Introduction
The glial cell line-derived neurotrophic factor
(GDNF) has been recognized as one of the most powerful neurotrophic
factors for developing and injured dopaminergic neurons and
motoneurons (MNs) (1,2). Moreover, GDNF has been proven to play
a pro-survival role in developing and injured MNs (1,3–8).
However, it remains uncertain as to which intracellular signaling
pathway interacting with GDNF is involved in MN development.
Previous studies have found that GDNF signals through a unique
multicomponent receptor system consisting of RET tyrosine kinase
and glycosylphosphatidylinositol-anchored co-receptor (GFRα1–4) in
the cell membrane (9). Notably,
the GDNF-TrkB signaling pathway has been proven to be involved in
the transcription, translation and trafficking of proteins during
neuronal and synaptic plasticity of the nervous system (10,11),
and these functions are carried out by a combination of the three
signaling cascades triggered when GDNF binds TrkB: the
mitogen-activated protein kinase (MAPK), phosphoinositide
phospholipase C-γ (PLCγ) and phosphatidylinositol 3-kinase (PI3K)
pathways (10–13). The brain-derived neurotrophic
factor (BDNF)-PLC-γ pathway has been found to widely participate in
many neuronal activities such as neurite outgrowth (14,15)
and differentiation (10,16). In addition, PLC-γ is involved in
the survival and growth of the developing nervous system of rats in
response to neurotrophin (17).
The GDNF-PLC-γ signaling pathway also plays a neuroprotective role
in Parkinson’s disease and in cognitive processes of the
hippocampus (18,19). Previous studies, including our own,
have proven that GDNF is capable of rescuing avulsion-injured
spinal MNs in adult rats (20–22).
However, GDNF is not capable of penetrating the brain-blood-barrier
due to its high molecular weight, and this creates an obstacle in
clinical practice (23).
Therefore, in this study, we aimed to investigate the GDNF-related
intracellular signals in the spinal MNs in order to find a more
useful molecular target for the treatment of avulsion-induced
spinal MN death. In the present study, we investigated whether the
GDNF-PLC-γ signaling pathway is involved in the survival of rat
spinal MNs.
Materials and methods
Primary spinal MN culture
The spinal MN cultures were established from 12- to
14-day-old embryos (E12–E14) of Sprague-Dawley rats. The ventral
portion of the cervical segments of the spinal cord was removed
using tungsten needles and was kept in ice-chilled,
sterile-filtered 0.01 M phosphate-buffered saline (PBS). The spinal
cord tissues were then treated with 0.5% trypsin prior to being
incubated at 37°C for 15 min with frequent agitation. The spinal
MNs were purified by centrifugation in a two-step metrizamide
gradient, whose purity typically was shown to achieve 75–90%
enrichment in MNs previously (24). Briefly, the partially dissociated
cells were added to Leibowitz L15 medium (Invitrogen, Melbourne,
Australia) and defined serum-free medium (Gibco, Grand Island, NY,
USA), and further dissociated by running the mixture through a 1-ml
pipette, followed by centrifugation (3,400 × g for 15 min; Beckman
GS-6R centrifuge; Beckman Instruments, Fullerton, CA, USA) over a
layer of 6.8% metrizamide (Sigma, St. Louis, MO, USA). Due to their
large size, spinal MNs remained in the top half of the metrizamide,
forming a visible white band, which was collected and added to 5 ml
of L15 medium. A 4% bovine serum albumin (BSA) cushion was then
gently added beneath the cells and centrifuged at 3,200 × g for 10
min (Beckman centrifuge). The supernatant was discarded, and the
pellet was resuspended in complete medium. The complete medium
(neurobasal medium; Gibco-BRL) was supplemented with 2 mM glutamate
(Sigma Pharmaceuticals, Melbourne, Australia), 1%
penicillin/streptomycin (CSL, Melbourne, Australia), 2% B-27
(Gibco-BRL), 7.2% glucose (BDH Chemicals, Australia), 2% horse
serum (CSL), 4% BSA (CSL), 10 ng/ml GDNF (Cytolab) and 25 μM
β-mercaptoethanol, and then filtered through a 50-μm nylon filter.
Cultures were incubated in a CO2 water-jacketed
incubator with 37°C and 5% CO2 for 24 h. To enhance
adhesion of the cells to the wall of the culture wells, the culture
plates were precoated with polyornithine-laminin (Sigma).
Immunocytochemistry for MN and astrocyte
markers
Several types of antibodies specific for the markers
of MNs and glial cells were chosen to identify the components of
the cultured cells. All antibodies used in the present study were
purchased from Sigma. At three days in vitro 3 (DIV) and 6
DIV, complete culture medium were removed gently, and the cultured
cells were washed once with 1–2 ml PBS before the cells were fixed
with 4% paraformaldehyde for 15 min at 4°C. The cells were then
washed three times with a washing buffer (0.2% Triton X-100 in PBS)
to remove any excess paraformaldehyde. The cultured cells were
incubated at room temperature for 1–2 h with each of the primary
antibodies, including neuron-specific enolase (NSE, 1:500),
anti-75-kDa low affinity neurotrophin receptor (anti-p75NTR,
1:100), neurofilament H non-phosphorylated (SMI-32, 1:5000) and
choline acetyltransferase (ChAT, 1:500). The presence of the glial
cells and astrocytes in the cell cultures was determined by
immunofluorescence of the astrocyte marker glial fibrillary acidic
protein (GFAP, 1:1000) (25).
After the cells were washed three times with washing buffer, the
cultured cells were incubated for 2 h at room temperature with
secondary antibodies. Following three additional rinses with
washing buffer, the cells were incubated for 2 h in an
avidin-biotin complex solution (Vectastain kit; Vector
Laboratories, Burlingame, CA, USA) and were stained with
diaminobenzidine (DAB; Vector Laboratories) for 2–5 min. Finally,
the cells were washed three times, and a coverslip was placed over
them for microscopic observation (Leica DM 2500B, Germany). For
immunofluorescence, the cells were incubated with
tetramethylrhodamine isothiocyanate (TRITC)-conjugated secondary
antibody at room temperature in the dark for 2 h. After three
additional washes with washing buffer, the sections were mounted
onto the slides, coverslipped in 0.5 M buffer bicarbonate (pH 9.5)
containing 50% glycerin, and then examined via an inverted
fluorescence microscopy (Leica DM 2500B). The negative controls
were treated in the same way, except that each primary antibody or
secondary antibody was omitted.
Spinal MN culture treatment with GDNF and
a specific inhibitor of PLC-γ,
1-[6-((17b-3-methoxyestra-1,3,5(10)-trien-17-yl)
amino)hexyl]-1H-pyrrole-2,5-dione (U73122)
GDNF was added to the complete medium followed by
plating cells into 96-well plates at the most optimal plating
density. According to the different concentrations of GDNF, the
cultured cells were divided into five groups as follows: 0.1, 1, 10
and 100 ng/ml. Another group of culture medium without GDNF was
used as a control. Twenty-four hours later (1 DIV), the cells of
each group were counted under the phase contrast optics of an
inverted microscope (Leica DMI4000B), and the survival rate of the
cultured MNs was compared among the cultures treated with various
concentrations of GDNF.
The inhibitor of PLC-γ, U73122 (Calbiochem), was
used to investigate the involvement of PLC-γ signaling in the
development of embryonic MNs facilitated by GDNF. U73122 was
dissolved in dimethyl sulfide (DMSO, 1 mg/ml, Sigma) and diluted in
normal saline to the working solution concentration (2.5 μmol/l)
prior to being applied to the cultured cells. The cultures were
then randomly assigned to the U73122 + DMSO + GDNF, GDNF + DMSO and
GDNF groups. U73122 was then added into the culture medium 30 min
prior to GDNF application.
Quantification of MN survival
MN counting was carried out by two investigators who
were blinded to the culture treatment groups. All attached cells
showing visible nuclei and a phase bright halo and clearly defined
limiting soma were considered to have survived (26,27).
The counting of surviving cells was carried out at ×400
magnification under an inverted microscope (Leica DM 2500B)
according to our previous study (28). Six random microscopic fields per
well were counted. The number of surviving MNs was counted in three
randomly selected fields per well, and the mean value was obtained.
In the present study, the surviving MNs at 4 h after initial
seeding (time required for complete neuronal attachment) in each
well were considered as the internal controls (27). Cells surviving at 24 h were
compared with those in each well initially at 4 h after plating,
which were taken arbitrarily as 100%. At 24 h after GDNF or GDNF +
U73122 treatment, the number of surviving MNs with a neurite
process length longer than the length which is two times the cell
diameter, was counted. Data are expressed as a percentage of the
internal control. Results were presented as the means ± standard
error of the mean of triplicate samples from at least six separate
experiments (n=6), as described in our previous studies (4,26,29).
Statistical analysis
The statistical calculations and data handling were
performed using SPSS version 16.0. All variables were expressed as
the means ± standard deviation (SD). A one-way ANOVA was applied to
detect differences among groups followed by Tukey-Kramer multiple
comparison tests. Differences were considered significant at p
values <0.05.
Results
Identification of the MN markers in the
cultured cells
Under a phase contrast microscope, the soma of these
cells were phase bright and attached to the plate of the well after
4 h (Fig. 1A). The MN-like cells
are predominantly solitary in their occurrence from culture 24 h
after initial cell seeding (Fig.
1B). The round and phase bright soma were associated with
several primary neurites, some of which have secondary and tertiary
branches, extending to twice the length of the cell soma in the
culture 72 h (Fig. 1C) after cell
seeding. The presence of these MN-like cells was sustained for a
period of up to seven days in the present study (Fig. 1D).
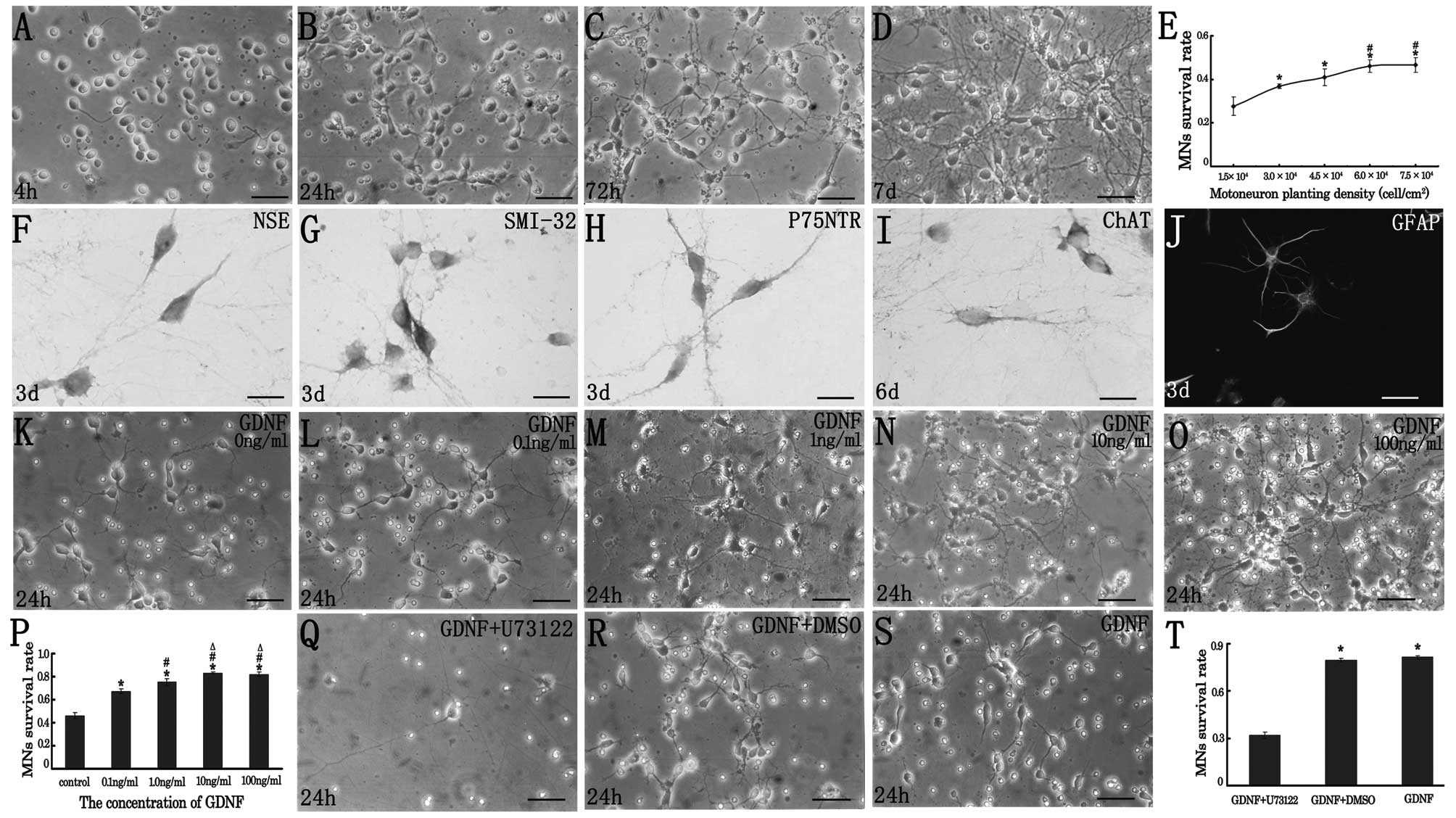 | Figure 1(A–D) Spinal motoneuron morphology
depicted using phase contrast microscopy at (A) 4 h, (B) 24 h, (C)
72 h and (D) 7 days after plating. The live cells with
motorneuronal morphology were predominantly from the 24 h culture,
and the round and phase bright soma were associated with several
primary neurites extending to twice the length of the cell soma
occurring in the culture 72 h after plating. The presence of these
MN-like cells was sustained for a period of up to 7 days. (E) When
cultured at a gradually elevated plating density, the survival rate
of the embryonic MNs at 24 h after plating increased
correspondingly. *Indicates values that were
significantly different compared with that at a density of
1.5×104 cell/cm2 (*p<0.05);
#indicated values that were significantly different
compared with that at a density of 3.0×104
cell/cm2 (#p<0.05); bar, 50 μm. (F–I)
Identification of the MN markers in the cultured cells by
immunocytochemistry or immunoflurorescence. The immuno-positive
particles were distributed mainly in the cytoplasm and the neurite
of the MNs. At day 3 after plating, the cultured embryonic spinal
MNs were identified by (F) the neuronal marker NSE; (G) MN markers
SMI-32 and (H) P75NTR. At day 6 after cell seeding, the cultured
embryonic spinal MNs were identified by (I) the MN marker ChAT, and
the presence of astrocytes was shown by (J) GFAP
immunoflurorescence. (F–I) Bar, 25 μm; (J) bar, 50 μm. (K–O) The
dose-dependent effect of GDNF on cultured embryonic spinal MNs.
Twenty four hours later, compared to the morphology of the cultured
MNs in the complete culture medium (K), the spinal motoneruons
cultured with GDNF at a concentration of (L) 0.1, (M) 1.0, (N) 10
or (O) 100 ng/ml typically possessed a phase bright, oval-shaped or
multi-angular shaped soma with extensive, relatively bipolar
neurites. Bar, 50 μm. (P) Twenty four hours later, the survival
rate of the cultured spinal MNs was compared and the result showed
that GDNF promoted spinal MN survival in vitro.
*Indicated values that were significantly different
compared with control (*p<0.05);
#indicated values that were significantly different
compared with the 0.1 ng/ml GDNF-treated group
(#p<0.05); △indicated values that were
significantly different compared with the 1 ng/mL GDNF-treated
group (△p<0.05). (Q–T) Effects of the PLC-γ inhibitor
on spinal MN survival. U-73122 at a concentration of 2.5 μM/l, was
added into the complete culture medium 30 min prior to GDNF (10
ng/ml) application. Twenty four hours later, the survival rate of
the cultured spinal MNs was decreased in the (Q) GDNF +
U73122-treated culture, while the survival rate was similar in the
(R) GDNF + DMSO treated culture as compared to that in the (S)
GDNF-treated culture. Bar, 50 μm. (T) Twenty four hours later, the
survival rate of the cultured spinal MNs was significantly
decreased by the PLC-γ inhibitor U73122. *Indicates
values that were significantly different compared with the GDNF +
U73122-treated culture (*p<0.05). |
In order to ensure that the live cells were MNs
in vitro, immunohistochemistry reactions for the neuronal
marker NSE, MNs markers including p75NTR, SMI-32 and ChAT were
carried out. The result revealed that the MN-like cells expressed
NSE (Fig. 1F), SMI-32 (Fig. 1G) and p75NTR (Fig. 1H) in culture 72 h after initial
plating, and expressed ChAT (Fig.
1I) in culture 6 d after initial plating. The immuno-positive
particles were distributed mainly in the cytoplasm and the neurite
of the MNs (Fig. F-I). Thereafter, on the sixth day of culture,
ChAT immuno-positive mature spinal MNs were characterized by darker
brown cytoplasm and longer axon-like processes. From day three
after initial plating, we also observed the presence of astrocytes,
which expressed GFAP (Fig.
1J).
GDNF promotes spinal MN survival
Since previous studies observed that the survival
rate of the cells in vitro was influenced by the plating
density of the cells, it was, therefore, necessary to choose an
optimal plating density prior to the application of GDNF and
U73122. At first in our study, the cells were diluted and plated at
five different densities: 1.5×104, 3.0×104,
4.5×104, 6.0×104 and 7.5×104
cells/cm2 in a 96-well plate. Twenty-four hours later,
the surviving cells were counted. The survival rates were
27.67±4.25%, 36.87±1.02%, 40.97±3.86%, 46.11±2.73% and 46.61±3.38%
at the densities of 1.5×104, 3.0×104,
4.5×104, 6.0× and 7.5×104
cells/cm2, respectively. Statistical analysis showed the
lowest survival rate was at the density of 1.5×104
cell/cm2 (Fig. 1E),
which was significantly lower than that at any of the other
densities (all P<0.05). The highest survival rate occurred at
the densities of 6.0×104 and 7.5×104
cell/cm2. The survival rate was higher with the increase
of the plating density. We took the density of 6.0×104
cell/cm2 as the most optimal plating density in the
following study, as the cells tended to cluster and were hard to
investigate at the density of 7.5× cell/cm2.
The GDNF, at a concentration of 0.1, 1.0, 10 or 100
ng/ml respectively, was added to the complete medium and applied to
the plating cells at a density of 6.0×104. The live
cells cultured with GDNF typically possessed MN-like morphology,
which showed a phase bright, oval-shaped or multi-angular shaped
soma with extensive, relatively bipolar neurites (Fig. 1K-O). Twenty four hours later, the
survival rate in the complete culture medium was 46.12±2.73%
(Fig. 1K), which was lower than
that in any of the GDNF-treated groups (all p<0.05). The
survival rate of the culture cells was 67.35±1.77% (Fig. 1L), 75.15±2.80% (Fig. 1M), 83.19±1.07% (Fig. 1N) and 81.88±2.12% (Fig. 1O) in the culture with GDNF at the
concentration of 0.1, 1.0, 10 or 100 ng/ml, respectively (Fig. 1P). The result showed that GDNF
promotes spinal MN survival in vitro. Among the GDNF-treated
groups, the survival rate was significantly higher at the
concentration of 10 and 100 ng/ml (all p<0.05). Therefore, we
chose 10 ng/ml as the optimal concentration of GDNF in the
following study.
U73122 blocks the pro-survival effect of
GDNF on spinal MNs
According to previous studies (30) and our preliminary experiment in
vitro, the inhibitor of PLC-γ, U-73122, at a concentration of
2.5 μM/l, was added into the complete culture medium 30 min prior
to GDNF (10 ng/ml) application. Twenty four hours later, the
survival rate of the culture cells was only 31.87±2.17% in GDNF +
U73122-treated cultures (Fig. 1Q),
which was significantly lower than 79.39±1.22% in GDNF + DMSO-
(Fig. 1R) and 81.38±1.13% in
GDNF-treated cultures (Fig. 1S)
(all p<0.05), while there was no significant difference of the
survival rate between GDNF + DMSO- and GDNF-treated cultures
(p>0.05). This result showed that the inhibitor of PLC-γ blocks
the pro-survival effect of GDNF on the spinal MNs in
vitro.
Discussion
The present data demonstrated a potent
survival-promoting effect of GDNF on developing spinal MNs in
culture. Furthermore, U73122, a pharmacological antagonist of
PLC-γ, reversed the neuroprotection of GDNF.
Cell counting, adopted as a major method to evaluate
MN survival, has been proven to be a credible and convincing tool
and has been widely used in previous studies (27,31–33).
In the present study, different MN survival rates were recorded by
means of cell counting and it was found that PLC-γ is required for
the embryonic MN survival in response to GDNF.
Neurotrophic factors play important roles in
survival, development and maintenance of the nervous system. GDNF,
one of the transforming growth factor (TGF)-β superfamily, was
reported to not only reverse 6-hydroxydopamine (6-OHDA)-induced
degeneration of dopaminergic neurons in vivo (34) but also rescue injured postnatal MNs
(35). The results from present
study further proved that GDNF exhibits a dose-dependent effect on
the survival of embryonic rat spinal MNs in culture. This result
was consistent with the previous study, which showed the ability of
GDNF to promote chicken MN survival in culture (36).
The important finding of the present study is that
the survival-promoting effect of GDNF on MN was reversed by the
inhibitor of the PLC-γ signal. Since previous studies have
demonstrated that PLC-γ acts as the downstream signal of the
GDNF-TrkB pathway, which is involved in the transcription,
translation and trafficking of proteins during neuronal and
synaptic plasticity of the nervous system (10,11),
our present results indicated that the GDNF-TrkB-PLC-γ signaling
pathway plays a pro-survival role in the developing spinal MNs. In
line with our results, many of the previous studies have also
identified that the PLC-γ signal plays a role in numerous types of
signaling transduction associated with neuroprotection. It has been
shown that PLC-γ plays a pivotal role in survival promotion for
many types of neurons in the central nervous system (CNS),
facilitated by neurotrophins, such as the hippocampal neurons
(37–39), cerebellar granule cells (17,40,41),
cerebral cortical neurons and PC12 cells (42). In addition, the role of PLC-γ has
been focused on the anti-apoptotic signaling pathway for neurons in
the CNS. In the research for preventing retinal ganglion neurons
from apoptosis induced by withdrawal of trophic additives, the
activation of PLC-γ is required for the anti-apoptotic signaling
pathway induced by apolipoprotein E-containing lipoproteins (LpE)
(43). It has been shown that
PLC-γ is one of the essential signals in the anti-apoptotic role of
the lithium ion to prevent cortical neurons from neuronal apoptosis
induced by neurotoxicity (44).
Furthermore, the literature suggests that PLC-γ is
capable of regulating the development process in the CNS. For
instance, the PLC-γ pathway is required for fibroblast growth
factor-2 to stimulate retinal ganglion cell (RGC) axon outgrowth
in vitro and in vivo (45). Besides, Xenopus spinal neuron
growth cone turning in response to a netrin-1 gradient is dependent
on PLC-γ and PI3K co-activation (46). In the previous studies exploring
the underlying neuroprotective effect of GDNF on Parkinson’s
disease, PLC-γ has also been recognized as one of the second
messengers of intracellular signaling pathways activated by the
GDNF-GFR α1-Ret system (19).
Taken together, the present study suggested that PLC-γ is involved
in the survival-promoting effect of GDNF on developing MNs.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (81070995, 31171290).
Abbreviations:
|
6-OHDA
|
6-hydroxydopamine
|
|
BDNF
|
brain-derived neurotrophic factor
|
|
BSA
|
bovine serum albumin
|
|
ChAT
|
choline acetyltransferase
|
|
CNS
|
central nervous system
|
|
DAB
|
diaminobenzidine
|
|
DMSO
|
dimethyl sulfoxide
|
|
GDNF
|
glial cell line-derived neurotrophic
factor
|
|
GFAP
|
glial fibrillary acidic protein
|
|
GFL
|
GDNF family of ligands
|
|
GFRα1–4
|
glycosylphosphatidylinositol-anchored
co-receptor
|
|
MAPK
|
mitogen-activated protein kinase
|
|
MN
|
motoneuron
|
|
NSE
|
neuron-specific enolase
|
|
p75NTR
|
75-kDa low-affinity neurotrophin
receptor
|
|
PBS
|
phosphate-buffered saline
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
PLC-γ
|
phosphoinositide phospholipase C-γ
|
|
RGC
|
retinal ganglion cell
|
|
TGF
|
transforming growth factor
|
|
U73122
|
1-[6-((17b-3-methoxyestra-1,3,5(10)-trien-17-yl)
amino)hexyl]-1H-pyrrole-2,5-dione
|
References
|
1
|
Pezeshki G, Franke B and Engele J: GDNF
elicits distinct immediate-early gene responses in cultured
cortical and mesencephalic neurons. J Neurosci Res. 71:478–484.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rangasamy SB, Soderstrom K, Bakay RA and
Kordower JH: Neurotrophic factor therapy for Parkinson’s disease.
Prog Brain Res. 184:237–264. 2010.
|
|
3
|
Houenou LJ, Oppenheim RW, Li L, Lo AC and
Prevette D: Regulation of spinal motoneuron survival by GDNF during
development and following injury. Cell Tissue Res. 286:219–223.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Oppenheim RW, Houenou LJ, Johnson JE, et
al: Developing motor neurons rescued from programmed and
axotomy-induced cell death by GDNF. Nature. 373:344–346. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rakowicz WP, Staples CS, Milbrandt J,
Brunstrom JE and Johnson EM Jr: Glial cell line-derived
neurotrophic factor promotes the survival of early postnatal spinal
motor neurons in the lateral and medial motor columns in slice
culture. J Neurosci. 22:3953–3962. 2002.
|
|
6
|
Chu TH and Wu W: Neurotrophic factor
treatment after spinal root avulsion injury. Cent Nerv Syst Agents
Med Chem. 9:40–55. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki M, McHugh J, Tork C, et al: GDNF
secreting human neural progenitor cells protect dying motor
neurons, but not their projection to muscle, in a rat model of
familial ALS. PLoS One. 2:e6892007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vyas A, Li Z, Aspalter M, et al: An in
vitro model of adult mammalian nerve repair. Exp Neurol.
223:112–118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takahashi M: The GDNF/RET signaling
pathway and human diseases. Cytokine Growth Factor Rev. 12:361–373.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee RH, Wong WL, Chan CH and Chan SY:
Differential effects of glial cell line-derived neurotrophic factor
and neurturin in RET/GFRalpha1-expressing cells. J Neurosci Res.
83:80–90. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshii A and Constantine-Paton M:
Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity,
and disease. Dev Neurobiol. 70:304–322. 2010.PubMed/NCBI
|
|
12
|
Sciarretta C, Fritzsch B, Beisel K, et al:
PLCgamma-activated signalling is essential for TrkB mediated
sensory neuron structural plasticity. BMC Dev Biol. 10:1032010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Arevalo JC and Wu SH: Neurotrophin
signaling: many exciting surprises! Cell Mol Life Sci.
63:1523–1537. 2006.PubMed/NCBI
|
|
14
|
Nakagawara A, Azar CG, Scavarda NJ and
Brodeur GM: Expression and function of TRK-B and BDNF in human
neuroblastomas. Mol Cell Biol. 14:759–767. 1994.PubMed/NCBI
|
|
15
|
Iwasaki Y, Ishikawa M, Okada N and Koizumi
S: Induction of a distinct morphology and signal transduction in
TrkB/PC12 cells by nerve growth factor and brain-derived
neurotrophic factor. J Neurochem. 68:927–934. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Du Y, Lercher LD, Zhou R and Dreyfus CF:
Mitogen-activated protein kinase pathway mediates effects of
brain-derived neurotrophic factor on differentiation of basal
forebrain oligodendrocytes. J Neurosci Res. 84:1692–1702. 2006.
View Article : Google Scholar
|
|
17
|
Rankin SL, Guy CS, Rahimtula M and Mearow
KM: Neurotrophin-induced upregulation of p75NTR via a protein
kinase C-delta-dependent mechanism. Brain Res. 1217:10–24. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giralt A, Rodrigo T, Martin ED, et al:
Brain-derived neurotrophic factor modulates the severity of
cognitive alterations induced by mutant huntingtin: involvement of
phospholipaseCgamma activity and glutamate receptor expression.
Neuroscience. 158:1234–1250. 2009. View Article : Google Scholar
|
|
19
|
Poteryaev D, Titievsky A, Sun YF, et al:
GDNF triggers a novel ret-independent Src kinase family-coupled
signaling via a GPI-linked GDNF receptor alpha1. FEBS Lett.
463:63–66. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou LH and Wu W: Survival of injured
spinal motoneurons in adult rat upon treatment with glial cell
line-derived neurotrophic factor at 2 weeks but not at 4 weeks
after root avulsion. J Neurotrauma. 23:920–927. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Li L, Yick LW, et al: GDNF and BDNF
alter the expression of neuronal NOS, c-Jun, and p75 and prevent
motoneuron death following spinal root avulsion in adult rats. J
Neurotrauma. 20:603–612. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watabe K, Ohashi T, Sakamoto T, et al:
Rescue of lesioned adult rat spinal motoneurons by adenoviral gene
transfer of glial cell line-derived neurotrophic factor. J Neurosci
Res. 60:511–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Windisch M, Gschanes A and Hutter-Paier B:
Neurotrophic activities and therapeutic experience with a brain
derived peptide preparation. J Neural Transm Suppl. 53:289–298.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Houenou LJ, D’Costa AP, Li L, et al:
Pigment epithelium-derived factor promotes the survival and
differentiation of developing spinal motor neurons. J Comp Neurol.
412:506–514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vandenberghe W, Van Den Bosch L and
Robberecht W: Tissue-type plasminogen activator is not required for
kainate-induced motoneuron death in vitro. Neuroreport.
9:2791–2796. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu W, Li Y and Schinco FP: Expression of
c-jun and neuronal nitric oxide synthase in rat spinal motoneurons
following axonal injury. Neurosci Lett. 179:157–161. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu X and An L: A serum- and
antioxidant-free primary culture model of mouse cortical neurons
for pharmacological screen and studies of neurotrophic and
neuroprotective agents. Cell Mol Neurobiol. 22:197–206. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li M, Wang L, Peng Y, Wang JC and Zhou LH:
Knockdown of the neuronal nitric oxide synthase gene retard the
development of the cerebellar granule neurons in vitro. Dev Dyn.
239:474–481. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou LH, Han S, Xie YY, Wang LL and Yao
ZB: Differences in c-jun and nNOS expression levels in motoneurons
following different kinds of axonal injury in adult rats. Brain
Cell Biol. 36:213–227. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang S, Polo-Parada L and Landmesser LT:
Characterization of rhythmic Ca2+ transients in early
embryonic chick motoneurons: Ca2+ sources and effects of
altered activation of transmitter receptors. J Neurosci.
29:15232–15244. 2009.
|
|
31
|
Chaudieu I and Privat A: Neuroprotection
of cultured foetal rat hippocampal cells against glucose
deprivation: are GABAergic neurons less vulnerable or more
sensitive to TCP protection? Eur J Neurosci. 11:2413–2421. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
D’Souza SD, Alinauskas KA and Antel JP:
Ciliary neurotrophic factor selectively protects human
oligodendrocytes from tumor necrosis factor-mediated injury. J
Neurosci Res. 43:289–298. 1996.PubMed/NCBI
|
|
33
|
Lukasiuk K and Pitkanen A:
GABA(A)-mediated toxicity of hippocampal neurons in vitro. J
Neurochem. 74:2445–2454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lindholm P, Voutilainen MH, Lauren J, et
al: Novel neurotrophic factor CDNF protects and rescues midbrain
dopamine neurons in vivo. Nature. 448:73–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bilak MM, Corse AM and Kuncl RW:
Additivity and potentiation of IGF-I and GDNF in the complete
rescue of postnatal motor neurons. Amyotroph Lateral Scler Other
Motor Neuron Disord. 2:83–91. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soler RM, Dolcet X, Encinas M, Egea J,
Bayascas JR and Comella JX: Receptors of the glial cell
line-derived neurotrophic factor family of neurotrophic factors
signal cell survival through the phosphatidylinositol 3-kinase
pathway in spinal cord motoneurons. J Neurosci. 19:9160–9169.
1999.
|
|
37
|
Beazely MA, Lim A, Li H, et al:
Platelet-derived growth factor selectively inhibits NR2B-containing
N-methyl-D-aspartate receptors in CA1 hippocampal neurons. J Biol
Chem. 284:8054–8063. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marsh HN and Palfrey HC: Neurotrophin-3
and brain-derived neurotrophic factor activate multiple signal
transduction events but are not survival factors for hippocampal
pyramidal neurons. J Neurochem. 67:952–963. 1996. View Article : Google Scholar
|
|
39
|
Song X, Wu B, Takata T, et al:
Neuroprotective effect of D-fructose-1,6-bisphosphate against
beta-amyloid induced neurotoxicity in rat hippocampal organotypic
slice culture: involvement of PLC and MEK/ERK signaling pathways.
Kobe J Med Sci. 51:73–83. 2005.
|
|
40
|
Zirrgiebel U, Ohga Y, Carter B, et al:
Characterization of TrkB receptor-mediated signaling pathways in
rat cerebellar granule neurons: involvement of protein kinase C in
neuronal survival. J Neurochem. 65:2241–2250. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nonomura T, Kubo T, Oka T, et al:
Signaling pathways and survival effects of BDNF and NT-3 on
cultured cerebellar granule cells. Brain Res Dev Brain Res.
97:42–50. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamada M, Numakawa T, Koshimizu H, et al:
Distinct usages of phospholipase C gamma and Shc in intracellular
signaling stimulated by neurotrophins. Brain Res. 955:183–190.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hayashi H, Campenot RB, Vance DE and Vance
JE: Protection of neurons from apoptosis by apolipoprotein
E-containing lipoproteins does not require lipoprotein uptake and
involves activation of phospholipase Cgamma1 and inhibition of
calcineurin. J Biol Chem. 284:29605–29613. 2009. View Article : Google Scholar
|
|
44
|
Kang HJ, Noh JS, Bae YS and Gwag BJ:
Calcium-dependent prevention of neuronal apoptosis by lithium ion:
essential role of phosphoinositide 3-kinase and phospholipase
Cgamma. Mol Pharmacol. 64:228–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lom B, Hopker V, McFarlane S, Bixby JL and
Holt CE: Fibroblast growth factor receptor signaling in
Xenopus retinal axon extension. J Neurobiol. 37:633–641.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ming G, Song H, Berninger B, Inagaki N,
Tessier-Lavigne M and Poo M: Phospholipase C-gamma and
phosphoinositide 3-kinase mediate cytoplasmic signaling in nerve
growth cone guidance. Neuron. 23:139–148. 1999. View Article : Google Scholar : PubMed/NCBI
|















