Introduction
Rotenone is a natural hydrophobic pesticide derived
from the roots and barks of the Derris and
Lonchorcarpus species. Rotenone has been used as a botanical
insecticide for over 150 years to control crop pests (1). The pesticidal activity of rotenone is
attributed to irreversible binding and inactivation of NADH
ubiquinone reductase (complex I) in the mitochondrial electron
transport chain (2). When
incorporated in the brain, rotenone decreases intracellular ATP
levels, increases production of reactive oxygen species (ROS)
(3,4), releases glutamate from presynaptic
terminals (5,6), elevates intracellular Ca2+
concentration ([Ca2+]i) (7,8) and
initiates neurodegenerative processes. In addition, rotenone is
known to cause Parkinson's disease-associated motor dysfunction
(9,10) and degeneration of dopaminergic
neurons via apoptotic pathways by activation of mitogen-activated
protein kinases (MAPKs) and caspases (CASPs) (11–13).
Previous studies have revealed that calcium channel
blockers play a role as neuroprotectants in neurodegenerative
disorders (14–16). Nicardipine is a hepatically
metabolized dihydropyridine-type calcium channel blocker that
causes vasodilation through blockade of L-type calcium channels in
vascular smooth muscle cells. Nicardipine is widely employed for
the treatment of specific cardiovascular and cerebrovascular
disorders and esophageal cancer in animals (17,18).
In the brain, nicardipine has been demonstrated to block
voltage-gated calcium channels (VGCCs) in the neuronal terminus
which modulate glutamate release (19). Indeed, in ischemia and reperfusion
rats, nicardipine improved motor neurological outcomes and reduced
infarction and edema volume (20).
Nicardipine also decreased plasma levels of the neuron-specific
enolase, a specific marker for the incidence of neuronal injury
(20). Therefore, we hypothesized
that nicardipine may exhibit protective effects against
rotenone-induced apoptosis in neuronal cells.
In the current study, the protective effect of
nicardipine in rotenone-treated SH-SY5Y neuroblastoma cells was
investigated. We specifically focused on the c-Jun N-terminal
protein kinase (JNK)/p38 MAPK and CASP pathways, which have been
hypothesized to be important for rotenone-induced apoptosis in
neuronal cells (11–13).
Materials and methods
Materials
Rotenone, nicardipine, Fluo-4 AM, the CASP3 assay
kit, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) and 4,6-diamidino-2-phenylindole (DAPI) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Dulbecco's modified Eagle's
medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin
were obtained from Gibco-BRL (Grand Island, NY, USA). The in
situ cell death detection terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) kit was
obtained from Roche Diagnostics (Indianapolis, IN, USA). Anti-JNK,
anti-phospho-JNK (Thr 183 and Tyr 185), anti-p38 MAPK,
anti-phospho-p38 MAPK (Thr 180 and Tyr 182), anti-cleaved CASP9,
anti-cleaved CASP3, anti-cleaved poly (ADP-ribose) polymerase-1
(PARP) and anti-β-actin antibodies were purchased from Cell
Signaling Technology (Beverly, MA, USA). Anti-B-cell lymphoma
protein 2 (Bcl2) and anti-Bcl2-associated X protein (BAX)
antibodies and horseradish peroxidase-conjugated anti-mouse and
anti-rabbit IgG were obtained from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Cell culture and treatment
SH-SY5Y cells were obtained from American Type
Culture Company (Rockville, MD, USA). Cells were grown in DMEM
supplemented with 10% FBS and 100 U/ml penicillin/streptomycin.
Cultures were maintained in a humidified incubator at 37°C in an
atmosphere of 5% CO2 and 95% air. Cell culture medium
was changed every 2 days.
Rotenone was freshly prepared in dimethyl sulfoxide
(DMSO) prior to each experiment. Nicardipine was freshly prepared
in saline and was added 4 h prior to rotenone addition to the
cultures.
Cell viability assay
Cell viability was determined by MTT assay. For
detecting cytotoxicity of rotenone on SH-SY5Y cells, cells were
seeded in triplicate at a concentration of 2×105
cells/ml on a 96-well plate. Cells were exposed to rotenone (0.1,
1, 5, 10 and 20 μM) containing 0.1% (v/v) DMSO or vehicle for 24 h.
In the experiment for detecting the effect of nicardipine, cells
were pretreated with nicardipine or saline for 4 h prior to
rotenone treatment with concentrations of 0.1, 0.5, 1 and 5 μM.
Following this, cells were exposed to rotenone (10 μM) containing
0.1% (v/v) DMSO or vehicle for 24 h. MTT (0.5 mg/ml) was added to
each group and the cells were incubated for 4 h. Then, cells were
incubated for an additional 1 h in the solution in which MTT was
dissolved. Viability was read with an absorbance microplate reader
(Molecular Devices, Toronto, ON, Canada) at a test wavelength of
595 nm with a reference wavelength of 690 nm. Optical density (OD)
was calculated as the difference between the reference and test
wavelength. Percent viability was calculated as
(ODdrug/ODcontrol) × 100.
DAPI staining
SH-SY5Y cells (1×105 cells/ml) were
cultured on four-chamber slides (Nalge Nunc International,
Naperville, IL, USA). Following pretreatment with nicardipine (5
μM, 4 h) or saline, cells were incubated with rotenone (10 μM) or
vehicle for 24 h. Then, cells were fixed in methanol and incubated
in 1 μg/ml DAPI solution for 30 min in the dark. The stained cells
were observed with a fluorescence microscope (Carl Zeiss,
Oberköchen, Germany).
TUNEL assay
TUNEL assays were performed according to the
manufacturer's instructions. SH-SY5Y cells (1×105
cells/ml) pretreated with 5 μM nicardipine (4 h) or saline were
exposed to 10 μM rotenone or vehicle for 24 h and then fixed in
acetic acid at −20°C. Fixed cells were incubated with the TUNEL
reaction mixture (terminal deoxynucleotidyl transferase and
nucleotide) for 1 h at 37°C, followed by the addition of
peroxidase-conjugated detection antibody. DNA fragments were
stained using 3,3′-diaminobenzidine as the substrate for the
peroxidase.
Measurement of
[Ca2+]i
Cells were pretreated with nicardipine (5 μM) for 4
h, incubated with 3 μM Fluo-4 AM (dissolved in DMSO) in serum-free
growth medium at 37°C for 45 min in a CO2 incubator and
then washed with calcium-free Hank's balanced salt solution
(Gibco-BRL). Ca2+ fluorescence was assessed at intervals
of 10 sec in an absorption spectrum (488 nm) and an emission
wavelength (515 nm) using laser scanning confocal microscope (Leica
Lasertechnik, Heidelberg GmbH, Wetzlar, Germany). Baseline
[Ca2+]i was observed for 100 sec and 10 μM
rotenone was added and changes in [Ca2+]i
were measured. Results are expressed as relative fluorescence
intensity (21).
Western blot analysis
Cells were lysed in RIPA buffer containing protease
inhibitors. The protein content was measured using a Bio-Rad
colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Equal
amounts of protein (80 μg) were separated on sodium dodecyl
sulfate-polyacrylamide gels and transferred onto a nitrocellulose
membrane (Schleicher & Schuell, Postfach, Germany). Following
blocking with 5% skimmed milk, membranes were probed with rabbit
anti-JNK, anti-phospho-JNK, anti-p38, anti-phospho-p38 MAPK,
anti-cleaved CASP9, anti-cleaved CASP3, anti-cleaved PARP or mouse
anti-β-actin antibodies overnight at 4°C. Horseradish
peroxidase-conjugated anti-mouse or anti-rabbit IgG were used as
the secondary antibodies. Band detection was performed using the
Enhanced Chemiluminescence detection system (Amersham Biosciences,
Uppsala, Sweden). Results of western blot analysis were quantified
using ImageJ image analysis software (v1.4; National Institutes of
Health, Bethesda, MD, USA).
CASP3 activity assay
CASP3 activity was measured using an assay kit
according to the manufacturer's instructions. SH-SY5Y cells
(2×105 cells/ml) were lysed following treatment with 5
μM nicardipine and 10 μM rotenone for 24 h. The CASP3 substrate
(Ac-DVED-p-NA) was added to cell lysates and the mixtures were
incubated overnight in a humidified environment at 37°C. Control
lysates were preincubated with the CASP3 inhibitor Ac-DEVD-CHO to
determine on-specific background substrate breakdown. The
concentration of p-NA released from the CASP3 substrate was
measured using an absorbance microplate reader (Molecular Devices)
as absorbance values at 405 nm and calculated from a calibration
curve of p-NA standards.
Statistical analysis
Data are presented as mean ± SEM from three
independent experiments. All experiments were performed at least
three times independently. Data were analyzed by one-way ANOVA,
followed by Tukey's HSD post hoc test, using the SPSS software
(v13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of nicardipine on rotenone-induced
cell death in SH-SY5Y cells
SH-SY5Y cells were treated with rotenone of various
concentrations (0.1–20 μM) for 24 h. Rotenone revealed significant
cytotoxic effects in a dose-dependent manner in SH-SY5Y cells
(Fig. 1A; P<0.05 vs.
non-treated cells). The viability of SH-SY5Y cells exposed to
rotenone at concentrations of 10 and 20 μM was 58.3±3.7 and
33.7±3.3% of the control value, respectively.
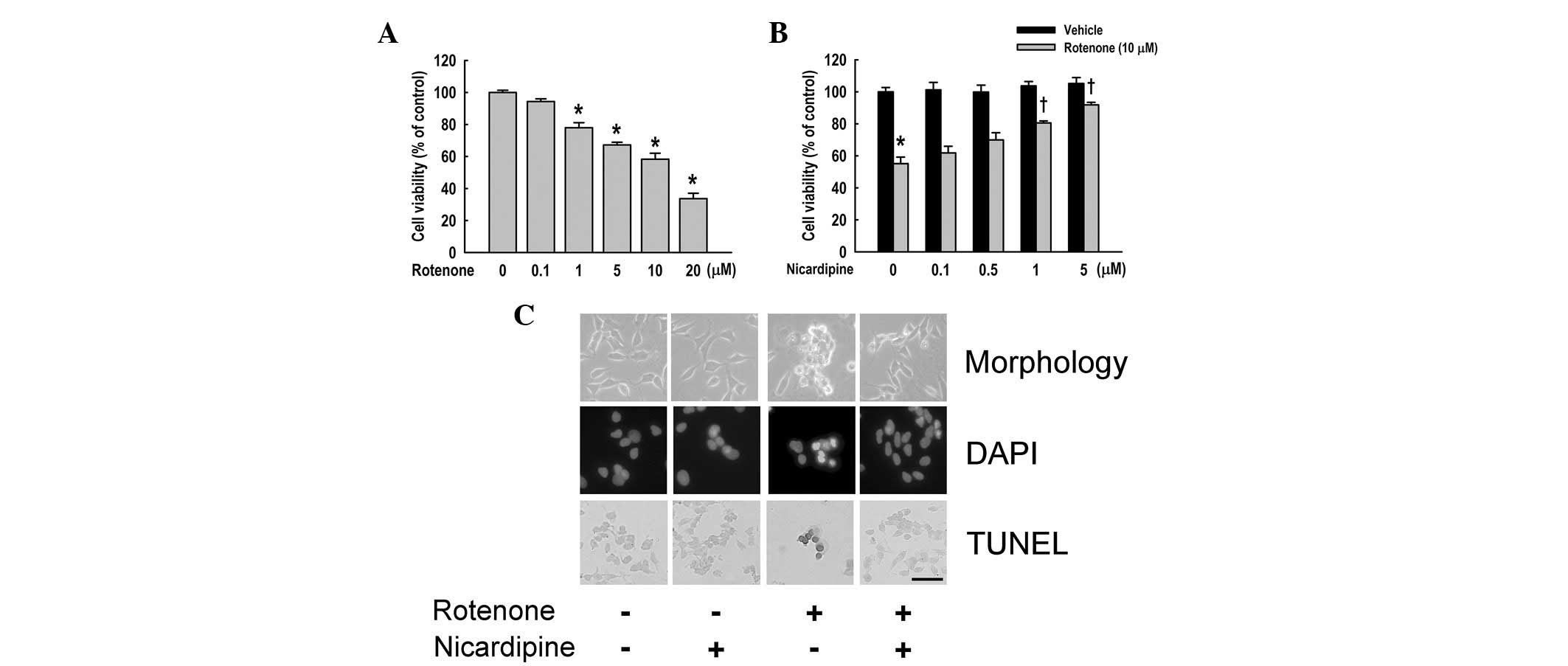 | Figure 1Effect of nicardipine on
rotenone-induced apoptosis. (A) SH-SY5Y cells were treated with
various concentrations of rotenone for 24 h prior to determination
of cellular viability using the MTT assay. (B) Effect of
nicardipine was examined in SH-SY5Y cells treated with 10 μM
rotenone for 24 h. Nicardipine was pretreated at various
concentrations 4 h prior to rotenone treatment. Results are
presented as mean ± SEM. *P<0.05, vs. non-treated
cells; †P<0.05, vs. rotenone-treated cells. (C)
SH-SY5Y cells were cultured with and/or without rotenone (10 μM, 24
h) and nicardipine (5 μM, 4 h prior to rotenone treatment).
Phase-contrast microscopy revealed that nicardipine decreases
rotenone-induced cell shrinkage, shape irregularity and cellular
detachment (upper panel). DAPI staining indicated that nicardipine
suppresses rotenone-induced nuclear condensation (middle panel).
TUNEL assay revealed that nicardipine attenuates TUNEL-positive
cells. Condensed and marginated chromatin is stained dark gray
(lower panel). Scale bar, 100 μm. MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; DAPI,
4,6-diamidino-2-phenylindole; TUNEL, terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling. |
To examine the effect of nicardipine on
rotenone-induced cytotoxicity, cells were treated with various
concentrations of nicardipine with or without rotenone (10 μM). As
demonstrated in Fig. 1B,
nicardipine pretreatment (4 h prior to rotenone treatment)
increased cell viability in rotenone-treated cells in a
dose-dependent manner. The viability of SH-SY5Y cells was 61.8±4.1,
69.9±4.5, 80.5±1.3 and 91.8±1.6% at concentrations of 0.1, 0.5, 1
and 5 μM nicardipine, respectively. Further experiments were
performed using 5 μM nicardipine to determine the precise effects
of nicardipine.
Effect of nicardipine on rotenone-induced
apoptosis
Apoptosis of rotenone-treated cells was determined
by DAPI staining and TUNEL assay. As revealed in Fig. 1C (upper panel), 5 μM nicardipine
protected against shrinkage of SH-SY5Y cells treated with 10 μM
rotenone for 24 h. DAPI staining demonstrated nuclear condensation,
DNA fragmentation and perinuclear apoptotic bodies upon treatment
of rotenone, whereas nicardipine pretreatment inhibited these
apoptotic features (Fig. 1C,
middle panel). TUNEL assay also revealed DNA strand breaks,
indicative of rotenone-induced apoptosis. By contrast, nicardipine
prevented the induction of apoptosis by rotenone (Fig. 1C, lower panel).
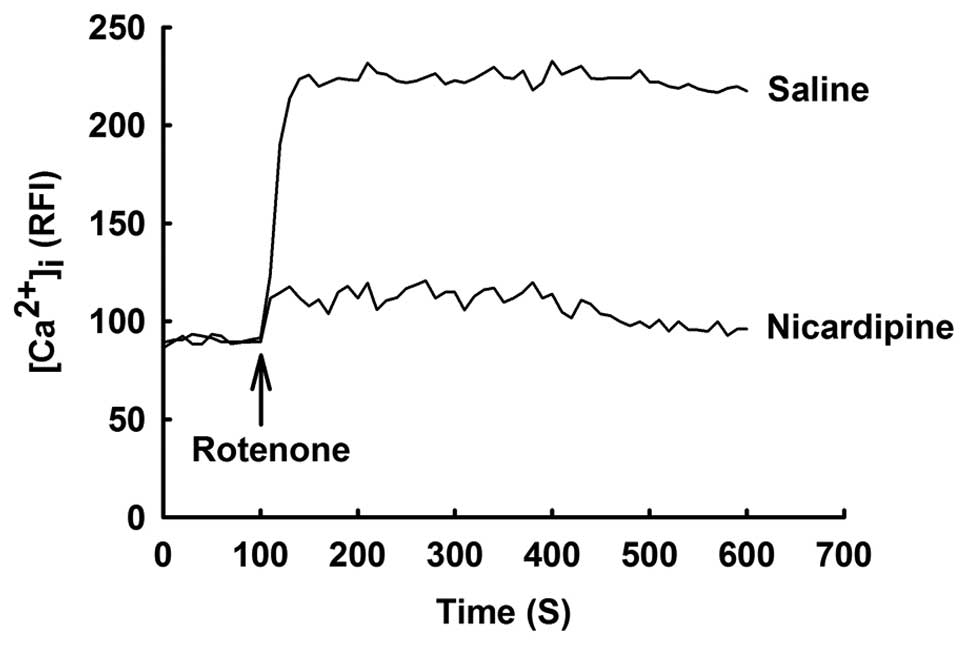
Effect of nicardipine on rotenone-induced
elevation of [Ca2+]i
Increased [Ca2+]i has been
postulated to be associated with rotenone-induced cell death in
previous studies (7,8). As demonstrated in Fig. 2, [Ca2+]i was
rapidly increased by treatment with 10 μM rotenone and increased
[Ca2+]i was sustained to the end of the
experiment. By contrast, nicardipine (5 μM) prevented
rotenone-induced elevation of [Ca2+]i.
Nicardipine did not affect [Ca2+]i in
rotenone-untreated cells.
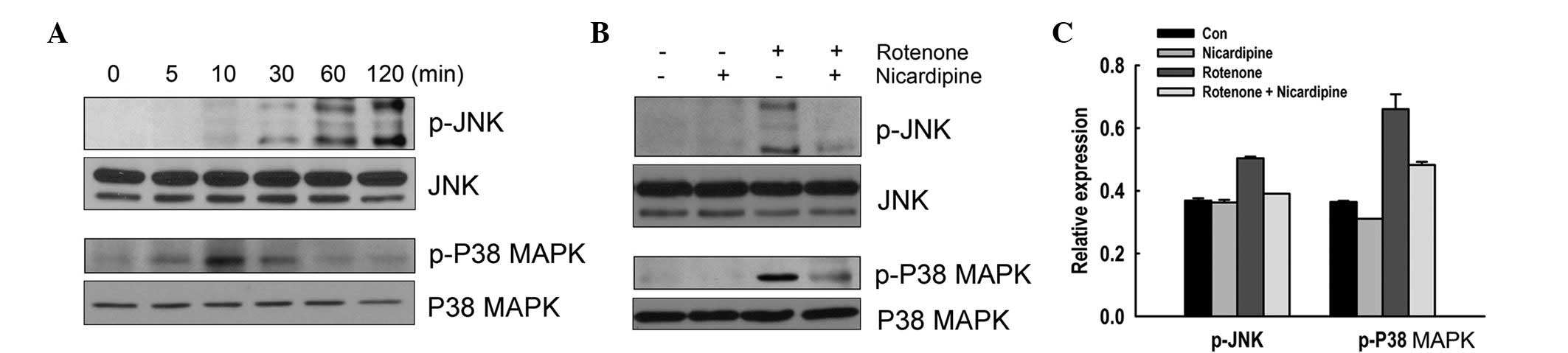
Effect of nicardipine on rotenone-induced
increase of phosphorylation of JNK and p38 MAPK
The effect of nicardipine on the phosphorylation of
JNK and p38 MAPK in SH-SY5Y cells treated with rotenone was
investigated. Cells were treated with 10 μM rotenone for up to 120
min. Fig. 3A indictates that
rotenone induced phosphorylation of JNK in a time-dependent manner.
Phosphorylation of p38 MAPK was also transiently upregulated
following exposure to rotenone, with peak expression observed at 10
min. The effect of nicardipine on phosphorylation of JNK and p38
MAPK was examined in cells exposed to rotenone for 120 and 10 min,
respectively. In nicardipine (5 μM)-pretreated cells, the
phosphorylation of JNK and p38 MAPK triggered by rotenone was
abrogated (Fig. 3B and C).
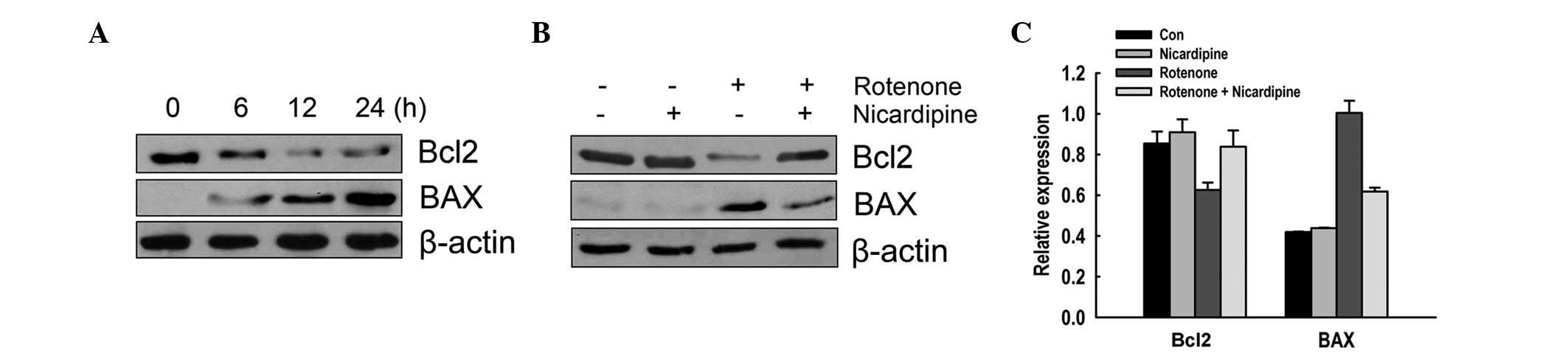
Effect of nicardipine on the decrease of
Bcl2 and increase of BAX by rotenone
The effect of nicardipine on Bcl2 family proteins,
including Bcl2 and BAX, was analyzed in SH-SY5Y cells exposed to
rotenone through western blot analysis. Rotenone (10 μM, 6–24 h)
decreased the expression of the anti-apoptotic protein Bcl2 and
increased the expression of pro-apoptotic protein BAX in a
time-dependent manner (Fig. 4A).
As demonstrated in Fig. 4B and C,
5 μM nicardipine pretreatment prevented the reduction of Bcl2 and
the elevation of BAX induced by rotenone (10 μM, 24 h).
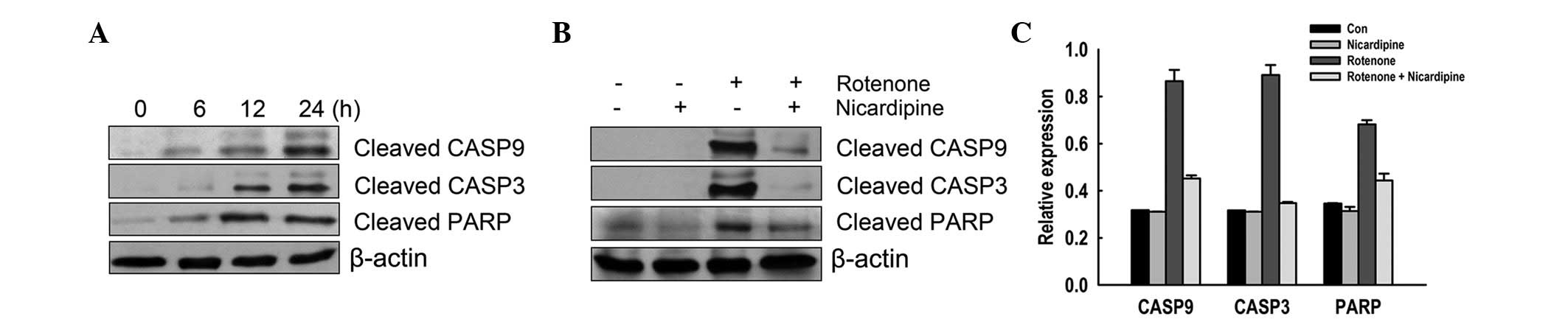
Effect of nicardipine on rotenone-induced
activation of caspases
The effect of nicardipine on CASP9 and 3 and PARP
was assessed in rotenone-treated SH-SY5Y cells. Rotenone (10 μM,
6–24 h) increased cleavage of CASP9 and 3 and PARP in a
time-dependent manner (Fig. 5A).
As revealed in Fig. 5B and C,
rotenone-induced cleavage of CASP9 and 3 and PARP was prevented by
pretreatment with 5 μM nicardipine.
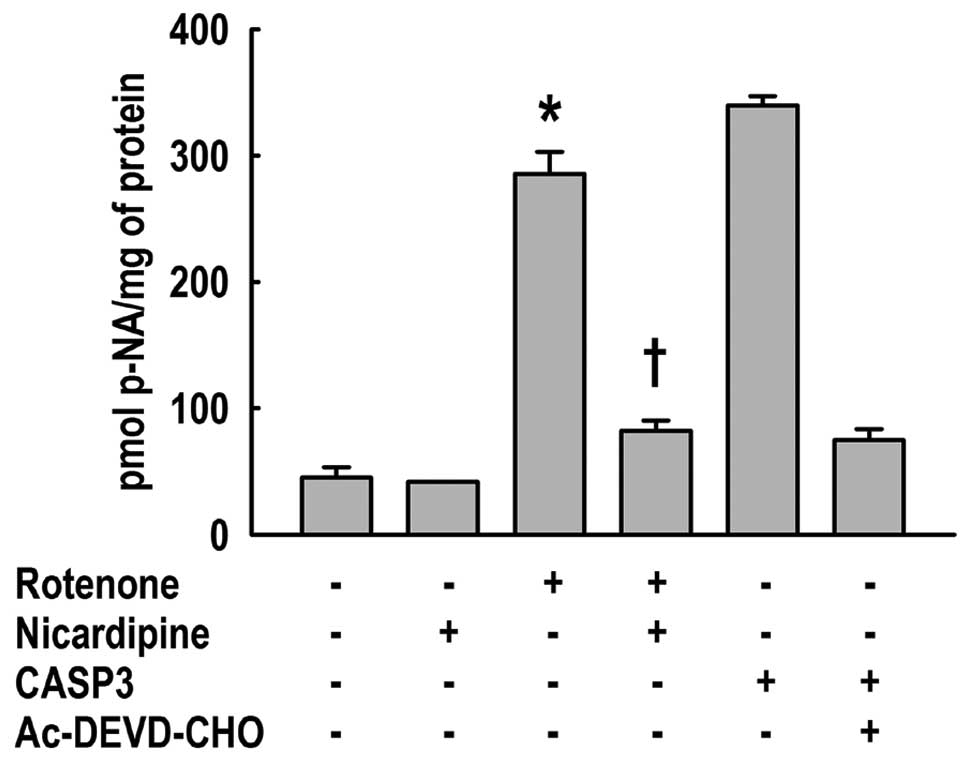
In addition, the effect of nicardipine on CASP3
enzyme activity, the primary executioner of apoptosis, was analyzed
in SH-SY5Y cells treated with 10 μM rotenone for 24 h. The activity
was measured by hydrolysis of the peptide substrate, Ac-DEVD-p-NA.
Nicardipine pretreatment significantly attenuated the cleavage of
Ac-DEVD-p-NA elevated by rotenone (Fig. 6).
Discussion
In the present study, nicardipine reduced
rotenone-induced cytotoxicity, apoptosis and elevation of
[Ca2+]i in SH-SY5Y cells. Rotenone increased
the phosphorylation of JNK and p38 MAPK, whereas nicardipine
inhibited these increases. Nicardipine also inhibited the
upregulation of Bcl2 expression and downregulation of BAX
expression by rotenone. In addition, nicardipine protected
rotenone-induced cleavage of CASP9 and 3 and PARP and increased
CASP3 enzyme activity. These results indicate that nicardipine
exhibits a protective effect against rotenone-induced apoptotic
cell death in SH-SY5Y cells.
Ca2+ has been implicated in the induction
of apoptosis and the regulation of the apoptotic signaling
pathways. Negre-Salvayre and Salvayre (22) demonstrated the importance of
Ca2+ for apoptosis as well as the protective effect of
Ca2+ channel blockers and chelators. A number of studies
have also reported that rotenone elevates
[Ca2+]i by inducing Ca2+ influx
into cells (7,8). Wang and Xu (8) identified that rotenone (10 μM)
induced an elevation in [Ca2+]i through
Ca2+ influx by opening VGCCs in SH-SY5Y cells, inducing
apoptotic events, including ROS production, G2/M cell
cycle arrest and CASP activation. In addition, treatment with the
intracellular Ca2+ chelator
1,2-bis-(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid
tetrakis/acetoxymethyl ester prevented rotenone-induced apoptosis
(8). Therefore, it was
hypothesized that the increase in Ca2+ influx via VGCCs
was associated with rotenone-induced apoptotic events. Freestone
et al(7) identified
rotenone-induced elevation of [Ca2+]i in
substantia nigra pars compacta neurons, hypothesizing that the
increase in Ca2+ influx resulted from transient receptor
potential M2 channels activated by increased ROS production.
However, a low toxin concentration (5 nM) was used in the study. In
the present study, rotenone increased [Ca2+]i
and induced apoptosis, decreasing anti-apoptotic protein Bcl2
expression and increasing pro-apoptotic protein BAX expression and
CASP activation. Nicardipine inhibited rotenone-induced elevation
of [Ca2+]i and changes in expression of these
apoptotic proteins. These results, together with previous studies,
indicate that rotenone-induced apoptosis may be suppressed by
blocking Ca2+ influx.
JNK and p38 MAPK play diverse roles in neuronal
differentiation, survival and death and are activated in response
to a variety of cellular stresses and toxicants (11). Prolonged activation of these
signaling pathways has been implicated in several forms of neuronal
apoptosis (12,23). Activation of JNK and p38 MAPK is
responsible for inhibition of Bcl2 and induction of phosphorylation
of c-Jun, a nuclear transcription factor and a known target of JNK,
which further promotes the release of cytochrome c from the
mitochondria to the cytoplasm and leads to activation of CASPs
(11,24). In addition, rotenone-induced
apoptosis has been found to require activation of JNK and p38 MAPK
via phosphorylation and may mediate the regulation of Bcl2 family
proteins and activation of CASPs (12,13).
Previous studies have also reported that MAPKs are activated by
Ca2+ influx through VGCCs (25,26).
Thus, we hypothesized that calcium channel blockers may inhibit the
activation of MAPKs by rotenone leading to Ca2+ influx
via VGCCs. In our study, nicardipine reduced the rotenone-induced
phosphorylation of JNK and p38 MAPK in SH-SY5Y cells. Results
indicate that nicardipine protects rotenone-induced apoptosis
through regulation of MAPKs as well as Bcl2 family proteins and
CASPs.
In conclusion, the present study demonstrates that
nicardipine has a potent protective effect on rotenone-induced
apoptosis in SH-SY5Y cells, inhibiting phosphorylation of JNK and
p38 MAPK and activation of CASPs with modulation of Bcl2 family
proteins. These observations indicate that nicardipine may be
useful for impairment of rotenone-induced neurotoxicity.
Acknowledgements
The present study was supported in part by the
Soonchunhyang University Research Fund.
References
|
1
|
Lee J, Huang MS, Yang IC, et al: Essential
roles of caspases and their upstream regulators in rotenone-induced
apoptosis. Biochem Biophys Res Commun. 371:33–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Singer TP and Ramsay RR: The reaction
sites of rotenone and ubiquinone with mitochondrial NADH
dehydrogenase. Biochim Biophys Acta. 1187:198–202. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alam ZI, Jenner A, Daniel SE, et al:
Oxidative DNA damage in the parkinsonian brain: an apparent
selective increase in 8-hydroxyguanine levels in substantia nigra.
J Neurochem. 69:1196–1203. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jenner P: Oxidative stress in Parkinson's
disease. Ann Neurol. 53(Suppl 3): S26–36; discussion S36–38. 2003.
View Article : Google Scholar
|
|
5
|
Kahlert S, Zundorf G and Reiser G:
Glutamate-mediated influx of extracellular Ca2+ is
coupled with reactive oxygen species generation in cultured
hippocampal neurons but not in astrocytes. J Neurosci Res.
79:262–271. 2005.PubMed/NCBI
|
|
6
|
Kilbride SM, Telford JE, Tipton KF and
Davey GP: Partial inhibition of complex I activity increases
Ca-independent glutamate release rates from depolarized
synaptosomes. J Neurochem. 106:826–834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Freestone PS, Chung KK, Guatteo E, Mercuri
NB, Nicholson LF and Lipski J: Acute action of rotenone on nigral
dopaminergic neurons - involvement of reactive oxygen species and
disruption of Ca2+ homeostasis. Eur J Neurosci.
30:1849–1859. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang XJ and Xu JX: Possible involvement of
Ca2+ signaling in rotenone-induced apoptosis in human
neuroblastoma SH-SY5Y cells. Neurosci Lett. 376:127–132. 2005.
|
|
9
|
Klein A, Gidyk DC, Shriner AM, et al:
Dose-dependent loss of motor function after unilateral medial
forebrain bundle rotenone lesion in rats: a cautionary note. Behav
Brain Res. 222:33–42. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mulcahy P, Walsh S, Paucard A, Rea K and
Dowd E: Characterisation of a novel model of Parkinson's disease by
intra-striatal infusion of the pesticide rotenone. Neuroscience.
181:234–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Newhouse K, Hsuan SL, Chang SH, Cai B,
Wang Y and Xia Z: Rotenone-induced apoptosis is mediated by p38 and
JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci.
79:137–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pei W, Liou AK and Chen J: Two
caspase-mediated apoptotic pathways induced by rotenone toxicity in
cortical neuronal cells. FASEB J. 17:520–522. 2003.PubMed/NCBI
|
|
14
|
Alps BJ and Hass WK: The potential
beneficial effect of nicardipine in a rat model of transient
forebrain ischemia. Neurology. 37:809–814. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grotta JC, Pettigrew LC, Rosenbaum D, Reid
C, Rhoades H and McCandless D: Efficacy and mechanism of action of
a calcium channel blocker after global cerebral ischemia in rats.
Stroke. 19:447–454. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miyazaki H, Tanaka S, Fujii Y, et al:
Neuroprotective effects of a dihydropyridine derivative,
1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic
acid methyl 6-(5-phenyl-3-pyrazolyloxy)hexyl ester (CV-159), on rat
ischemic brain injury. Life Sci. 64:869–878. 1999. View Article : Google Scholar
|
|
17
|
Sorkin EM and Clissold SP: Nicardipine. A
review of its pharmacodynamic and pharmacokinetic properties and
therapeutic efficacy, in the treatment of angina pectoris,
hypertension and related cardiovascular disorders. Drugs.
33:296–345. 1987.
|
|
18
|
Varon J and Marik PE: The diagnosis and
management of hypertensive crises. Chest. 118:214–227. 2000.
View Article : Google Scholar
|
|
19
|
Miller RJ: Multiple calcium channels and
neuronal function. Science. 235:46–52. 1987. View Article : Google Scholar
|
|
20
|
Kittaka M, Giannotta SL, Zelman V, et al:
Attenuation of brain injury and reduction of neuron-specific
enolase by nicardipine in systemic circulation following focal
ischemia and reperfusion in a rat model. J Neurosurg. 87:731–737.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nguyen TT, Cho SO, Ban JY, et al:
Neuroprotective effect of Sanguisorbae radix against
oxidative stress-induced brain damage: in vitro and in vivo. Biol
Pharm Bull. 31:2028–2035. 2008.
|
|
22
|
Negre-Salvayre A and Salvayre R:
UV-treated lipoproteins as a model system for the study of the
biological effects of lipid peroxides on cultured cells. 4 Calcium
is involved in the cytotoxicity of UV-treated LDL on lymphoid cell
lines. Biochim Biophys Acta. 1123:207–215. 1992. View Article : Google Scholar
|
|
23
|
Namgung U and Xia Z: Arsenite-induced
apoptosis in cortical neurons is mediated by c-Jun N-terminal
protein kinase 3 and p38 mitogen-activated protein kinase. J
Neurosci. 20:6442–6451. 2000.PubMed/NCBI
|
|
24
|
Junn E and Mouradian MM: Apoptotic
signaling in dopamine-induced cell death: the role of oxidative
stress, p38 mitogen-activated protein kinase, cytochrome c and
caspases. J Neurochem. 78:374–383. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rosen LB, Ginty DD, Weber MJ and Greenberg
ME: Membrane depolarization and calcium influx stimulate MEK and
MAP kinase via activation of Ras. Neuron. 12:1207–1221. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wood KW, Sarnecki C, Roberts TM and Blenis
J: ras mediates nerve growth factor receptor modulation of three
signal-transducing protein kinases: MAP kinase, Raf-1 and RSK.
Cell. 68:1041–1050. 1992. View Article : Google Scholar : PubMed/NCBI
|