Introduction
Ovarian cancer is the sixth most common cancer
worldwide in women (1). Although
the incidence rate varies widely among different geographic regions
and ethnic groups, ovarian cancer is more common in industrialized
nations, such as Northern Europe and the United States, but it is
exceptionally low in Japan (2).
The exact cause of ovarian cancer remains unknown. Thus, the
mechanisms involved in ovarian epithelial cell malignant
transformation remain to be defined. However, hormones, genetic
factors, such as mutations of BRCA1 and 2 genes, or family history
may play a role in ovarian carcinogenesis. At the molecular level,
accumulated data indicate that integrin-regulated cell adhesion,
motility and signaling may be involved in ovarian carcinoma cell
proliferation (3–6).
Integrin-linked kinase (ILK), a serine-threonine
kinase, associates with the cytoplasmic domains of integrin-β1 and
-β3 (7,8). ILK is recognized as a multifunctional
intracellular effector of cell-matrix interactions and regulates
various important signaling pathways that may be involved in the
regulation of tumor cell growth, proliferation,
epithelial-mesenchymal transition, migration, invasion, survival
and angiogenesis (9). Indeed, the
expression of ILK is often upregulated in various human
malignancies and is correlated with advanced tumor stage and grade
(9). In ovarian cancer, ILK
expression was reported to be induced and was found to be
associated with tumor progression (10). In addition, inhibition of ILK
expression by small molecule inhibitors or RNA interference
inhibited the growth of various cancer cells in vitro,
including human bladder cancer cells (11), breast cancer cells (12) and hepatocellular carcinoma (HCC)
cells (13). In ovarian cancer,
knockdown of ILK expression using short hairpin RNA (shRNA) induced
growth inhibition and apoptosis of ovarian cancer SKOV3 cells
(14). These studies indicate that
ILK may be a novel target for cancer therapeutics (15). Nevertheless, prior to clinical
translation, the effects of ILK gene silencing on the regulation of
tumor formation and growth of ovarian cancer cells need to be
established in animal models.
In the present study, we determined the inhibitory
effects of ILK gene knockdown on the regulation of tumor formation
and growth by inoculating human ovarian cancer HO-8910 cells
transfected with an ILK antisense oligonucleotide (ILK-ASO) into
nude mice. Our results may provide useful insights into the
antitumor activities of ILK gene knockdown.
Materials and methods
Reagents
The ILK antisense oligonucleotide (ILK-ASO) sequence
(5′-ATGTCGTCCATAGCAGCGTC-3′) and the PCR primers were synthesized
by the Shanghai Invitrogen Biotechnology Co., Ltd. (Shanghai,
China). Lipofectamine™ 2000 transfection reagent was purchased from
Invitrogen (Carlsbad, CA, USA). A rabbit polyclonal anti-human ILK
antibody was obtained from Cell Signaling Technology, Inc.
(Beverly, MA, USA). The cell cycle analysis kit was purchased from
Becton-Dickinson (East Rutherford, NJ, USA).
Cell line, culture and oligonucleotide
transfection
The human ovarian carcinoma cell line HO-8910 was
obtained from the Laboratory of Medical Genetics, Harbin Medical
University (Harbin, China). Cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin, and
incubated at 37°C in a 5% CO2 and 95% air incubator.
Oligonucleotide transfection into cells was carried out as
previously described with a few modifications (16). Briefly, cells in the log phase were
digested by 0.25% trypsin (containing 0.02% EDTA), seeded onto
100-ml culture flasks and grown for 24 h. Prior to transfection,
cells were washed twice with DMEM free of serum and antibiotics.
ILK-ASO was mixed with Lipofectamine 2000 in DMEM and incubated for
20 min at room temperature. This mixture was then added to cells
with a final ILK-ASO concentration of 100 nM. Cells treated with
Lipofectamine 2000 but without ILK-ASO were used as a negative
control. After 8 h, the transfection medium was replaced with
culture medium for up to 72 days. At the end of the experiments,
the cells were subjected to cell viability and gene expression
analyses.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Expression of ILK mRNA in cells was determined by
RT-PCR. In brief, 72 h after oligonucleotide transfection, cells
were collected and resuspended at a density of 1×107
cells/ml. Total RNA was collected from 1 ml of the cell suspension
and was reverse transcribed using a Promega RT kit (Madison, WI,
USA). PCR amplification was then performed using the ILK gene
upstream primer (5′-TGGACGACATTTTCACTCAG-3′) and downstream primer
(5′-CATCAATCATTACACTA CGG-3′), producing an amplified fragment of
984 bp. Amplification of the β-actin gene upstream primer
(5′-GCTGGCCGGGACCTGACTGA-3′) and downstream primer (5′-AAGCATTTGCGG
TGGACGAT-3′) produced an amplified fragment with a length of 584
bp. PCR amplification conditions were as follows: pre-denaturation
at 95°C for 5 min and then 35 cycles of denaturation at 95°C for 45
sec, annealing at 49°C for 45 sec, and extension at 72°C for 60
sec. All protocols had an extra 10-min extension at 72°C.
Subsequently, PCR products were separated by agarose gel
electrophoresis, and then ethidium bromide-stained bands were
visualized by ultraviolet transillumination. The fluorescence
intensity was quantified using a Gel Imaging System (Kodak, USA).
The level of ILK mRNA was semi-quantified by calculating the
ILK/β-actin ratio. Data were summarized from three independent
experiments.
Protein extraction and western
blotting
To determine the levels of ILK protein, cells were
lysed for 30 min at 4°C and protein was collected and quantified
using the Bradford protocol. Denatured protein (30 μg) was then
separated on acrylamide gels by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene fluoride (PVDF) membranes.
Membranes were blocked, probed with an anti-ILK primary antibody
for 1 h at room temperature, and then blotted with a secondary
antibody for another 1 h. Blots were then visualized by
3,3′-diaminobenzidine (DAB) staining. Quantification of western
blotting was performed by normalizing the signal intensity of each
band to that of the β-actin control. Data were summarized from
three independent experiments.
Cell cycle analysis
Seventy-two hours after transfection, cells were
collected, washed with ice-cold phosphate-buffered saline (PBS) and
fixed in 70% ethanol at 4°C overnight. The next day, cells were
washed with PBS and stained with propidium iodide (PI) in the dark
for 30 min and subjected to FACScan flow cytometry (FC 500MPL,
Beckman Coulter, USA) analysis. For data generation, 10,000 cells
were analyzed with CXP Acquisition and CXP Analysis software
packages (Beckman Coulter).
Nude mouse xenograft assay
Four to six week-old female specific pathogen-free
(SPF) nude mice of the BALB/c strain (20–22 g) were purchased from
the Vital River Laboratory Animal Technology Co., Ltd. (Beijing,
China). Animals were routinely housed and had free access to water
and food. All efforts were made to minimize animal suffering and to
reduce the number of animals used. All animal procedures and the
study were approved by the Ethics Committee of the Harbin Medical
University Cancer Hospital. Twenty nude mice were randomly divided
into two groups: a control group (n=10) and an ILK-ASO group
(n=10). Animals in the control group were inoculated with negative
control cells, while animals in the ILK-ASO group were inoculated
with cells transfected with ILK-ASO.
After transfection, tumor cells were collected by
centrifugation, and the cell density was adjusted to
1×107 cells/ml. The cell suspension (0.3 ml) was
subcutaneously injected into the back of the right shoulder of 10
nude mice (ILK-ASO group). Tumor growth was monitored every day
following inoculation. Tumor volume was measured using a caliper on
the indicated days after tumor cell injection and was calculated by
the following formula: V (mm3) = π × a ×
b2/6, where a is the longest diameter of the tumor and b
is the shortest diameter of the tumor. Additionally, the body
weight of each nude mouse was recorded up to 30 days following
inoculation. At the end of the experiments, the animals were
sacrificed and tumor tissues were carefully removed and weighed.
The tumor growth inhibition rates were determined by the following
equations: tumor size inhibition rate (%) = (1 -
VILK-ASO/VControl) × 100; and tumor weight
inhibition rate (%) = (1 - MILK-ASO/MControl)
× 100.
Statistical analysis
Data were analyzed with the Statistical Package for
the Social Sciences (SPSS) version 13.0 software (SPSS, Inc.,
Chicago, IL, USA) and were expressed as the means ± standard
deviation (SD). The Student’s t-test was used to compare the
quantitative data and the χ2 test was used to analyze
enumeration data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of ASO transfection on the
knockdown of ILK gene expression
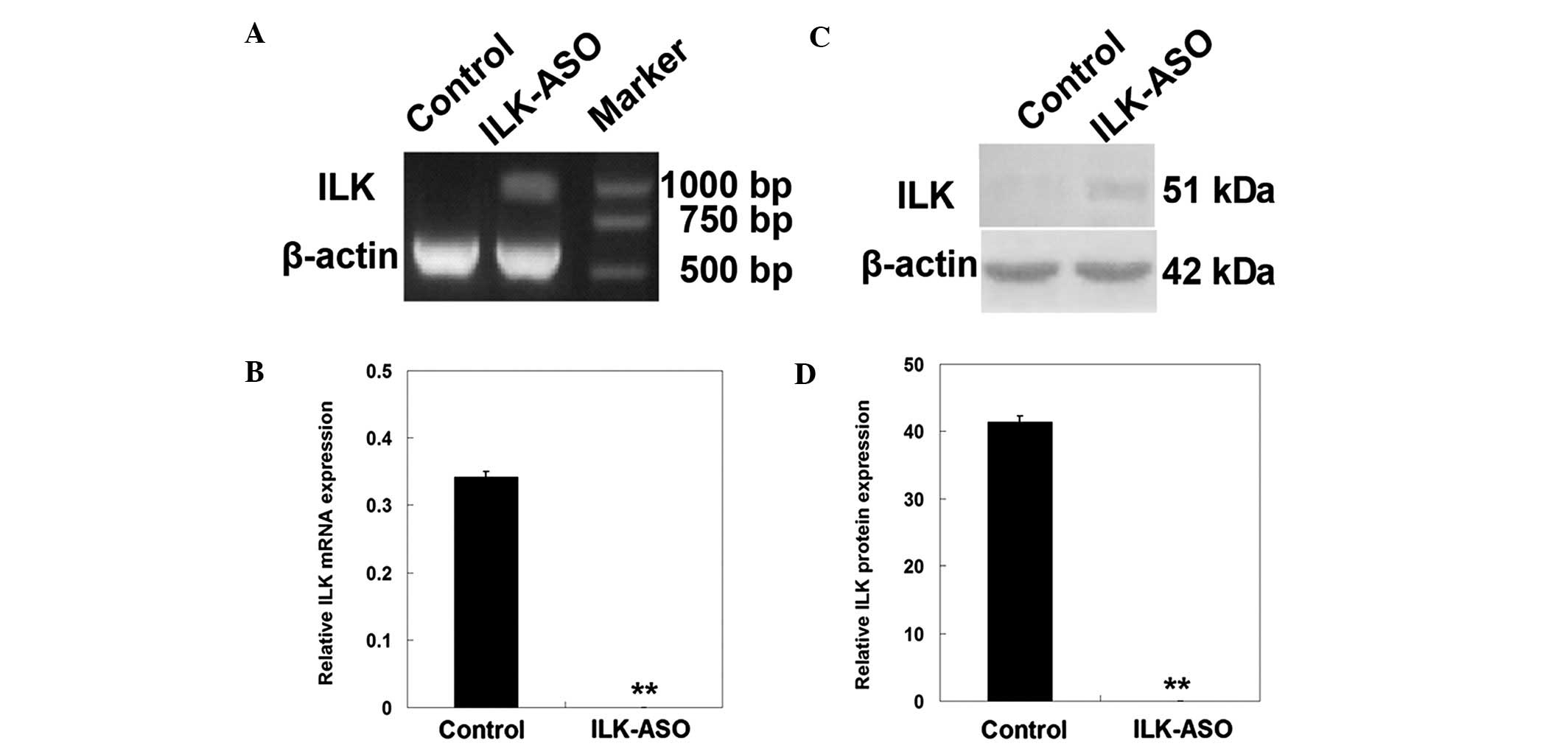
In this study, we first determined the in
vitro effect of ILK knockdown on the regulation of tumor growth
using ILK-ASO transiently transfected into human ovarian carcinoma
HO-8910 cells. The gene silencing efficiency was examined by RT-PCR
and western blotting. As shown in Fig.
1, three days after the ASO transfection, the expression of ILK
mRNA and protein was greatly decreased in ILK-ASO-transfected
HO-8910 cells as compared with the control cells (ILK mRNA levels,
0 vs. 0.34±0.01, respectively; ILK protein levels, 0 vs.
41.44±0.87, respectively; P<0.01). These results demonstrated
that ILK-ASO transfection efficiently downregulated the expression
of ILK mRNA and protein in HO-8910 cells.
Effect of ILK knockdown on the regulation
of HO-8910 cell cycle distribution
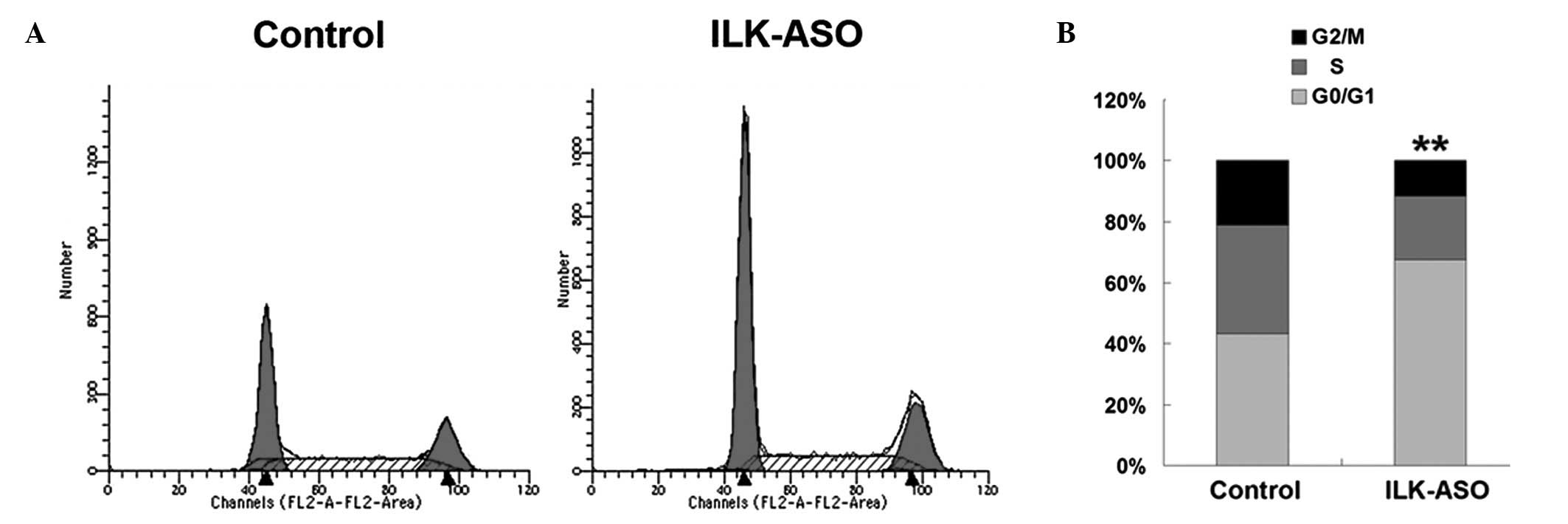
We then observed phenotypic changes of HO-8910 cells
after ILK knockdown and performed flow cytometric analyses to
determine cell cycle re-distribution. As shown in Fig. 2, the number of cells in the G0/G1
phase was significantly increased as a result of ILK gene silencing
(67.61 vs. 43.29% in the control cells; χ2=1197.15,
P<0.01). ILK silencing significantly reduced the cell numbers in
the S and G2/M phase (S phase, 20.69 vs. 35.52% in the control
cells, χ2=544.21, P<0.01; G2/M phase, 11.70 vs.
21.19% in the control cells, χ2=327.71, P<0.01),
indicating that ILK silencing inhibits ovarian cancer cell
proliferation through G0/G1 phase arrest.
Effect of ILK knockdown on the regulation
of tumor formation and growth in nude mouse xenografts
We then performed nude mouse xenograft assays to
test the effect of ILK gene knockdown on the regulation of tumor
formation and growth in vivo. Our data showed that all of
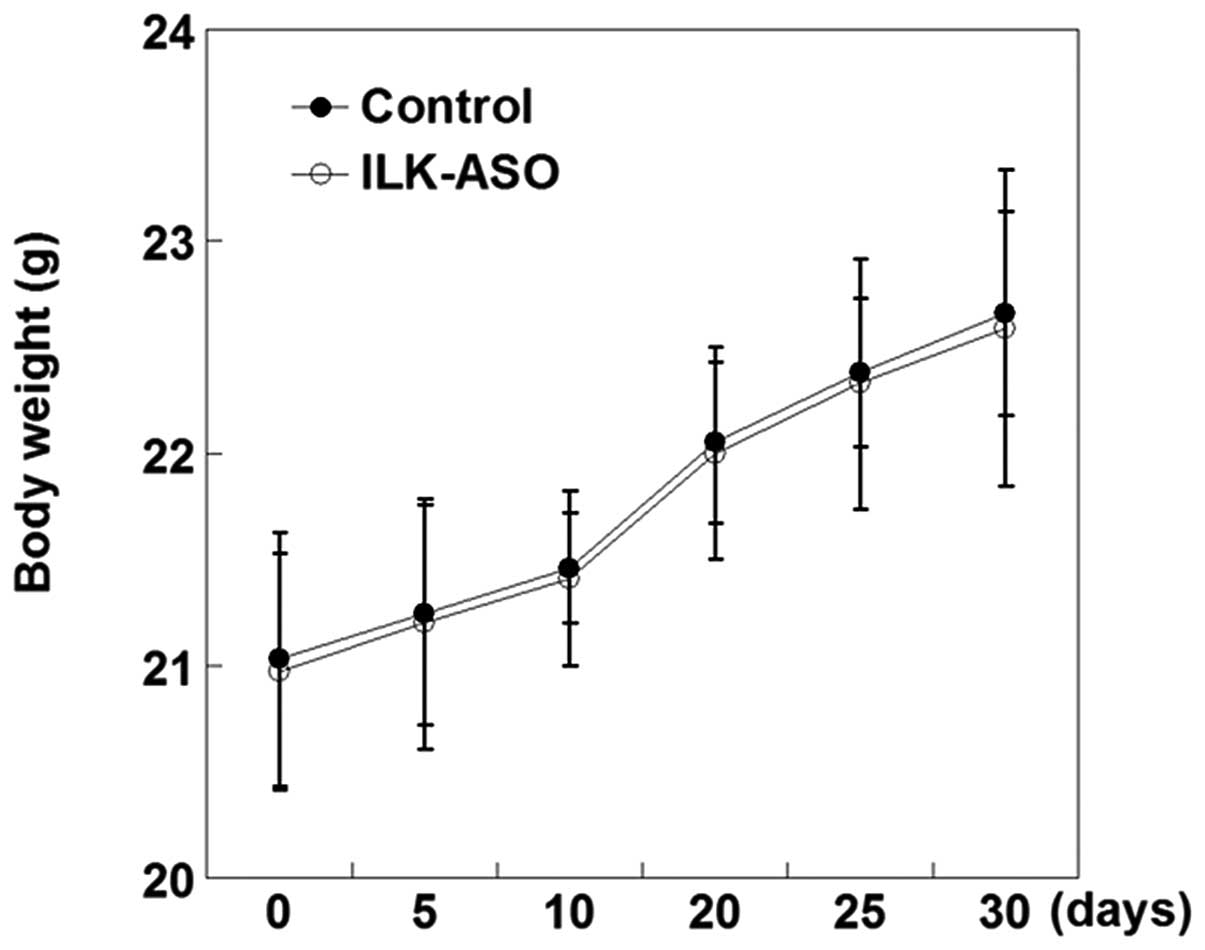
the nude mice survived 30 days following tumor cell inoculation.
Moreover, the average mouse body weight gradually increased after
tumor cell injection; however, no statistical difference was
detected in the body weight between control mice and
ILK-ASO-silenced mice at each time point following inoculation
(P>0.05; Fig. 3).
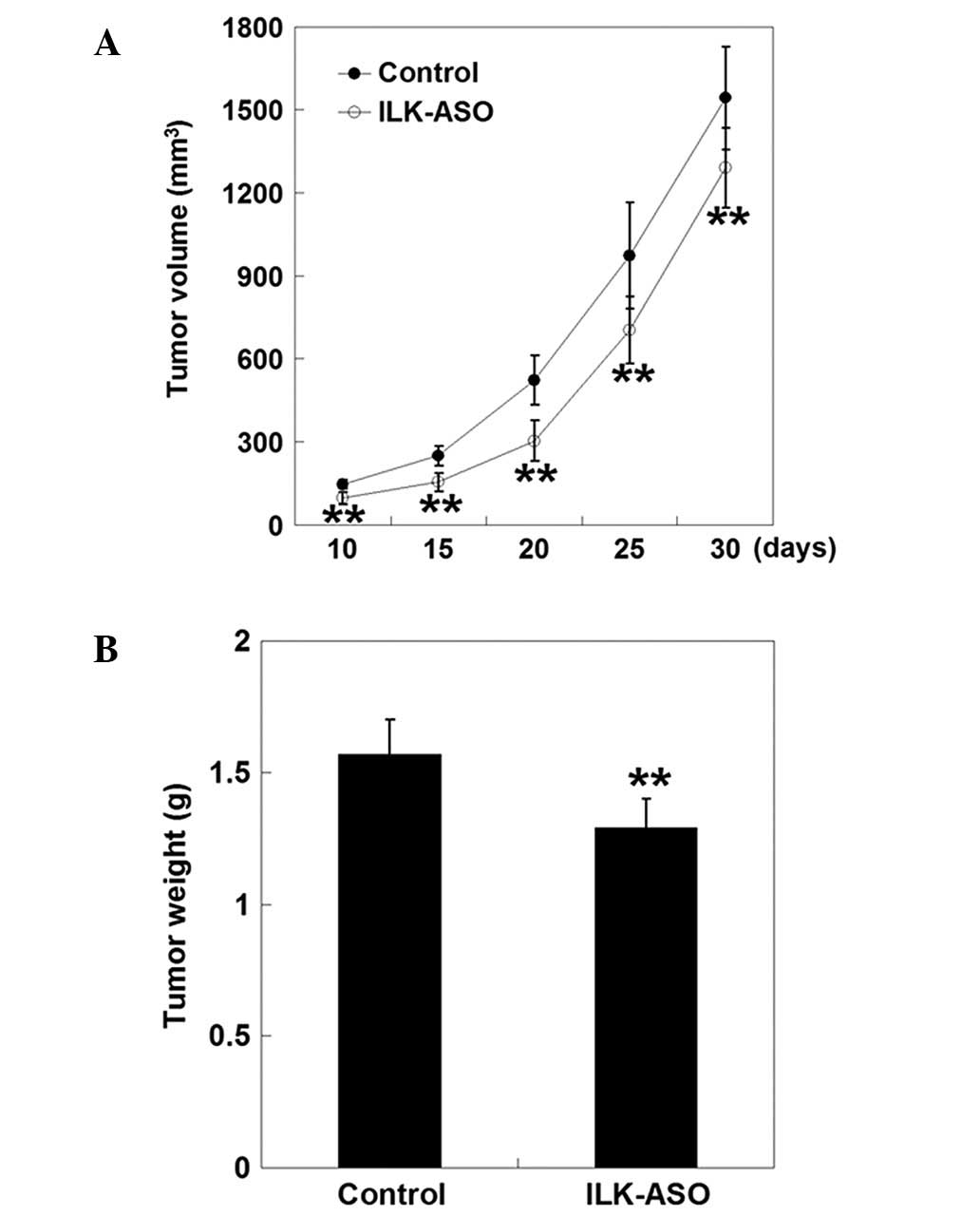
Furthermore, a single subcutaneous xenotransplanted
mass was formed in each nude mouse after tumor cell inoculation,
indicating that ILK gene silencing could not inhibit tumorigenesis
in nude mice. However, inoculation of cells transfected with
ILK-ASO markedly delayed the tumor formation when compared to
control mice (9.10±0.74 days vs. 5.30±0.67, respectively,
P<0.01). Thus, the tumor size was markedly decreased in mice
injected with tumor cells transfected with ILK-ASO (P<0.01,
Fig. 4). The inhibition rate
gradually elevated 10 days after inoculation, peaked at 20 days,
and then decreased thereafter (10 days, 33.77%; 15 days, 37.92%; 20
days, 41.79%; 25 days, 27.55%; and 30 days, 16.31%). Thirty days
after tumor cell inoculation, mice were sacrificed and tumor
tissues were removed and weighed. The average tumor weight in the
ILK-ASO group was statistically lower than that of the control
group (1.29±0.11 vs. 1.57±0.13 g, respectively, P<0.01). The
tumor weight inhibition rate for mice inoculated with ILK-ASO cells
was 17.83%. These observations indicated that inoculation of cells
transfected with ILK-ASO suppressed tumor growth in
vivo.
Discussion
ILK protein is a unique intracellular adaptor and
kinase that links the cell-adhesion receptors, integrins and growth
factors to the cytoskeleton. In addition, ILK regulates a variety
of intracellular signaling pathways (15). Thus, ILK plays an essential role in
the regulation of various cellular processes that are critical for
tumor progression, including tumor cell proliferation, migration,
invasion and survival, epithelial-mesenchymal transition and
angiogenesis (9). Moreover, the
regulatory activity of ILK protein is controlled by a network of
intracellular and intercellular processes that result in aberrant
ILK expression and signaling in a number of human malignancies
(17). This study explored the
role of ILK in mediating tumor cell proliferation in human ovarian
carcinoma by using ILK-ASO. ASO is an established and mature
technique in molecular biology, which is designed to
complementarily bind to specific mRNA, resulting in the degradation
of the message encoding the targeted protein (18). In particular, this method may be
used as a novel therapeutic tool in preclinical or future clinical
research. In this present study, an ASO specifically targeting
against ILK was synthesized and transfected into human ovarian
carcinoma HO-8910 cells. Our data showed that three days after
transfection, expression of ILK mRNA and protein was markedly
downregulated in HO-8910 cells, indicating the successful silencing
of ILK gene expression.
A previous study demonstrated that overexpression of
ILK protein promoted anchorage-independent cell cycle progression
(19). In this study, we knocked
down ILK expression and flow cytometric analyses revealed that ILK
silencing inhibited tumor cell proliferation via G0/G1 phase arrest
in HO-8910 cells. Our current data are in accordance with a
previous report, which demonstrated that transfection of a
kinase-deficient, dominant-negative form of ILK induced G1 phase
cycle arrest and enhanced tumor cell apoptosis in PTEN-mutant
prostate cancer cells (20). Taken
together, these observations indicate that ILK-induced tumor cell
proliferation was mediated by cell cycle progression.
Since silencing of the ILK gene by shRNA induced
growth inhibition and apoptosis in ovarian cancer SKOV3 cells
(14), we aimed to determine the
inhibitory effects of ILK gene silencing on tumorigenesis in
vivo. HO-8910 cells transfected with ILK-ASO or without
transfection (control) were subcutaneously injected into nude mice.
Inoculation of tumor cells transfected with ILK-ASO significantly
delayed tumor formation and suppressed tumor growth. These findings
are consistent with previous reports in other types of human
cancer. For example, Chan et al(13) suppressed tumorigenesis in
vivo after subcutaneously injecting ILK knockdown HCC cells
into the right flank of nude mice. Moreover, nude mice injected
with bladder BIU-87 cells, which were transfected with ILK small
interference RNA (siRNA), showed a significant inhibition in tumor
growth, as well as decreased tumor weight and microvessel density
and an increased apoptosis rate when compared with the control
groups (11). However, to date,
the underlying molecular mechanisms of ILK silencing-induced tumor
growth suppression are poorly understood. Activation of Akt
(11,13), glycogen synthase kinase 3-β
(GSK-3β) (11), or other unknown
pathways may contribute to this process.
In our current study, it should be noted that tumor
size inhibition gradually elevated 10 days after inoculation,
peaked at 20 days and decreased thereafter, implying that the
degradation of ASO may have resulted in the failure of growth
suppression 20 days after inoculation. Thus, ASO degradation at
later time points may be a limitation of this study. In order to
yield a better therapeutic outcome, more frequent administration of
ASO may be required in future studies.
Although encouraging results from preclinical and
clinical studies have been obtained and significant progress has
been made in developing ASO as an antitumor drug, it is not yet
recognized as an effective therapeutic (21). Future studies should continue to
compare the anti-tumorigenic capabilities between ASO and RNA
interference techniques, including siRNA and shRNA.
Acknowledgements
This study was supported in part by a grant from the
Natural Science Foundation of Heilongjiang Province (QC
2010067).
References
|
1
|
Notani P: Global variation in cancer
incidence and mortality. Curr Sci. 81:465–475. 2001.
|
|
2
|
Holschneider CH and Berek JS: Ovarian
cancer: epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cannistra SA, Ottensmeier C, Niloff J,
Orta B and DiCarlo J: Expression and function of beta 1 and alpha v
beta 3 integrins in ovarian cancer. Gynecol Oncol. 58:216–225.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cruet-Hennequart S, Maubant S, Luis J,
Gauduchon P, Staedel C and Dedhar S: alpha(v) integrins regulate
cell proliferation through integrin-linked kinase (ILK) in ovarian
cancer cells. Oncogene. 22:1688–1702. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ruseva Z, Geiger PX, Hutzler P, Kotzsch M,
Luber B, Schmitt M, Gross E and Reuning U: Tumor suppressor KAI1
affects integrin alphavbeta3-mediated ovarian cancer cell adhesion,
motility, and proliferation. Exp Cell Res. 315:1759–1771. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur S, Kenny HA, Jagadeeswaran S,
Zillhardt MR, Montag AG, Kistner E, Yamada SD, Mitra AK and Lengyel
E: {beta}3-integrin expression on tumor cells inhibits tumor
progression, reduces metastasis, and is associated with a favorable
prognosis in patients with ovarian cancer. Am J Pathol.
175:2184–2196. 2009.
|
|
7
|
Mulrooney J, Foley K, Vineberg S,
Barreuther M and Grabel L: Phosphorylation of the beta1 integrin
cytoplasmic domain: toward an understanding of function and
mechanism. Exp Cell Res. 258:332–341. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dedhar S, Williams B and Hannigan G:
Integrin-linked kinase (ILK): a regulator of integrin and
growth-factor signalling. Trends Cell Biol. 9:319–323. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McDonald PC, Fielding AB and Dedhar S:
Integrin-linked kinase - essential roles in physiology and cancer
biology. J Cell Sci. 121:3121–3132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed N, Riley C, Oliva K, Stutt E, Rice
GE and Quinn MA: Integrin-linked kinase expression increases with
ovarian tumour grade and is sustained by peritoneal tumour fluid. J
Pathol. 201:229–237. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gao J, Zhu J, Li HY, Pan XY, Jiang R and
Chen JX: Small interfering RNA targeting integrin-linked kinase
inhibited the growth and induced apoptosis in human bladder cancer
cells. Int J Biochem Cell Biol. 43:1294–1304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pontier SM, Huck L, White DE, Rayment J,
Sanguin-Gendreau V, Hennessy B, Zuo D, St-Arnaud R, Mills GB,
Dedhar S, et al: Integrin-linked kinase has a critical role in
ErbB2 mammary tumor progression: implications for human breast
cancer. Oncogene. 29:3374–3385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan J, Ko FC, Yeung YS, Ng IO and Yam JW:
Integrin-linked kinase overexpression and its oncogenic role in
promoting tumorigenicity of hepatocellular carcinoma. PLoS One.
6:e169842011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Xiao L, Yuan D, Shi X and Li P:
Silencing of the integrin-linked kinase gene induces the apoptosis
in ovarian carcinoma. J Recept Signal Transduct Res. 32:120–127.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: a cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Fukui K, Koike T and Zheng X:
Induction of autophagy in neurite degeneration of mouse superior
cervical ganglion neurons. Eur J Neurosci. 26:2979–2988. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McDonald PC and Dedhar S: The role of
integrin-linked kinase in cancer development and progression.
Cell-Extracellular Matrix Interactions in Cancer. Zent R and Pozzi
A: Springer; New York: pp. 245–273. 2010, View Article : Google Scholar
|
|
18
|
Monteith DK, Geary RS, Leeds JM, Johnston
J, Monia BP and Levin AA: Preclinical evaluation of the effects of
a novel antisense compound targeting C-raf kinase in mice and
monkeys. Toxicol Sci. 46:365–375. 1998.PubMed/NCBI
|
|
19
|
Radeva G, Petrocelli T, Behrend E,
Leung-Hagesteijn C, Filmus J, Slingerland J and Dedhar S:
Overexpression of the integrin-linked kinase promotes
anchorage-independent cell cycle progression. J Biol Chem.
272:13937–13944. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Persad S, Attwell S, Gray V, Delcommenne
M, Troussard A, Sanghera J and Dedhar S: Inhibition of
integrin-linked kinase (ILK) suppresses activation of protein
kinase B/Akt and induces cell cycle arrest and apoptosis of
PTEN-mutant prostate cancer cells. Proc Natl Acad Sci USA.
97:3207–3212. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rayburn ER and Zhang R: Antisense, RNAi,
and gene silencing strategies for therapy: mission possible or
impossible? Drug Discov Today. 13:513–521. 2008. View Article : Google Scholar : PubMed/NCBI
|