Introduction
Testicular germ cell tumors tend to affect young
males, representing the most common tumor in males aged from 20 to
40 years and the incidence has been on the increase over the last
decades (1). Bleomycin has been
approved by the FDA to be used alone or with other drugs as a
palliative treatment of testicular cancer. Bleomycin is used in
combination with etoposide and cisplatinum (BEP therapy) for the
treatment of adult and childhood testicular germ cell tumors. It is
known that drug combinations usually work with greater efficacy
compared with monotherapy since different drugs kill cancer cells
in different ways. In our study, we did not use BEP, but applied
bleomycin alone as our aim was to investigate the mechanism of
action of bleomycin. Bleomycin is an essential component of the
cisplatin-based chemotherapy regimens used effectively in the
treatment of testicular cancer (2). Bleomycin generates oxygen radicals
via its ferrous binding site, and induces the oxidative cleavage of
DNA strands and cancer cell apoptosis (3). Bleomycin induces a high level of
oxidative stress. This is due to the unique ability of bleomycin to
generate reactive oxygen species (ROS) in mitochondria. One of its
effects is breaking the DNA double helix via the production of free
radicals, a process that is oxygen and iron-dependent. Bleomycin
forms complexes with iron that reduce molecular oxygen to
superoxide and hydroxyl radicals which cause single- and
double-stranded breaks in DNA. Moreover, these ROS induce lipid
peroxidation, carbohydrate oxidation and alterations in
prostaglandin synthesis and degradation.
Numerous in vitro studies (4–6) have
demonstrated that a wide range of anticancer agents induce
programmed cell death (apoptosis) in malignant cells by generating
ROS which is an important therapeutic interventional approach in
cancer. Oxidative stress has been shown to decrease the
LD50 (lethal dose that kills 50% of cells) of several
types of antineoplastic agents and induce cancer cell apoptosis.
ROS are essential for life due to their role in numerous vital
processes, including normal mitochondrial metabolism, signal
transduction and the bactericidal activity of phagocytes. These
molecules are formed in vivo via oxidation-reduction
reactions. ROS includes free radicals, such as hydroxyl and
superoxide radicals, and non-radicals, including
H2O2 and singlet oxygen (7). Hydrogen peroxide yields the highly
toxic hydroxyl radical (•OH) in the presence of reduced
iron or copper via Fenton or Haber-Weiss reactions. Hydrogen
peroxide easily diffuses into and out of the cells, and modulates
cell proliferation, signal transduction pathways, gene expression
and induces DNA damage, apoptosis and necrosis (8,9).
There is an intense debate on the concurrent use of
antioxidants during conventional cancer treatments. This argument
is based on the fact that some chemotherapy drugs generate ROS
which may kill cancer cells by inducing apoptosis. The induction of
apoptosis via ROS is potentially an alternative mechanism for the
cytotoxic effect of chemotherapeutic agents. It has been suggested
that antioxidants prevent cancer cell death from ROS by inhibiting
ROS and preventing ROS-induced apoptosis (7). Studies in the literature
investigating the effects of various antioxidants on ROS-induced
apoptosis in cancer are available (10,11).
So far, only three antioxidants, NAC with cisplatinum and
doxorubicin, tangeretin with tamoxifen, and β-carotene with
5-fluorouracil have been shown to decrease the effectiveness of
conventional cancer therapy in vivo(12,13).
Curcumin (diferuloylmethane) is the chief component
of the spice turmeric and is isolated from Curcuma longa.
Curcumin is responsible for the yellow color of the spice as well
as the majority of turmeric’s therapeutic effects (14). The effects of curcumin have been
investigated in other cancer cell types, but not in germ cell
tumors. Although curcumin has been demonstrated to have anticancer
activities both in vitro in numerous cancer cell lines and
in vivo models, no study has been performed investigating
the effects of curcumin on oxidative stress in testicular germ cell
tumors. For this reason, we studied effects of curcumin on
oxidative stress in NTera-2 and NCCIT testicular cancer cells
(intrinsic) incubated with curcumin alone or in combination with
bleomycin, and compared these results with oxidative stress
generated by incubation with H2O2. We
determined the levels of oxidative stress markers including protein
carbonyl content, thiobarbituric acid reactive substances (TBARS),
glutathione (GSH), 8-isoprostane, lipid hydroperoxide (LPO) levels
and total antioxidant capacity in two testicular cancer cell lines
incubated with curcumin, bleomycin, bleomycin+curcumin,
H2O2 and
H2O2+curcumin.
Materials and methods
Cell lines
NTera-2 and NCCIT cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). NTera-2 and
NCCIT cells were grown to confluence at 37°C in a humidified
atmosphere containing 5% CO2 in air in DMEM and RPMI
medium, respectively, supplemented with 10% fetal bovine serum, 100
IU/ml penicilin and 10 μg/ml streptomycin (Invitrogen, Carlsbad,
CA, USA). Results of our previous experiments (15), showed the LD50 of
H2O2 (Sigma, St. Louis, MO, USA) on NCCIT
cell viability to be 35 μM, for bleomycin (Nippon Kayaku,
Co., Ltd., Tokyo, Japan) as 120 μg/ml and for curcumin (Sigma) as 5
μM. Moreover, we determined LD50 of curcumin on NTera-2
cell viability as 20 μM, for bleomycin as 20 μg/ml, and for
H2O2 as 400 μM (15). We applied these doses in the
incubations.
8-Isoprostane assay
As a measure of cellular oxidation, the
8-epi-prostaglandin F2α level was measured in 100 μl
samples of cell culture media using an enzyme immunoassay kit (Cat.
#516351 Cayman Chemical, Ann Arbor, MI, USA) according to the
manufacturer’s instructions. A standard curve utilizing authentic
8-epi-prostaglandin F2α standard was prepared and values
for 8-epi-prostaglandin F2α were reported as pg/ml of
media.
TBARS assay
Lipid peroxidation was determined measuring TBARS
using the TBARS assay kit (Cat. #10009055 Cayman Chemical),
according to the manufacturer’s instructions. Cell suspensions were
centrifuged at 1,000 rpm for 5 min and washed twice with
phosphate-buffered saline (PBS). Supernatants were discarded and
cell pellets were resuspended in 1 ml PBS and sonicated using an
ultrasonic processor three times for 5-sec intervals at the 40 V
setting over ice. TBARS levels are expressed as nmol/ml.
GSH assay
Cells were scraped and collected by centrifugation.
The cell pellet was resuspended in 1 ml of PBS containing 1 mM
ethylenediaminetetraacetic acid (EDTA) and sonicated using an
ultrasonic processor three times for 5-sec intervals at the 40 V
setting over ice. The supernatant was deproteinized using 10%
metaphosphoric acid (Sigma) and collected to determine total GSH
levels according to the manufacturer’s instructions (Cat. #703002
Cayman Chemical). The total GSH levels were expressed as nmol/mg of
protein.
Protein carbonyl assay
Protein-bound carbonyl levels were measured using a
protein carbonyl assay kit (Cat. #1005020 Cayman Chemical). The
method was based on the covalent reaction of the carbonylated
protein side chain with 2,4-dinitrophenylhydrazine (DNPH) and
detection of the protein-hydrazone product at an absorbance of 370
nm. The results were calculated using the extinction coefficient of
22 M−1cm−1 for aliphatic hydrazones and were
expressed as a nmol/mg protein.
Total antioxidant capacity assay
The total antioxidant status in cell culture lysates
was determined using the commercial total antioxidant assay kit
(Cat. #709001 Cayman Chemical) according to the manufacturer’s
instructions. The assay relies on the ability of antioxidants
present in the samples to inhibit the oxidation of ABTS
[2,2′-azino-di-(3-ethylbenzthiazoline sulfonate)] to
ABTS•+. The amount of ABTS•+ produced is
monitored by reading the absorbance at 405 nm. Total antioxidant
capacity levels were expressed in mM.
LPO assay
The LPO assay measures hydroperoxides in isolated
lipid-phase of samples directly following ferrous ion reduction.
This assay was performed using the LPO kit (Cat. #705003 Cayman
Chemical Company). LPO levels were expressed in nM.
Statistical analysis
Data were presented as the mean ± standard error.
Statistical analysis was performed using SPSS packed program
version 10 (SPSS Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant result. Comparison of the
non-parametric data among the groups was performed using the
Mann-Whitney U test.
Results
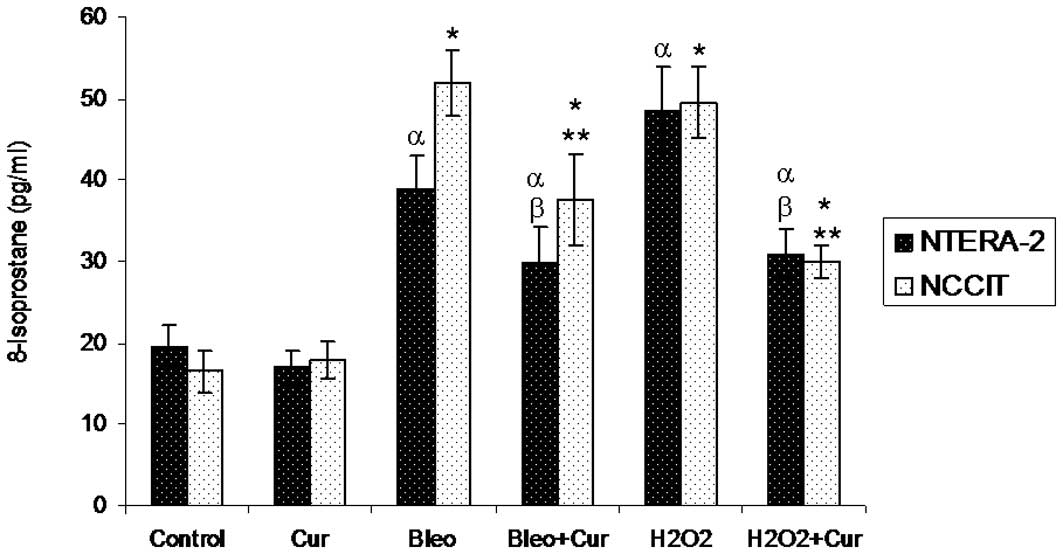
8-Isoprostane levels
Following incubation with curcumin, bleomycin,
bleomycin+curcumin, H2O2 or
H2O2+curcumin for 72 h, cellular
8-isoPGF2α levels were measured in NTera-2 and NCCIT
cells. Incubation with bleomycin or H2O2
significantly increased 8-isoprostane levels in the two cell lines
compared with the control cells and cells incubated with curcumin
alone. Co-incubation of the two cell lines with bleomycin+curcumin
or H2O2+curcumin decreased 8-isoprostane
levels significantly compared with the cells incubated with
bleomycin or H2O2 alone, respectively
(Fig. 1).
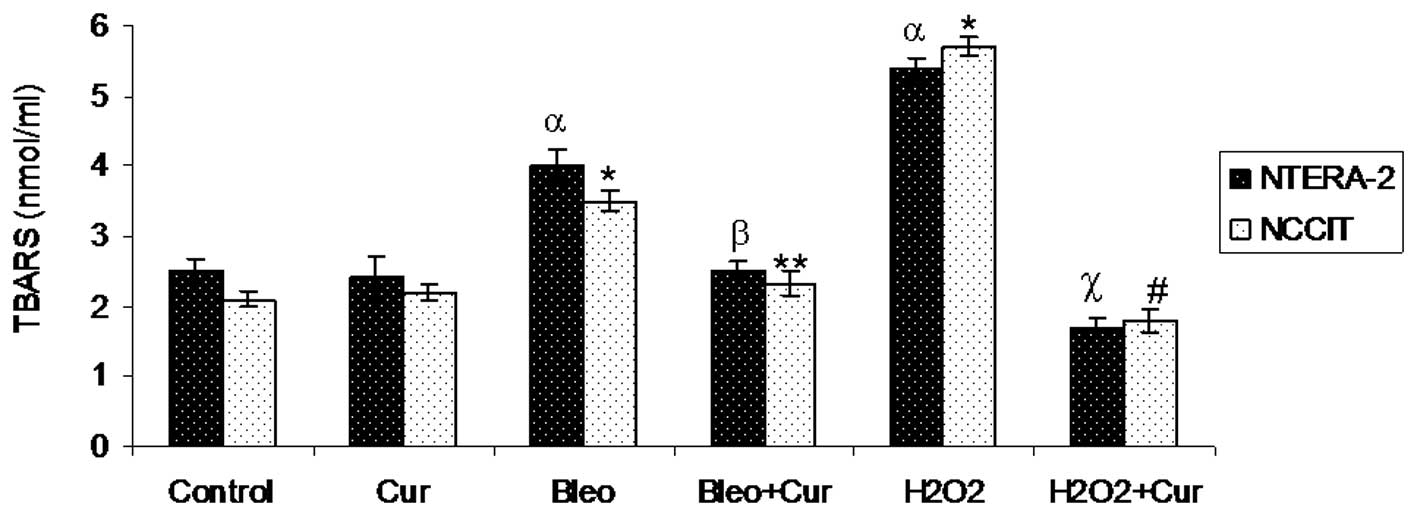
TBARS levels
TBARS levels were measured in NTera-2 and NCCIT
cells following incubation with curcumin, bleomycin,
bleomycin+curcumin, H2O2 or
H2O2+curcumin for 72 h. TBARS levels were
significantly increased in bleomycin and
H2O2-treated groups when compared with the
control cells. No significant difference was observed in TBARS
levels measured in cells incubated with curcumin when compared with
the control cells. Co-incubation of cells treated with
bleomycin+curcumin or H2O2+curcumin
significantly reduced TBARS levels compared with the NTera-2 and
NCCIT cells incubated with bleomycin or H2O2
alone, respectively (Fig. 2).
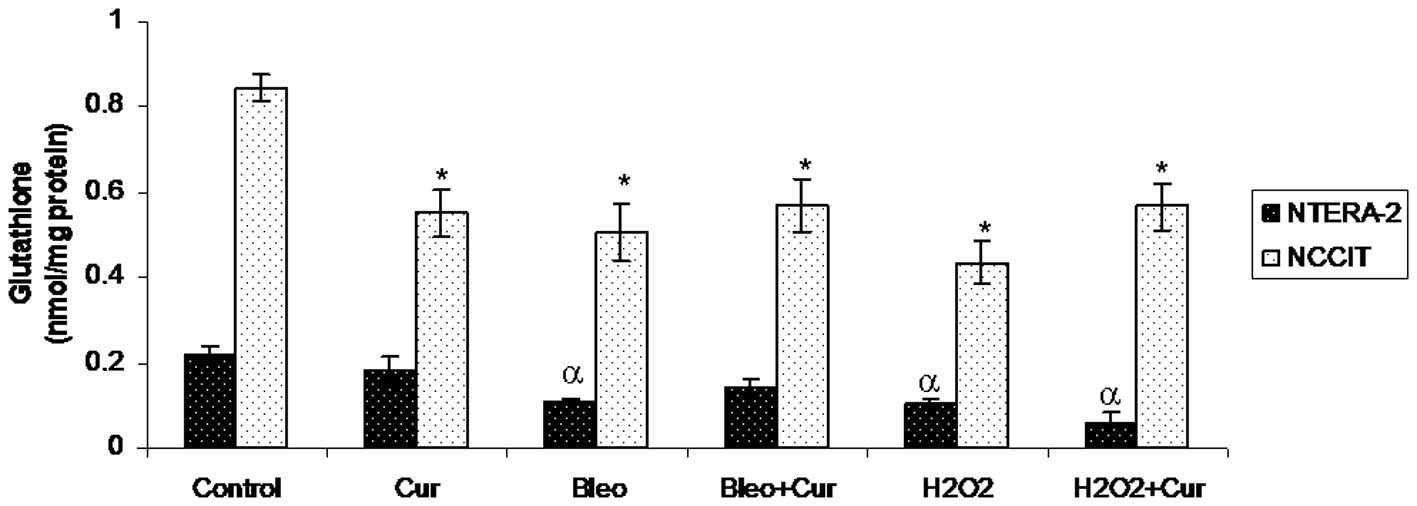
GSH levels
Cellular GSH levels were measured in NTera-2 and
NCCIT cells following incubation with curcumin, bleomycin,
bleomycin+curcumin, H2O2 or
H2O2+curcumin for 72 h. GSH levels were not
significantly different in NTera-2 cells incubated with curcumin
compared with control NTera-2 cells. Bleomycin,
H2O2 or H2O2+curcumin
significantly reduced GSH levels in NTera-2 cells compared with the
control NTera-2 cells. Co-incubation with curcumin and bleomycin
did not significantly increase GSH levels in NTera-2 cells compared
with the NTera-2 cells incubated with bleomycin alone. GSH levels
were significantly lower in NCCIT cells incubated with curcumin,
bleomycin, bleomycin+curcumin, H2O2 or
H2O2+curcumin compared with the control NCCIT
cells (Fig. 3).
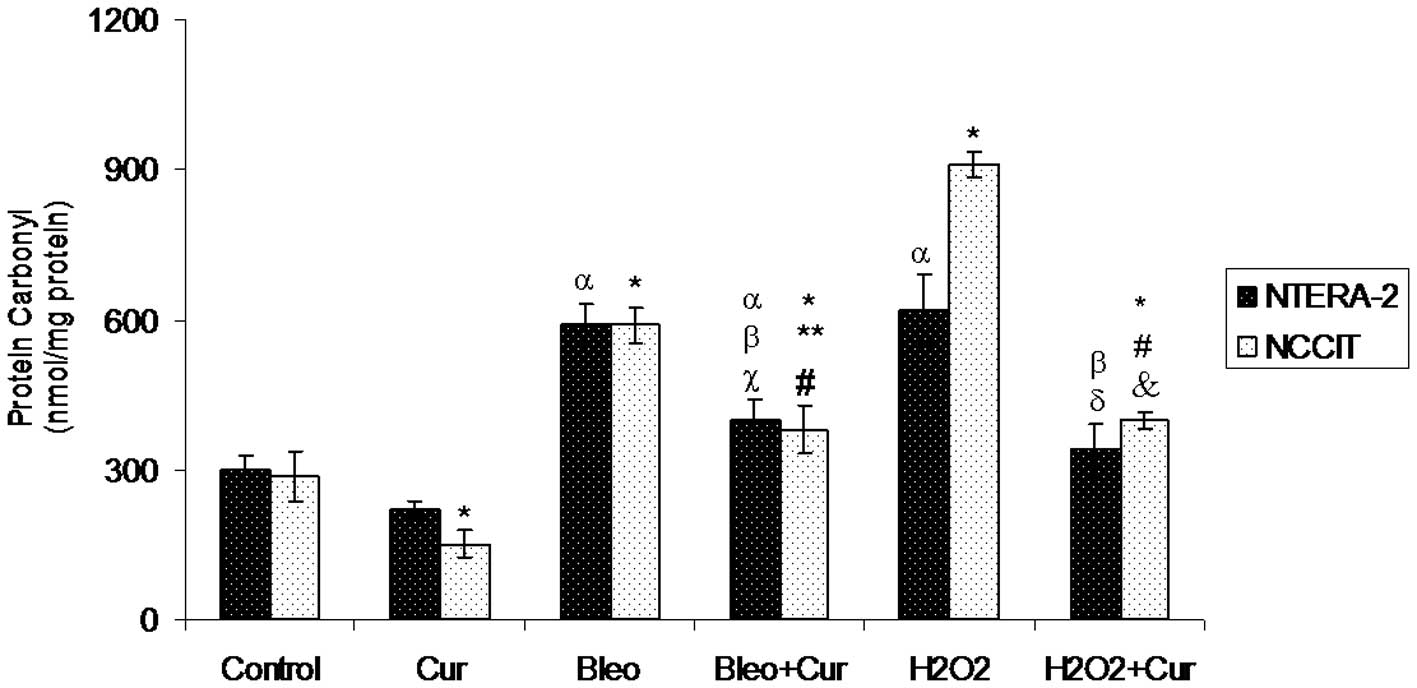
Protein carbonyl levels
Protein carbonyl content was measured in NTera-2 and
NCCIT cells following incubation with curcumin, bleomycin,
bleomycin+curcumin, H2O2 or
H2O2+curcumin for 72 h. Curcumin decreased
the protein carbonyl level significantly in NCCIT cells, but not
significantly in NTera-2 cells compared with the control cells and
other cell groups. The lowest significant protein carbonyl level
was found in NCCIT cells incubated with curcumin. The protein
carbonyl level increased in the two cell lines incubated with
bleomycin or H2O2. The protein carbonyl
content was double (600 nmol/mg) in NTera-2 cells incubated with
bleomycin compared with the level (300 nmol/mg) in control NTera-2
cells. Compared with control NCCIT cells, NCCIT cells incubated
with H2O2 showed a 3-fold increase in protein
carbonyl content. Co-incubation with curcumin and bleomycin or
curcumin and H2O2 significantly decreased
protein carbonyl content in the two cell lines incubated with
bleomycin or H2O2 alone, respectively
(Fig. 4).
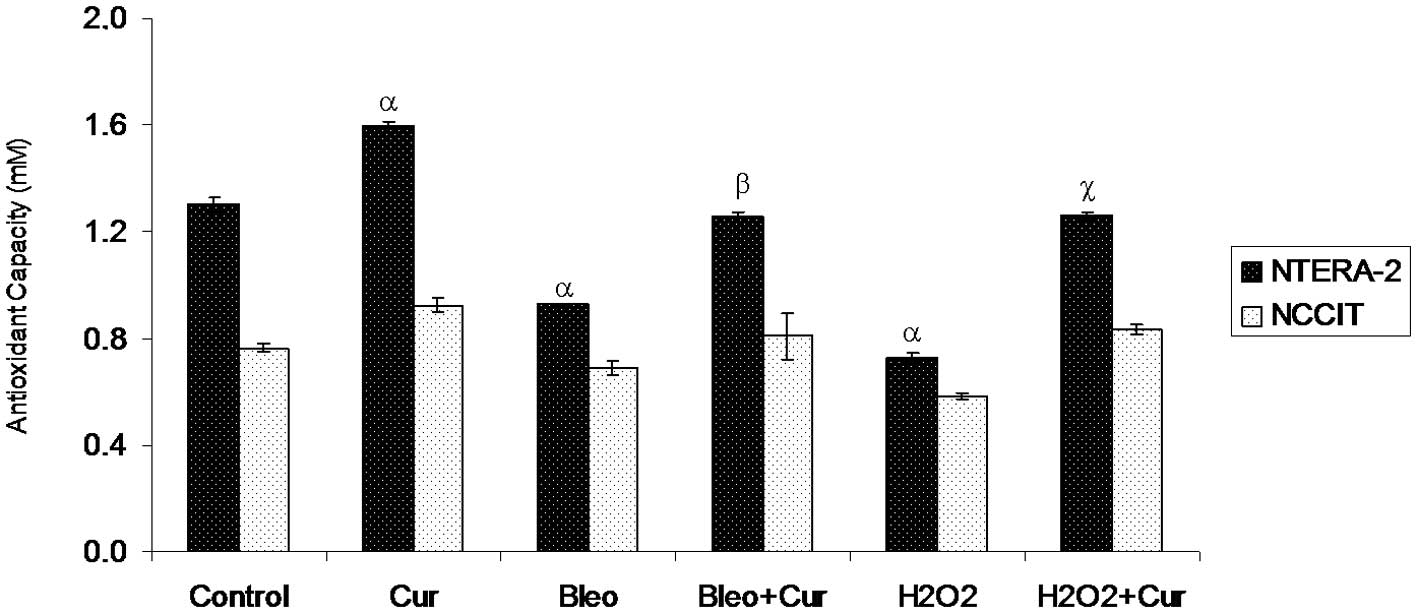
Antioxidant capacity levels
Antioxidant capacity increased significantly in
NTera-2 cells incubated with curcumin in comparison with the
untreated NTera-2 cells. The highest significant antioxidant
capacity level was found in NTera-2 cells incubated with curcumin.
By contrast, NTera-2 cells incubated with bleomycin or
H2O2 exhibited a significant decrease in
antioxidant levels compared with the control NTera-2 cells.
However, co-incubation of NTera-2 cells with curcumin and bleomycin
or curcumin and H2O2 significantly increased
antioxidant levels compared with NTera-2 cells incubated with
bleomycin or H2O2 alone, respectively. No
significant difference was observed in the antioxidant levels in
the NCCIT cells incubated with different agents compared with the
control NCCIT cells (Fig. 5).
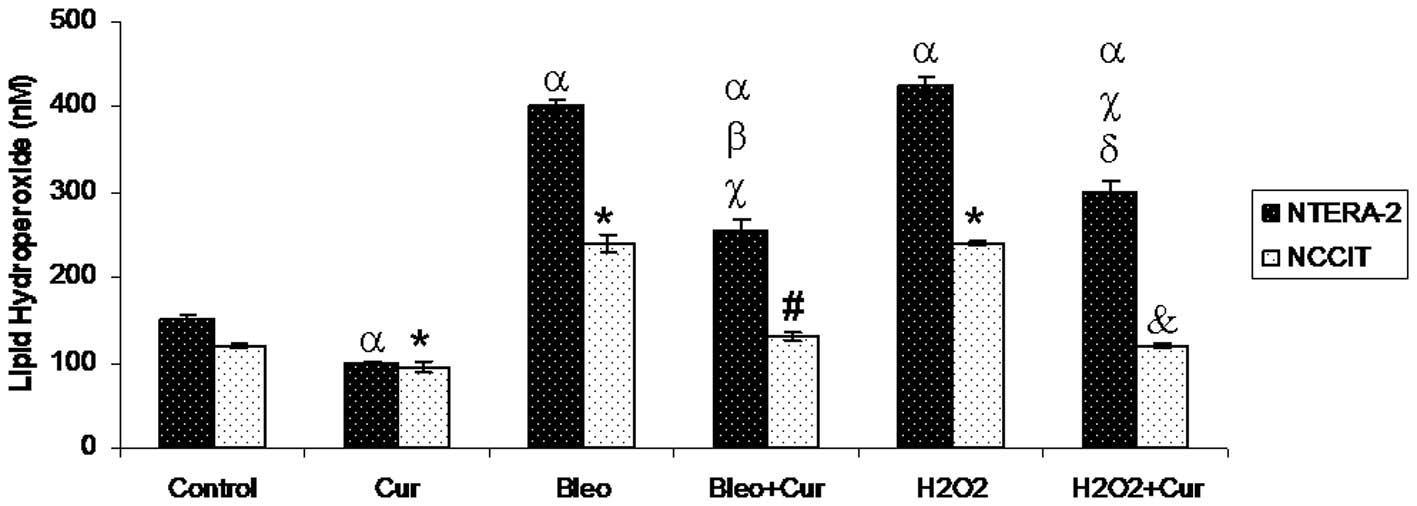
LPO levels
Curcumin treatment significantly reduced LPO levels
in the two cell lines compared with the control cells. Incubation
with bleomycin or H2O2 in the two cell lines
caused a significant increase in LPO levels compared with the
control cells. However, co-incubation with curcumin and bleomycin
or curcumin and H2O2 significantly decreased
LPO levels in the two cells compared with the cells incubated with
bleomycin or H2O2 alone, respectively
(Fig. 6).
Discussion
Curcumin is currently being evaluated as a potential
chemotherapeutic agent in several clinical trials (16,17).
Animal studies have shown that curcumin prevents carcinogenesis in
the colon (18) and breast
(19). Curcumin exhibits potent
in vitro antiproliferative and apoptosis-inducing activities
in a range of human cancer cell lines, including those derived from
cancers of the prostate, breast, ovary and colon (20–22),
but no study has been carried out with regard to the effects of
curcumin on oxidative stress in testicular cancer cells.
We used a curcumin concentration in our experiments
relevant to the antioxidant (curcumin) uptake concentration in
humans. There are conflicting studies in the literature with regard
to the oral intake of curcumin and its serum and urine
concentrations. Evidence suggests that orally administered curcumin
accumulates in gastrointestinal tissues. For instance, when
colorectal cancer patients were administered 3.6 g/day of curcumin
orally for seven days prior to surgery, curcumin was detected in
malignant and normal colorectal tissue (23). By contrast, curcumin was not
detected in the liver tissue of patients with liver metastases of
colorectal cancer following the same oral dose of curcumin
(24), suggesting that oral
curcumin administration may not effectively deliver curcumin to
tissues outside the gastrointestinal tract. Results of a clinical
study (25) have shown that no
curcumin was detected in the serum of participants administered
with 500; 1,000; 2,000; 4,000; 6,000 or 8,000 mg curcumin. The
presence of curcumin was only detected in two subjects (one
receiving 10,000 mg and one receiving 12,000 mg). No plasma
concentrations of curcumin were detected in the remaining subjects
receiving 10,000 or 12,000 mg dose levels. By contrast, in a
clinical trial conducted in Taiwan, the serum concentration of
curcumin was usually found to peak at 1–2 h following oral intake
of curcumin and gradually declined within 12 h (26). The average peak serum
concentrations following the administration of 4,000; 6,000 and
8,000 mg curcumin were 0.51±0.11, 0.63±0.06 and 1.77±1.87 μM,
respectively. Urinary excretion of curcumin was undetectable. We
incubated two types of testicular cancer cells with various
concentrations of curcumin and selected the doses of 20 μM for
NTera-2 cells and 5 μM for NCCIT cells. These doses were higher
than the doses used in the study in Taiwan (26). The reason for using higher curcumin
doses in our study was that other investigators (25) did not detect curcumin in serum with
the same doses used by the investigators in Taiwan (26). To eliminate these conflicting
results, we selected effective curcumin concentrations based on our
previous experimental results (15).
Our data revealed that bleomycin and
H2O2 significantly increased 8-isoprostane,
protein carbonyl, TBARS and LPO levels in the two cell types and
significantly decreased antioxidant capacity and GSH levels in
NTera-2 cells. Incubation with bleomycin or
H2O2 did not affect antioxidant capacity, but
significantly decreased GSH levels in NCCIT cells. Previous studies
have reported that bleomycin catalyzes the formation of ROS with
ultimate progression to lipid peroxidation (27). This effect is likely a secondary
event, following bleomycin-induced increase in free radical
generation. Quantification of lipid peroxidation is essential to
assess the role of oxidative injury in cancer. The increase in
lipid peroxidation via bleomycin and the suppressive effect of
curcumin were demonstrated by Venkatesan et al in rat lung
injury (28). This is the first
study showing the suppressive effect of curcumin on bleomycin and
H2O2-induced increases in LPO levels in
testicular cancer cells. 8-Isoprostane is a reliable marker of
oxidative stress. Bleomycin and H2O2
significantly increased 8-isoprostane levels in NTera-2 and NCCIT
cells. Increases in 8-isoPGF2α levels as a function of
bleomycin and H2O2 exposure have not been
previously reported in testicular cancer cells. Co-incubation with
curcumin significantly decreased 8-isoprostane levels in the two
cell lines incubated with bleomycin and H2O2.
Similar to our findings, curcumin was reported to induce a decrease
in 8-iso-prostaglandin levels following exposure to radiation in
breast cancer cells (29). The
measurement of TBARS is a well-established method for screening and
monitoring lipid peroxidation. In the present study, we
demonstrated that bleomycin and H2O2
increased TBARS levels in the two cell lines compared with the
control cells. Co-incubation of curcumin with bleomycin or
H2O2 decreased TBARS levels compared with the
cells incubated with bleomycin or H2O2 alone.
Increased TBARS formation was reported in human hepatoma G2 cells
following exposure to high levels of curcumin. By contrast,
exposure to low curcumin concentration did not cause any increase
in TBARS levels, similar to our findings (30).
The most general indicator and by far the most
commonly used marker of protein oxidation is protein carbonyl
content, based on the fact that free radicals convert amino acid
side chains to carbonyl moieties in vitro. In our study, we
used the method of protein 2,4-DNPH post labeling, originally
introduced by Levine et al(31), for isolated proteins as a useful
monitor of bleomycin and H2O2-mediated
oxidative protein damage. Our data clearly demonstrate that
curcumin treatment inhibits bleomycin and
H2O2-induced protein oxidation as monitored
by measuring the formation of protein reactive carbonyl contents in
testicular cancer cells and provides further indication that
curcumin protects cancer cells from oxidative stress via its
antioxidant property. Bleomycin or
H2O2-mediated increases in protein carbonyl
content likely indicates a predisposition of testicular cancer
cells to cell death. Biswas et al showed that curcumin did
not have a significant effect on protein carbonyl content in
arsenic carcinogenicity in humans (32). By contrast, Dance-Barnes et
al reported that curcumin increased oxidative damage in mouse
lung tissue by inducing protein carbonylation (33). In another study performed by Biswas
et al, curcumin treatment reduced ROS generation, lipid
peroxidation and protein carbonyl content, which were elevated by
arsenic in Swiss albino mice (34). Bleomycin and
H2O2 significantly decreased GSH levels in
NTera-2 cells. Bleomycin, H2O2, curcumin,
bleomycin+curcumin or H2O2+curcumin led to a
decrease in GSH levels in NCCIT cells. The curcumin-induced
depletion of GSH was demonstrated in previous studies (35,36).
Hilchie et al reported that the curcumin treatment of
prostate cancer cells caused depletion of GSH. The authors reported
that GSH depletion was not due to curcumin-induced ROS production
(35). Curcumin-GSH interactions
were demonstrated in another study performed by Awasthi et
al(37). Previously, it was
found that GSH S-transferase catalyzes a reaction between curcumin
and GSH in Caco-2 colon cancer cells, leading to the formation of
monoglutathionyl curcumin conjugates (38). The antioxidant capacity is a
measure of total protective antioxidant mechanisms both for
preventing the production of free radicals and for repairing
oxidative damage (39). Curcumin
has been shown to have beneficial effects on the antioxidant
defense system, scavenge free radicals and/or prevent lipid
peroxidation and it is at least 10 times more active as an
antioxidant than vitamin E. In our study, bleomycin and
H2O2 decreased total antioxidant capacity in
the two testicular cancer cell lines but incubation with curcumin
enhanced total antioxidant capacity. Anti-carcinogenic action of
curcumin by activation of antioxidant defence system was reported
in animal models and cell lines (29,40).
This is the first study showing that curcumin has an inhibitory
effect on bleomycin and H2O2-induced
oxidative stress.
The probability that antioxidants interfere with the
conventional cancer treatments, which are designed to prevent the
mortality of cancer patients, is a complex issue. Although numerous
chemotherapy drugs induce the formation of ROS, their anticancer
effects do not, in general, depend on the formation of these free
radicals. Antioxidant supplementation may in certain circumstances
aid the prevention of free-radical-induced side effects without
inhibiting the positive effects of the chemotherapy and provide a
safe and effective means of enhancing the response to cancer
chemotherapy. The cancer cells should divide rapidly for the
cytotoxic effect of anticancer agents. Excess ROS in cancer cells
slows or arrests cell growth and interferes with the effectiveness
of chemotherapy since anticancer drugs are effective only when
there is rapid cell proliferation. Antioxidant supplementation
during chemotherapy may overcome the growth-inhibiting effects of
oxidative stress and maintain responsiveness to anti-neoplastic
agents (12,13). Our findings with curcumin supports
these hypotheses. We found that curcumin decreases oxidative stress
in germ cells induced by bleomycin, however, this does not mean
that curcumin decreases the chemotherapeutic effect of bleomycin.
By decreasing oxidative stress, curcumin may increase the response
to bleomycin since bleomycin does not exert its chemotherapeutic
action only by generating oxidative stress. Our results demonstrate
the precise molecular pathways of the inhibitory effect of curcumin
on oxidative stress in human testicular cancer cells induced by
bleomycin. Although curcumin decreased oxidative stress in germ
cells induced by bleomycin, this does not mean that curcumin
decreases the chemotherapeutic effect of bleomycin. By contrast, by
decreasing oxidative stress, curcumin may increase the response to
bleomycin. It can be concluded that curcumin has certain inhibitory
effects on oxidative stress and its concomitant use with bleomycin
should be followed closely during the treatment of testicular
cancer.
Acknowledgements
This study was supported by TUBITAK, Turkey
(COST-CM0603-15; 107S291) and Akdeniz University.
References
|
1
|
Chieffi P: New prognostic markers and
potential therapeutic targets in human testicular germ cell tumors.
Curr Med Chem. 18:5033–5040. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Wit R, Stoter G, Kaye SB, et al:
Importance of bleomycin in combination chemotherapy for
good-prognosis testicular nonseminoma: a randomized study of the
European Organization for Research and Treatment of Cancer
Genitourinary Tract Cancer Cooperative Group. J Clin Oncol.
15:1837–1843. 1997.
|
|
3
|
Burger RM, Peisach J and Horwitz SB:
Activated bleomycin. A transient complex of drug, iron, and oxygen
that degrades DNA. J Biol Chem. 256:11636–11644. 1981.PubMed/NCBI
|
|
4
|
Yen CY, Chiu CC, Haung RW, et al:
Antiproliferative effects of goniothalamin on Ca9-22 oral cancer
cells through apoptosis, DNA damage and ROS induction. Mutat Res.
747:253–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ullah MF, Ahmad A, Zubair H, et al: Soy
isoflavone genistein induces cell death in breast cancer cells
through mobilization of endogenous copper ions and generation of
reactive oxygen species. Mol Nutr Food Res. 55:553–559. 2011.
View Article : Google Scholar
|
|
6
|
Yu JS and Kim AK: Wogonin induces
apoptosis by activation of ERK and p38 MAPKs signaling pathways and
generation of reactive oxygen species in human breast cancer cells.
Mol Cells. 31:327–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conklin KA: Cancer chemotherapy and
antioxidants. J Nutr. 134:3201S–3204S. 2004.PubMed/NCBI
|
|
8
|
Nakamura H, Nakamura K and Yodoi J: Redox
regulation of cellular activation. Annu Rev Immunol. 15:351–369.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cantoni O, Cattabeni F, Stocchi V, Meyn
RE, Cerutti P and Murray D: Hydrogen peroxide insult in cultured
mammalian cells: relationships between DNA single-strand breakage,
poly(ADP-ribose) metabolism and cell killing. Biochim Biophys Acta.
1014:1–7. 1989. View Article : Google Scholar
|
|
10
|
Kim KY, Yu SN, Lee SY, et al:
Salinomycin-induced apoptosis of human prostate cancer cells due to
accumulated reactive oxygen species and mitochondrial membrane
depolarization. Biochem Biophys Res Commun. 413:80–86. 2011.
View Article : Google Scholar
|
|
11
|
Bejarano I, Espino J, Marchena AM, et al:
Melatonin enhances hydrogen peroxide-induced apoptosis in human
promyelocytic leukaemia HL-60 cells. Mol Cell Biochem. 353:167–176.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ozben T: Oxidative stress and apoptosis:
impact on cancer therapy. J Pharm Sci. 96:2181–2196. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akbas HS, Timur M and Ozben T: Concurrent
use of antioxidants in cancer therapy: an update. Expert Rev Clin
Immunol. 2:931–939. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duvoix A, Blasius R, Delhalle S, et al:
Chemopreventive and therapeutic effects of curcumin. Cancer Lett.
223:181–190. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cort A, Timur M, Ozdemir E, Kucuksayan E
and Ozben T: Synergistic anticancer activity of curcumin and
bleomycin: An in vitro study using human malignant
testicular germ cells. Mol Med Rep. 5:1481–1486. 2012.PubMed/NCBI
|
|
16
|
Thomasset SC, Berry DP, Garcea G, Marczylo
T, Steward WP and Gescher AJ: Dietary polyphenolic phytochemicals -
promising cancer chemopreventive agents in humans? A review of
their clinical properties. Int J Cancer. 120:451–458. 2007.
View Article : Google Scholar
|
|
17
|
Strimpakos AS and Sharma RA: Curcumin:
preventive and therapeutic properties in laboratory studies and
clinical trials. Antioxid Redox Signal. 10:511–545. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu G, Ren G, Xu X, et al: Combination of
curcumin and green tea catechins prevents dimethylhydrazine-induced
colon carcinogenesis. Food Chem Toxicol. 48:390–395. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bachmeier B, Nerlich AG, Iancu CM, et al:
The chemopreventive polyphenol Curcumin prevents hematogenous
breast cancer metastases in immunodeficient mice. Cell Physiol
Biochem. 19:137–152. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Watson JL, Hill R, Yaffe PB, et al:
Curcumin causes superoxide anion production and p53-independent
apoptosis in human colon cancer cells. Cancer Lett. 297:1–8. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Watson JL, Greenshields A, Hill R, et al:
Curcumin-induced apoptosis in ovarian carcinoma cells is
p53-independent and involves p38 mitogen-activated protein kinase
activation and downregulation of Bcl-2 and survivin expression and
Akt signaling. Mol Carcinog. 49:13–24. 2010.
|
|
22
|
Aggarwal BB, Banerjee S, Bharadwaj U, Sung
B, Shishodia S and Sethi G: Curcumin induces the degradation of
cyclin E expression through ubiquitin-dependent pathway and
up-regulates cyclin-dependent kinase inhibitors p21 and p27 in
multiple human tumor cell lines. Biochem Pharmacol. 73:1024–1032.
2007. View Article : Google Scholar
|
|
23
|
Garcea G, Berry DP, Jones DJ, et al:
Consumption of the putative chemopreventive agent curcumin by
cancer patients: assessment of curcumin levels in the colorectum
and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers
Prev. 14:120–125. 2005.
|
|
24
|
Garcea G, Jones DJ, Singh R, et al:
Detection of curcumin and its metabolites in hepatic tissue and
portal blood of patients following oral administration. Br J
Cancer. 90:1011–1015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lao CD, Ruffin MT IV, Normolle D, et al:
Dose escalation of a curcuminoid formulation. BMC Complement Altern
Med. 6:102006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng AL, Hsu CH, Lin JK, et al: Phase I
clinical trial of curcumin, a chemopreventive agent, in patients
with high-risk or pre-malignant lesions. Anticancer Res.
21:2895–2900. 2001.PubMed/NCBI
|
|
27
|
Ali EN and Mansour SZ: Boswellic acids
extract attenuates pulmonary fibrosis induced by bleomycin and
oxidative stress from gamma irradiation in rats. Chin Med.
6:362011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Venkatesan N, Punithavathi V and
Chandrakasan G: Curcumin protects bleomycin-induced lung injury in
rats. Life Sci. 61:PL51–PL58. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calaf GM, Echiburú-Chau C, Roy D, Chai Y,
Wen G and Balajee AS: Protective role of curcumin in oxidative
stress of breast cells. Oncol Rep. 26:1029–1035. 2011.PubMed/NCBI
|
|
30
|
Cao J, Jia L, Zhou HM, Liu Y and Zhong LF:
Mitochondrial and nuclear DNA damage induced by curcumin in human
hepatoma G2 cells. Toxicol Sci. 91:476–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Levine RL, Williams JA, Stadtman ER and
Shacter E: Carbonyl assays for determination of oxidatively
modified proteins. Methods Enzymol. 233:346–357. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Biswas J, Sinha D, Mukherjee S, Roy S,
Siddiqi M and Roy M: Curcumin protects DNA damage in a chronically
arsenic-exposed population of West Bengal. Hum Exp Toxicol.
29:513–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dance-Barnes ST, Kock ND, Moore JE, et al:
Lung tumor promotion by curcumin. Carcinogenesis. 30:1016–1023.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Biswas J, Roy S, Mukherjee S, Sinha D and
Roy M: Indian spice curcumin may be an effective strategy to combat
the genotoxicity of arsenic in Swiss albino mice. Asian Pac J
Cancer Prev. 11:239–247. 2010.PubMed/NCBI
|
|
35
|
Hilchie AL, Furlong SJ, Sutton K, et al:
Curcumin-induced apoptosis in PC3 prostate carcinoma cells is
caspase-independent and involves cellular ceramide accumulation and
damage to mitochondria. Nutr Cancer. 62:379–389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Atsumi T, Tonosaki K and Fujisawa S:
Comparative cytotoxicity and ROS generation by curcumin and
tetrahydrocurcumin following visible-light irradiation or treatment
with horseradish peroxidase. Anticancer Res. 27:363–371. 2007.
|
|
37
|
Awasthi S, Pandya U, Singhal SS, et al:
Curcumin-glutathione interactions and the role of human glutathione
S-transferase P1-1. Chem Biol Interact. 128:19–38. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Usta M, Wortelboer HM, Vervoort J, et al:
Human glutathione S-transferase-mediated glutathione conjugation of
curcumin and efflux of these conjugates in Caco-2 cells. Chem Res
Toxicol. 20:1895–1902. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Koracevic D, Koracevic G, Djordjevic V,
Andrejevic S and Cosic V: Method for the measurement of antioxidant
activity in human fluids. J Clin Pathol. 54:356–361. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Das L and Vinayak M: Anti-carcinogenic
action of curcumin by activation of antioxidant defence system and
inhibition of NF-κB signalling in lymphoma-bearing mice. Biosci
Rep. 32:161–170. 2012.PubMed/NCBI
|