
| Gastrointestinal
tract | | ++/++ | ++/++ | −/− | −/− | −/− | −/− | −/− |
| Liver | | −/− | −/− | −/− | −/− | −/− | −/− | −/− |
| Pancreas | | NA | −/+++ | −/+++ | −/+++ | −/+++ | −/+++ | −/+++ |
| Genitourinary
system |
| Kidney |
| Glomerulus | NA | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ |
| Proximal
tubule | NA | ++/+++ | ++/+++ | ++/+++ | ++/+++ | ++/+++ | ++/+++ |
| Distal tubule | NA | +++/++ | +++/++ | +++/++ | +++/++ | +++/++ | +++/++ |
| Collecting
duct | NA | +++/++ | +++/++ | +++/++ | +++/++ | +++/++ | +++/++ |
| Urothelium | | NA | NA | NA | −/− | −/− | −/− | −/− |
| Prostate
gland | | NA | NA | NA | −/− | −/− | −/− | −/− |
| Endocrine
system |
| Adrenal gland |
| Zona
glomerulosa | NA | ++/++ | ++/++ | +++/+++ | +++/+++ | +++/+++ | +++/+++ |
| Zona
fasciculata | NA | ++/++ | ++/++ | +/+++ | +/+++ | +/+++ | +/+++ |
| Zona
reticularis | NA | ++/++ | ++/++ | +++/+++ | +++/+++ | +++/+++ | +++/+++ |
| Medulla | NA | ++/++ | ++/++ | −/+++ | −/+++ | −/+++ | −/+++ |
| Thyroid gland | | NA | NA | NA | −/− | −/− | −/− | −/− |
| Pituitary | | NA | NA | NA | ++/++ | ++/++ | ++/++ | ++/++ |
| Immune system |
| Spleen | | NA | ++/++ | ++/++ | ++/++ | ++/++ | ++/++ | ++/++ |
| Lymphoid node | | NA | NA | NA | +/+ | +/+ | +/+ | +/+ |
| Thymus | | NA | NA | ++/++ | ++/++ | NA | NA | NA |
| Skin |
| Basal layer | NA | ++/++ | ++/++ | ++/++ | ++/++ | ++/++ | ++/++ |
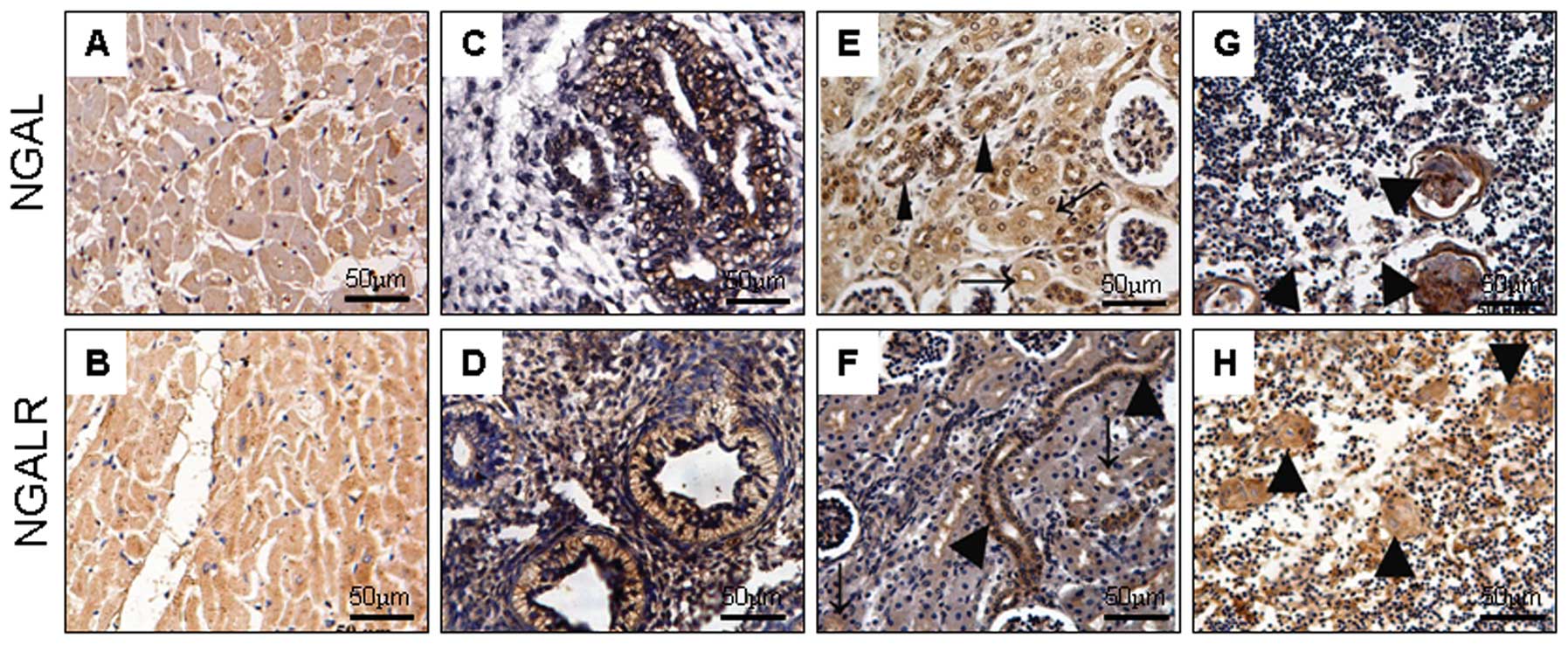
Nervous system
In the development of the nervous system, the
expression of NGAL was undetectable in the early embryos, but
detectable in the later developmental stages, and NGALR protein was
detected throughout human development. In the early embryos, the
neuroepithelium of the neural tube demonstrated NGAL-negative
(Fig. 1A) and NGALR-positive
expression (Fig. 1B). In the
fetuses and adults, NGAL and NGALR immunoreactivities were detected
in stellate cells of the cerebrum, but not in glial cells (Fig. 1C and D). In the cerebellum, no
immunoreactivity was found in any cells of the Purkinje cell,
granular or molecular layer at 8–12 weeks’ gestation (Fig. 1E and F); however, the Purkinje
cells demonstrated pronounced immunoreactivity in the later
developmental stages (Fig. 1G and
H).
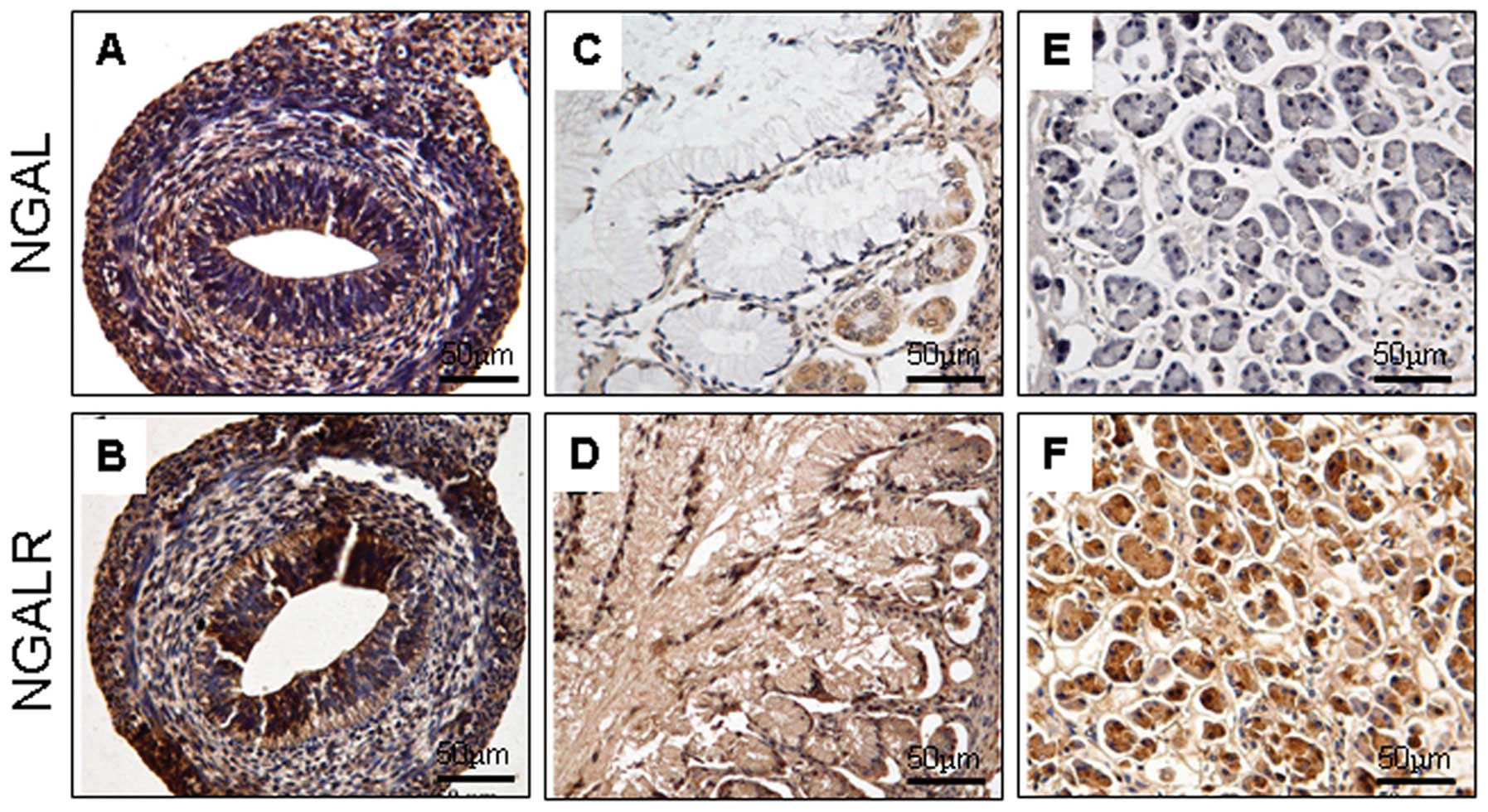
Alimentary system
The expression pattern of NGAL and NGALR was similar
to the gastrointestinal development. At 6–8 weeks’ gestation, the
two proteins were expressed in cells of the gastrointestinal tract
(Fig. 2A and B). In later
developmental stages, the proteins were observed in certain cells
of the glandular epithelium, including the Paneth’s and gastric
parietal cells (Fig. 2C and D). In
the liver, NGAL and NGALR were weakly positive. In the pancreas,
the acini and tubular epithelium of the glandular pancreatic tissue
exhibited NGAL-negative (Fig. 2E)
and NGALR-positive expression (Fig.
2F).
Endocrine system
During the development of the endocrine system, the
expression of NGAL and NGALR was detectable in certain cells of the
anterior pituitary, but undetectable in the follicular and C-cells
of the thyroid gland (Fig. 3A and
B). In the adrenal gland, the two proteins demonstrated a
moderately positive reaction at 8–22 weeks’ gestation in the cortex
and the medulla (Fig. 3C and D).
However, in later stages of development, NGAL was particularly
demonstrated in cells of the inner and outer layers of the cortex,
decreased in the zona fasciculata and entirely absent in the
medulla (Fig. 3E), while NGALR
demonstrated strong positivity in the cortex and the medulla
(Fig. 3F).
Expression of NGAL and NGALR in other
systems
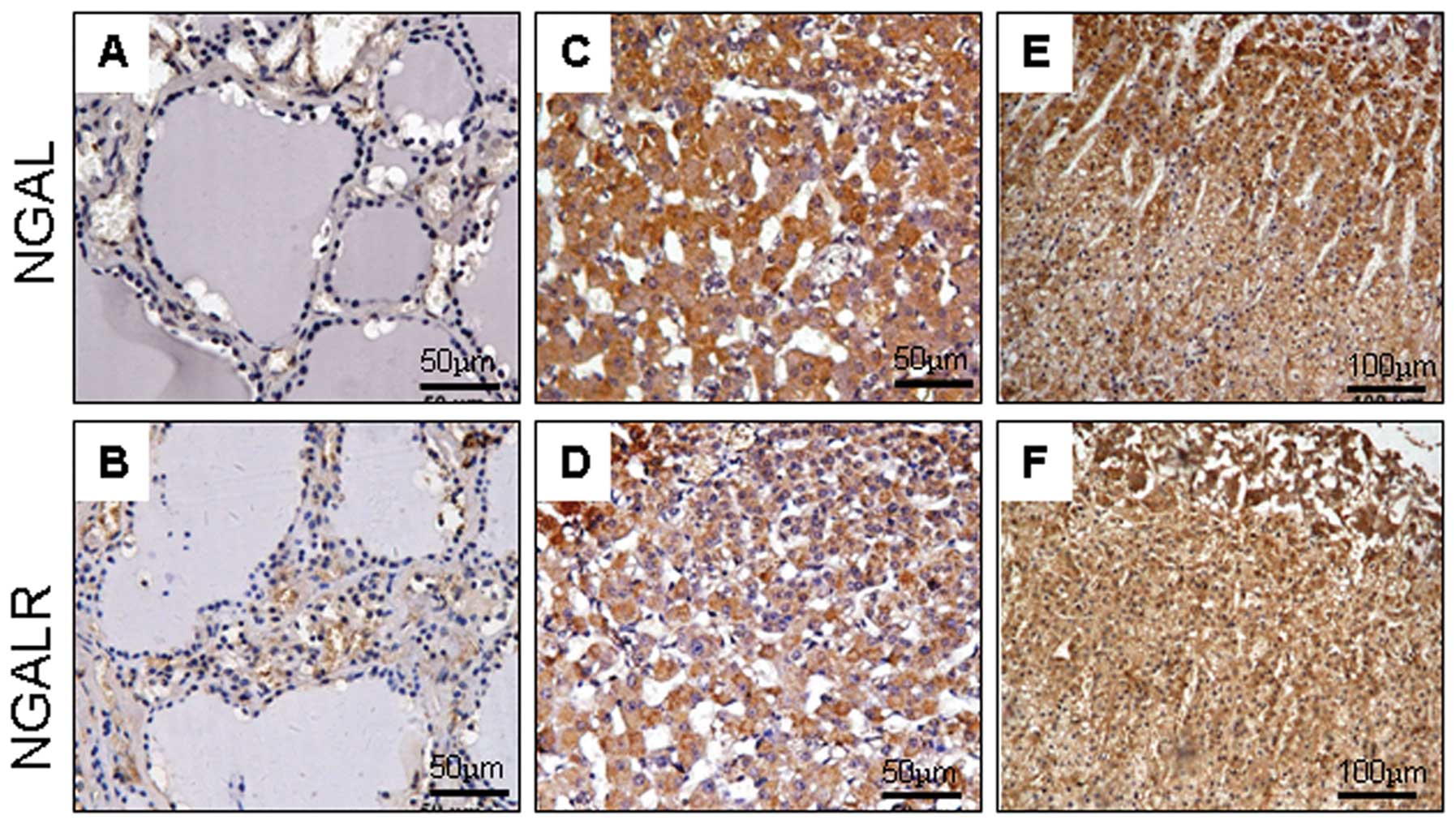
In the cardiovascular system, NGAL and NGALR
produced no or weak reactivity in the vascular endothelial and
myocardial cells (Fig. 4A and B).
In the respiratory system, NGAL and NGALR were most prevalent in
the epithelium of the alveolar gland in the early embryos (Fig. 4C and D), but not in the cells of
the lung during later developmental stages. In the development of
the genitourinary system, there was no evident difference between
NGAL and NGALR expression. In the kidney, the two proteins were
expressed in epithelial cells of the pars convoluta of the proximal
tubule, and strongly expressed in epithelium of the distal tubule
and the collecting duct (Fig. 4E and
F). Bladder epithelium and prostate tissues were all negative.
In the immune system, NGAL and NGALR were undetectable in the
lymphocytes, but evidently demonstrated in the Hassall’s corpuscle
epithelial cells of the thymus (Fig.
4G and H).
Discussion
In the present study, we demonstrated that NGAL and
NGALR were expressed in the epithelium of the renal tubule, the
kidney, the thymus and the adrenal gland, but were not detectable
in the heart, liver and thyroid gland, which was consistent with
findings from a previous study demonstrating NGAL expression in
normal human tissues (3). It was
reported that in developing muscle fibers, a NGAL homologue was
found in the hypertrophic cartilage during chicken embryo
development (13) and in the
hypertrophic region of growth plate cartilage in developing rat
embryos (14). Mallbris et
al investigated the expression of NGAL in human skin embryonic
development and reported that the embryonic expression of NGAL was
induced in the interfollicular epidermis at 20–24 weeks of
gestational age, but thereafter progressively receded towards the
hair follicles (21). In this
study, we revealed that NGAL was expressed in the gastrointestinal
tract during human embryo development. These findings suggest that
NGAL is an evolutionarily conserved protein.
We found that NGAL and NGALR demonstrated different
expression patterns in human embryonic, fetal and normal adult
tissues, and their expression was adapted to become tissue and
cell-specific throughout human development. The two proteins were
weakly-positive or negative in normal cells of the thyroid gland,
liver, heart and blood vessels, but they were abundant in the
spleen and adrenal gland, and at the cell level, they were specific
to lymphocytes and the epithelium of the renal tubule. In addition
to a broad range of simple epithelial cell types, we found that the
proteins were also expressed in stratified epithelia. For example,
in the skin, mature cells of the outermost stratified layers
exhibited a distribution of NGAL, which was inconsistent with
results from a previous study (11). The expression of NGAL and NGALR
does not appear to follow any morphogenetic pattern, however,
colocalization with regards to cell function can be identified at
closer examination.
Expression of NGAL and NGALR were also found to be
time-specific during human growth and development. The proteins
were expressed in the epithelium of the lung alveolus and
gastrointestinal tract in the embryos, but were almost undetectable
in the later developmental stages. In the embryonic adrenal gland,
the cortex and medulla demonstrated a moderately positive reaction.
In the adult tissues, NGAL was particularly present in the cells of
the inner and outer layers of the cortex, but was absent in the
medulla. Additionally, a pronounced expression pattern for NGAL was
undetectable throughout the neural tube, but was detected in the
stellate cells of the cerebrum and the Purkinje cells of the
cerebellum in postnatal stages. It has been reported that the
expression of NGAL was induced in the interfollicular epidermis at
20–24 weeks of gestational age, but thereafter progressively
receded towards the hair follicles (21). Although there is no precise
mechanism to explain the reason for the upregulation of the NGAL
protein level in a number of normal tissues, as observed at early
developmental stages, we speculate that NGAL might be involved in
cell-cell interactions, cell division and/or cell growth, due to
the high demand for those biological behaviors during
development.
Lipocalins have been extensively used as biochemical
markers for diseases. The clinical indications are associated with
almost any field of medicine, including inflammatory disease,
cancer, lipid disorders and kidney disease (22). Previous studies have demonstrated
that overexpression of NGAL is involved in the progression of
tumors (2,7–12).
In cancers of the alimentary system, NGAL was upregulated in human
colorectal and gastric cancer (2,12).
Results from our study revealed that the glandular epithelium of
the normal adult gastrointestinal tract is negative for NGAL
expression, but a pronounced positive staining was observed
throughout the gastrointestinal tract at early fetal stages.
Furthermore, NGAL was detected in the epithelium of the lung
alveolus in embryos and A549 cells, a lung carcinoma cell line
(6), but was almost undetectable
in the adult lung tissue. Thus, NGAL immunoreactivity was found in
cells of early fetal stages and in malignant cells, but was
negative in the corresponding adult tissues, suggesting that there
was a specific correlation between NGAL expression in certain
malignant cells and in the corresponding normal cells from early
developmental stages. We speculate that there may be a correlation
between NGAL expression and the processes of embryogenesis and
carcinogenesis.
In conclusion, the immunohistochemical observations
in the present study are the first systematic demonstration of the
immunoreactive presence of NGAL and NGALR in human embryonic, fetal
and normal adult tissues. Through analyses of the differential
expression, it was found that the expression of NGAL and NGALR is
highly tissue- and time-specific.
Acknowledgements
The authors are grateful to Professor Xiao-Jun Yu
from the Department of Forensic Medicine, Shantou University
Medical College for his assistance in providing autopsy specimens.
This study was supported by grants from the National High
Technology Research and Development Program of China (No.
2006AA02A403), the Natural Science Foundation of China-Guangdong
Joint Fund (No. U0932001) and the Foundation for Distinguished
Young Talents in Higher Education of Guangdong, China
(LYM09080).
References
|
1
|
Kjeldsen L, Johnsen AH, Sengeløv H and
Borregaard N: Isolation and primary structure of NGAL, a novel
protein associated with human neutrophil gelatinase. J Biol Chem.
268:10425–10432. 1993.PubMed/NCBI
|
|
2
|
Nielsen BS, Borregaard N, Bundgaard JR,
Timshel S, Sehested M and Kjeldsen L: Induction of NGAL synthesis
in epithelial cells of human colorectal neoplasia and inflammatory
bowel diseases. Gut. 38:414–420. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Friedl A, Stoesz SP, Buckley P and Gould
MN: Neutrophil gelatinase-associated lipocalin in normal and
neoplastic human tissues. Cell type-specific pattern of expression.
Histochem J. 31:433–441. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gwira JA, Wei F, Ishibe S, Ueland JM,
Barasch J and Cantley LG: Expression of neutrophil
gelatinase-associated lipocalin regulates epithelial morphogenesis
in vitro. J Biol Chem. 280:7875–7882. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mori K, Lee HT, Rapoport D, Drexler IR,
Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, et al:
Endocytic delivery of lipocalin-siderophore-iron complex rescues
the kidney from ischemia-reperfusion injury. J Clin Invest.
115:610–621. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tong Z, Wu X, Ovcharenko D, Zhu J, Chen CS
and Kehrer JP: Neutrophil gelatinase-associated lipocalin as a
survival factor. Biochem J. 391:441–448. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bauer M, Eickhoff JC, Gould MN, Mundhenke
C, Maass N and Friedl A: Neutrophil gelatinase-associated lipocalin
(NGAL) is a predictor of poor prognosis in human primary breast
cancer. Breast Cancer Res Treat. 108:389–397. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moniaux N, Chakraborty S, Yalniz M,
Gonzalez J, Shostrom VK, Standop J, Lele SM, Ouellette M, Pour PM,
Sasson AR, et al: Early diagnosis of pancreatic cancer: neutrophil
gelatinase-associated lipocalin as a marker of pancreatic
intraepithelial neoplasia. Br J Cancer. 98:1540–1547. 2008.
View Article : Google Scholar
|
|
9
|
Monier F, Surla A, Guillot M and Morel F:
Gelatinase isoforms in urine from bladder cancer patients. Clin
Chim Acta. 299:11–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lim R, Ahmed N, Borregaard N, Riley C,
Wafai R, Thompson EW, Quinn MA and Rice GE: Neutrophil
gelatinase-associated lipocalin (NGAL) an early-screening biomarker
for ovarian cancer: NGAL is associated with epidermal growth
factor-induced epithelio-mesenchymal transition. Int J Cancer.
120:2426–2434. 2007. View Article : Google Scholar
|
|
11
|
Zhang H, Xu L, Xiao D, Xie J, Zeng H, Wang
Z, Zhang X, Niu Y, Shen Z, Shen J, et al: Upregulation of
neutrophil gelatinase-associated lipocalin in oesophageal squamous
cell carcinoma: significant correlation with cell differentiation
and tumour invasion. J Clin Pathol. 60:555–561. 2007. View Article : Google Scholar
|
|
12
|
Du ZP, Yuan HM, Wu BL, Chang JX, Lv Z,
Shen J, Wu JY, Chen HB, Li EM and Xu LY: Neutrophil
gelatinase-associated lipocalin in gastric carcinoma cells and its
induction by TPA are controlled by C/EBPβ. Biochem Cell Biol.
89:314–324. 2011.PubMed/NCBI
|
|
13
|
Descalzi CF, Dozin B, Zerega B, Cermelli S
and Cancedda R: Ex-FABP: a fatty acid binding lipocalin
developmentally regulated in chicken endochondral bone formation
and myogenesis. Biochim Biophys Acta. 1482:127–135. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zerega B, Cermelli S, Michelis B, Cancedda
R and Cancedda FD: Expression of NRL/NGAL (neu-related
lipocalin/neutrophil gelatinase-associated lipocalin) during
mammalian embryonic development and in inflammation. Eur J Cell
Biol. 79:165–172. 2000. View Article : Google Scholar
|
|
15
|
Devireddy LR, Teodoro JG, Richard FA and
Green MR: Induction of apoptosis by a secreted lipocalin that is
transcriptionally regulated by IL-3 deprivation. Science.
293:829–834. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang WK, Xu LY, Lu XF, Liao LD, Cai WJ,
Shen ZY and Li EM: A novel alternative spliced variant of
neutrophil gelatinase-associated lipocalin receptor in esophageal
carcinoma cells. Biochem J. 403:297–303. 2007. View Article : Google Scholar
|
|
17
|
Devireddy LR, Gazin C, Zhu X and Green MR:
A cell-surface receptor for lipocalin 24p3 selectively mediates
apoptosis and iron uptake. Cell. 123:1293–1305. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui L, Xu LY, Shen ZY, Tao Q, Gao SY, Lv
Z, Du ZP, Fang WK and Li EM: NGALR is overexpressed and regulated
by hypomethylation in esophageal squamous cell carcinoma. Clin
Cancer Res. 14:7674–7681. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du ZP, Lv Z, Wu BL, Wu ZY, Shen JH, Wu JY,
Xu XE, Huang Q, Shen J, Chen HB, Li EM and Xu LY: Neutrophil
gelatinase-associated lipocalin and its receptor: independent
prognostic factors of oesophageal squamous cell carcinoma. J Clin
Pathol. 64:69–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu MF, Jin T, Shen JH, Shen ZY, Zheng ZC,
Zhang ZL, Xu LY, Li EM and Xu HX: NGAL and NGALR are frequently
overexpressed in human gliomas and are associated with clinical
prognosis. J Neurooncol. 104:119–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mallbris L, O’Brien KP, Hulthén A,
Sandstedt B, Cowland JB, Borregaard N and Ståhle-Bäckdahl M:
Neutrophil gelatinase- associated lipocalin is a marker for
dysregulated keratinocyte differentiation in human skin. Exp
Dermatol. 11:584–591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu S and Venge P: Lipocalins as
biochemical markers of disease. Biochim Biophys Acta. 1482:298–307.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie JJ, Zhang FR, Tao LH, et al:
Expression of ezrin in human embryonic, fetal, and normal adult
tissues. J Histochem Cytochem. 59:1001–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|