Introduction
In hemodialysis (HD) patients, vascular access (VA)
is a requirement for life and according to the K/DOQI guidelines,
an arteriovenous fistula (AVF) is the first choice of VA for
nephrologists. However, HD VA dysfunction affects the long-term
survival of dialysis patients as it is a major cause of inadequate
dialysis and cardiovascular disease (CVD) morbidity (1,2). The
mechanism behind VA failure is considered to be neointimal
hyperplasia of the vascular smooth muscle cells near the venous
anastomosis of the AVFs, resulting in vein wall thickening,
stenosis and, ultimately, occlusion (3). Chronic kidney disease (CKD) in itself
also accelerates the development of neointimal hyperplasia at the
anastomotic site of an AVF (4,5).
In previous years, a number of studies revealed that
the pathogenesis and manifestation of AVFs are similar to those of
atherosclerosis and that angiogensis occurs during typical
neointimal hyperplasia of the AVF (6–8).
Angiogenesis was thought to depend on a precise balance of positive
and negative regulation (9).
Angiopoietin-1 and angiopoietin-2 are antagonistic non-redundant
gatekeepers of endothelial activation and thus are potentially
significant factors in accelerated atherosclerosis (9–11).
Angiopoietin/Tie signaling is essential during embryonic vessel
assembly and maturation, as well as in functioning as a key
regulator of adult vascular homeostasis (12). In recent years, the
angiopoietin/Tie system, and angiopoietin-2 in particular, has
emerged as a predictive marker for cardiovascular risk in
hypertension, rheumatoid arthritis, dialysis and congestive heart
failure patients (10,13–15).
Sulodexide (KRX-101) is a mixture of GAGs composed
of 80% low-molecular mass heparin and 20% dermatan sulfate
(16). Sulodexide is effective in
partially reversing the thrombogenic coagulation profile without
increasing the risk of bleeding (17). Sulodexide has primarily been used
in treating peripheral occlusive arterial disease (18) and more recently to reduce albumin
excretion rates and improve microvasular function of diabetic
nephropathy. Sulodexide has also had a marked effect on plasma
viscosity and plasma fibrinogen concentrations (19). A previous study revealed that it
may be an effective adjunctive agent in myocardial
revascularization procedures as it is able to reduce myocardial
infarct size and the serum concentration of troponin I during
reperfusion (16).
Angiogenesis is implicated in endothelial and
cardiac injury. The present study provides a novel description and
characterization of neoangiogenesis occurring in the venous limb of
an AVF in the rat model which, as we previously demonstrated,
exhibits intimal hyperplasia and proinflammatory changes (20–23).
In the present study, the possible role of sulodexide in treating
the AVF model was tested to observe if there are any changes in the
fistula tissue. Angiopoietin-1, angiopoietin-2 and Tie-2 expression
were also emphasized in the angiogenesis process implicated in the
rat femoral AVF model.
Subjects and methods
Subjects
All experiments were approved by the local animal
humane board and were performed in accordance with Chinese
legislation on the protection of animals. Male 12-week-old Sprague
Dawley rats (250–300 g) were purchased from a commercial breeder
(Guangdong Medical Laboratory Animal Center, Guangdong, China). The
rats were kept in a climate-controlled room (21°C and 60% relative
humidity) with a 12-h cycle of light and darkness. All animals were
housed in normal cages with free access to water and food.
Surgery
Rats were anesthetized by sodium phenobarbital (60
mg/kg) and placed into a supine position on a heating pad (TR-200,
Fine Science Tools, Heidelberg, Germany) prior to performing an
end-to-side anastomosis of the femoral artery to the femoral vein.
The femoral vasculature was exposed by a 2-cm incision along the
left inguinal fold, and by retraction of the soft tissues and
abdominal musculature. The femoral artery and vein were freed from
the surrounding fascia and the femoral nerve by careful dissection
with the aid of a dissecting scope (Nikon Instruments, Melville,
NY, USA; ×10 to ×16 magnification). The branching vessels from the
femoral artery and vein were ligated doubly with sterile 6-0 silk
sutures and then divided. The artery was ligated at the distal end
of its exposure, clamped with a non-traumatic aneurysm clip at the
proximal end and transected just proximal to the ligation at a 45°
angle. Approximating clamps were situated on the vein, framing the
site of the anastomosis and a small longitudinal incision was made
with a microsurgical knife. The lumina of the two vessels were
rinsed with heparinized saline and the transected end of the artery
was attached to the opening in the adjacent vein using 11-0
monofilament Ethilon nylon sutures (Ethicon, Shanghai, China) to
make eight equidistant interrupted sutures. The approximating
clamps and the aneurysm clip were then removed and the arterial
flow into the femoral vein was verified by a visual inspection.
Finally, the femoral vein was ligated just distal to the
anastomosis and the skin was closed with 3-0 continuous sutures. In
a similar fashion, sham surgeries, consisting of the inguinal
incision and dissection of the vasculature from the surrounding
tissue, were performed on the control rats. At 8 weeks
post-surgery, the rats were euthanized for the harvest of the
vasculature of the AVF and to collect the femoral arteries and
veins from rats that underwent sham surgeries.
Study design
The rats were divided into 4 groups, the sham (n=6),
model (n=6), treatment (n=6) and treatment control groups (n=6).
Sulodexide (10 mg/kg) was injected subcutaneously six times a week
in the treatment and treatment control groups. Rats underwent the
AVF surgery in the model and treatment groups and were sacrificed 8
weeks later.
Measurements of systemic concentrations
of cytokines
Serum levels of angiopoietin-1, angiopoietin-2 and
soluble Tie-2 (sTie-2) were determined by ELISA kits (catalog no.
DANG10, DANG20 and DTE200, respectively).
Histology and immunoflurescence
Cardiac perfusion and tissue
preparation
Prior to tissue harvesting, anesthesia was
administered as described previously. The groinal incisions were
followed by cardiac puncture to flush the vessels, first with a
saline solution and then with 10% neutral formalin in
phosphate-buffered saline (PBS, pH 7.4). The femoral veins from the
four groups were gently removed and fixed in 10% neutral formalin
in PBS for histological and immunofluorescent analysis. Following
overnight fixation, the specimens were processed and embedded in
paraffin using standard techniques. The 4-μm longitudinal sections
were stained with hematoxylin-eosin (HE) for morphometrical
analysis and the 6-μm sections stained with HE for
immunofluorescent analysis at the site of the anastomosis. The rest
of tissues were stored at −80°C for further study.
Western blot analysis
Western blot analysis was performed as described in
the literature (8). Briefly,
proteins (25–60 μg) were separated on 10% Tris-HCl gels (Bio-Rad,
Hercules, CA, USA) and transferred to nitrocellulose membranes.
Primary antibodies for angiopoietin-1, angiopoietin-2, Tie-2, vWF
(catalog no. 612392, 610296 and 610431, respectively; Abcam,
Cambridge, MA, USA) or β-actin (catalog no. 2118; Cell Signaling
Technology Inc., Danvers, MA, USA) were used in overnight
incubations at 4°C. Horseradish peroxidase-conjugated secondary
antibodies were then used and bands were visualized using an
enhanced chemiluminescence method.
Immunofuorescence analysis
Immunofluorescence was performed using commercial
antibodies. Standard immunofuorescence protocols were used;
deparafinization and hydration was followed by antigen demasking
(2% citrate buffer in an autoclave) and nonspecific protein binding
with 10% fetal goat serum in 3% BSA (TBS) buffer for 60 min.
Primary antibody incubation took place overnight at 4°C and at room
temperature the next day. Secondary antibody blend incubation
followed for 60 min at room temperature and subsequent to washing
with PBS 3 times, another primary antibody was incubated for 60 min
followed by the relevent secondary antibody. Subsequent to this,
DAPI was used to stain the nuclei for 7 min and then samples were
embedded using a mounting medium.
Statistical analysis
Data are expressed as mean ± SEM. The Student’s
t-test and ANOVA were used for comparisons between the groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
General characteristics
AVF model rats were made by using an end-to-side
anastomosis of the femoral artery to the femoral vein. There were
no observable procedure-related complications. All animals survived
the procedures and were sacrificed at 8 weeks post-surgery. During
the observation period in between, there was no sign of any
peripheral ischemia resulting from steal syndrome or edema caused
by venous congestion.
Descriptive histology
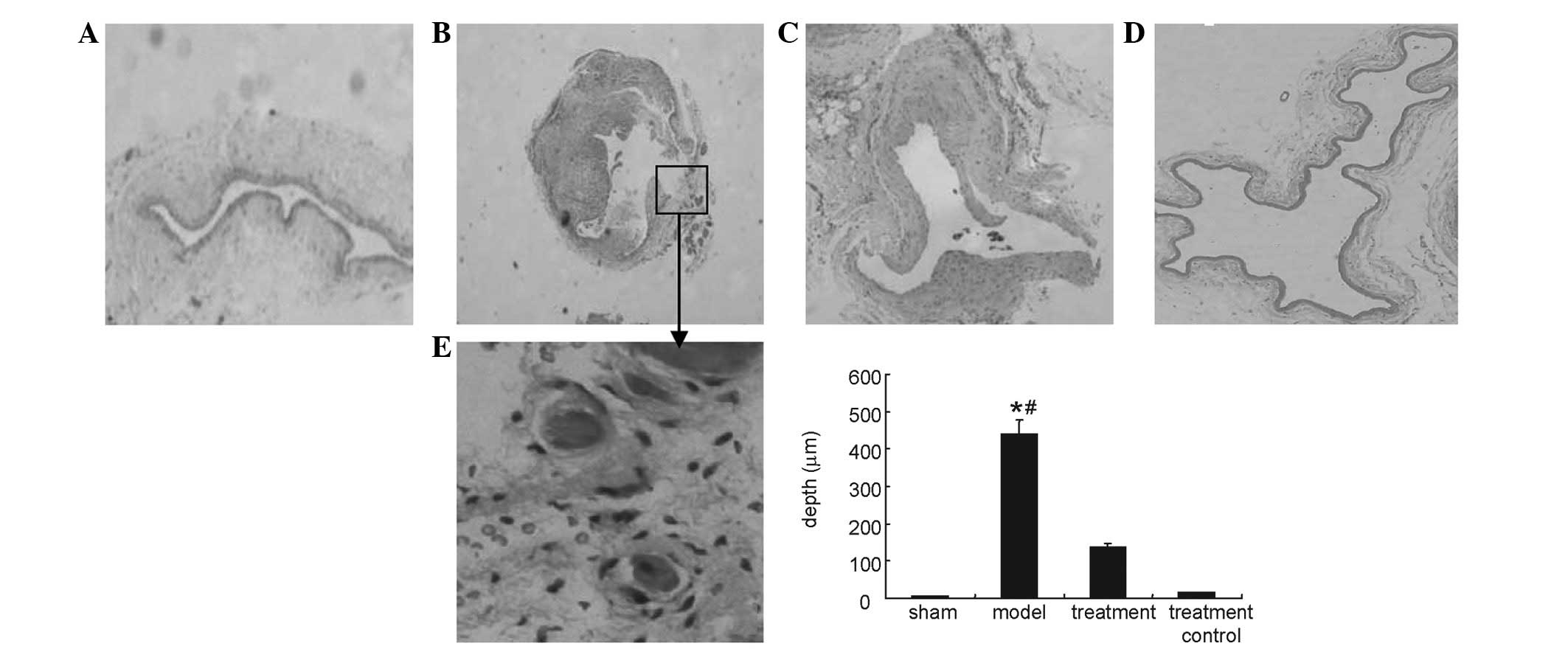
Fig. 1 shows
intimal hyperplasia of the venous limb as identified in the model
and treatment groups. Histological analysis revealed that intimal
hyperplasia was irregular with marked cellular proliferation and
with associated angiogenesis in certain areas. The depth of the
intima was decreased in the treatment group in comparison to that
of model group.
ELISA analysis
Serum levels of angiopoietin-1 and angiopoietin-2
were not detected by ELISA in the present study. However, serum
sTie-2 levels which were increased in the AVF model group were
significantly decreased in the sulodexide treatment group
(P<0.05). There was also a significant difference in serum
sTie-2 levels between the treatment and treatment control groups
(P<0.05), as depicted in Table
I.
 | Table ISerum concentrations of sTie-2 in the
four groups (ng/ml). |
Table I
Serum concentrations of sTie-2 in the
four groups (ng/ml).
| Variable | Sham group (n=6) | Model group
(n=6) | Treatment group
(n=6) | Treatment control
group (n=6) | P-value |
|---|
| sTie-2 | 0.030±0.010 | 0.094±0.034 | 0.055±0.022 | 0.029±0.005 | 0.015 |
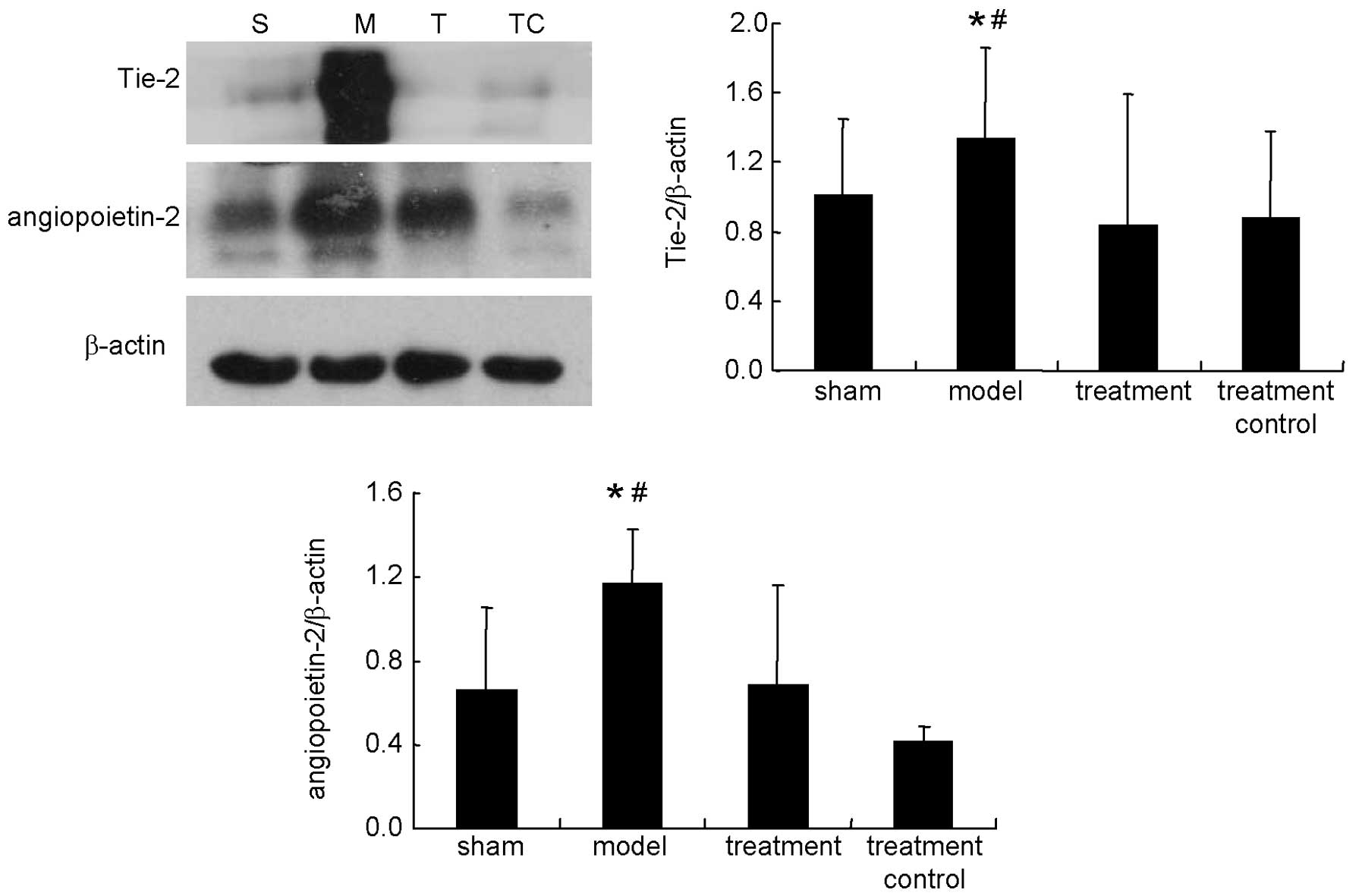
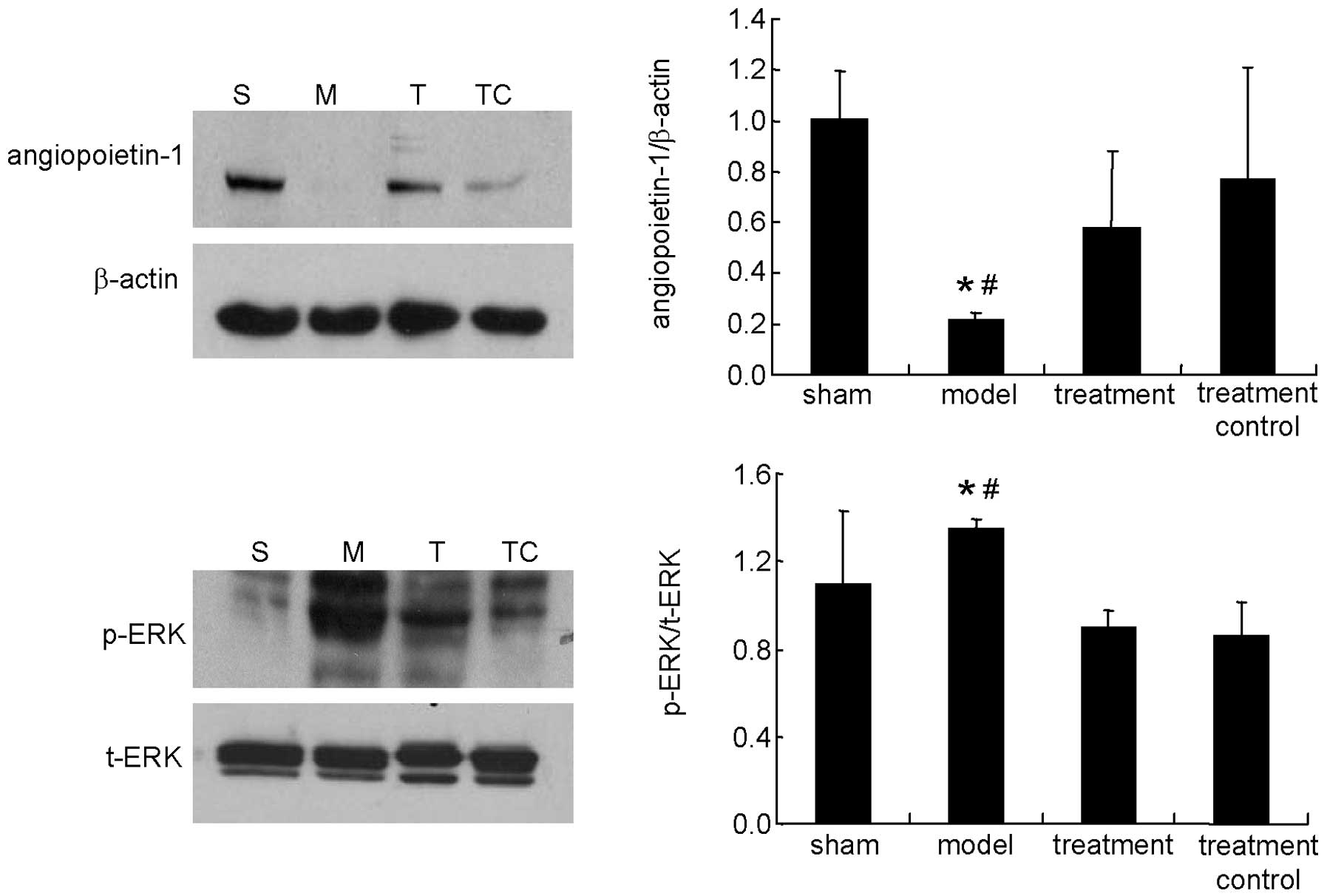
Western blot
As shown in Figs. 2
and 3, sulodexide decreased the
expression of angiopoietin-2 and Tie-2, which were upregulated in
the model (P<0.05), and increased the expression of
angiopoietin-1, which was downregulated in the model (P<0.05).
Sulodexide also inhibited the phosphorylation of ERK, which was
increased in the fistula tissue, when compared with tissue from the
sham group (P<0.05). There was no significant difference between
the sham and treatment control groups (P>0.05).
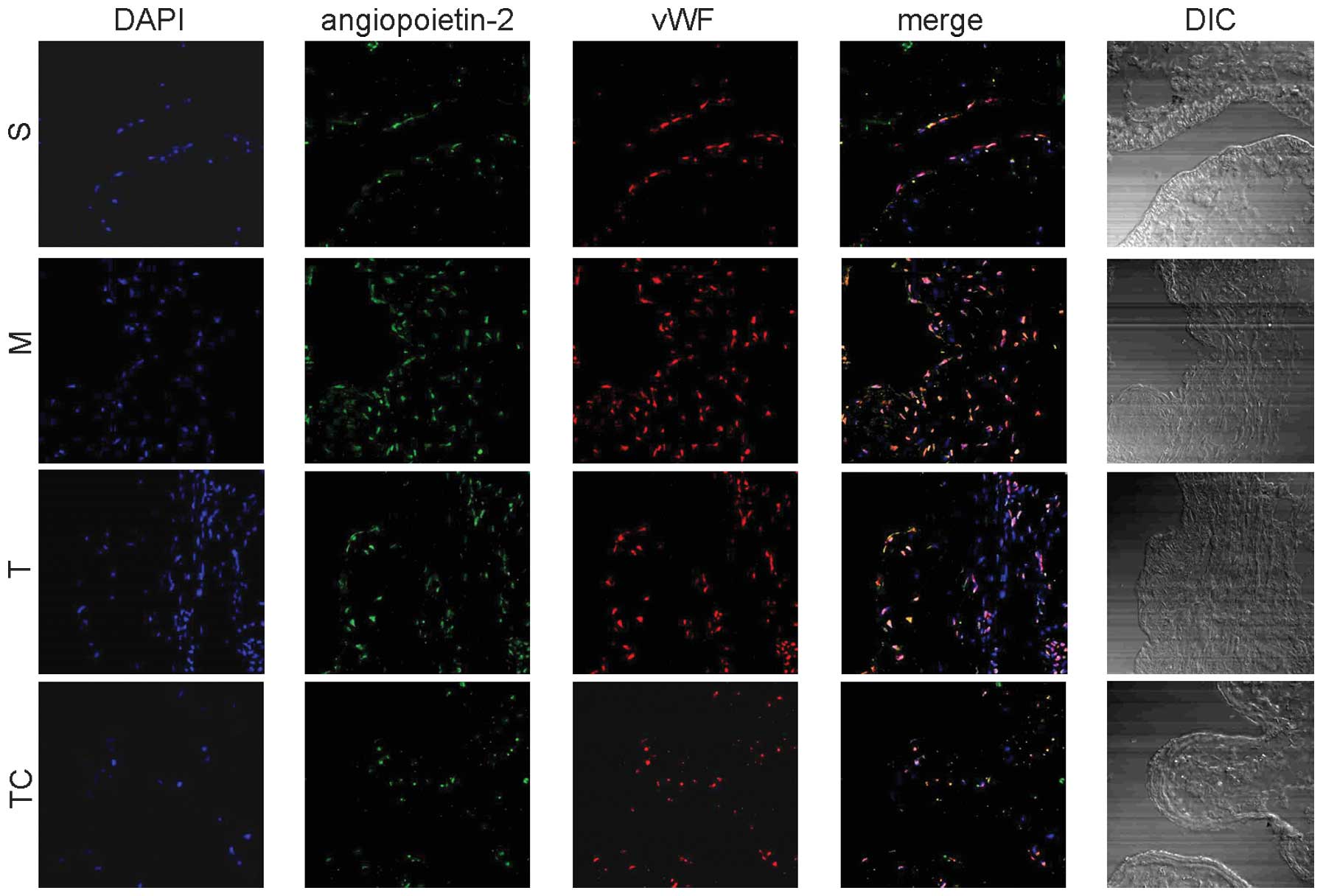
Immunoflorescent analysis
As shown in Fig. 4,
sulodexide was able to decrease angiopoietin-2 expression which was
markedly elevated in the model group. Angiopoietin-2 was
colocalized with vWF, an endothelial cell marker. It is possible
that sulodexide decreased angiopoietin-2 expression by inhibiting
endothelial cell proliferation to a certain extent.
Discussion
In an AVF, the venous vasulcature is exposed to the
arterial circulation and the veins are subjected to hemodynamic
stresses that they were not designed to cope with. This results in
venous intimal hyperplasia (7).
Oxygen and nutrient diffusion out of the veins and
arteries is impeded by hypertrophy and hyperplasticity.
Angiogenesis is able to circumvent this, but it may also promote
atherosclerotic plaque progression and ruptures. Neoangiogenesis
thus generates pathogenetically significant lesions and is the
focus of numerous investigations which aim to elucidate the role of
angiogenesis in hemodynamic change-induced cardiovascular
remodeling following the creation of an AVF.
Sulodexide is used for the prophylaxis and treatment
of thromboembolic diseases (peripheral vascular diseases, PVDs),
however, a recent study has also demonstrated the beneficial
effects of sulodexide in animal models of reperfusion injury
(16) and the treatment of
diabetic nephropathy (20),
suggesting it has cardiovascular protection properties. In the
present study, sulodexide was used to treat an animal model of an
AVF. Intimal hyperplasia was alleviated significantly by sulodexide
and a possible role for the drug was implicated in the process.
Previous studies have demonstrated that
angiopoietin-2/Tie-2 is part of the molecular response to shear
stress which may regulate angiogenesis. Therefore, the present
study measured the concentration of angiogenic species,
angiopoietin-1, angiopoietin-2 and sTie-2 in the circulation.
However, angiopoietin-1 and angiopoietin-2 levels were undetectable
in the serum, while sTie-2 upregulation was observed in the AVF
model group and downregulation occurred following sulodexide
treatment.
The present study also examined whether the
expression of angiopoietin-1, angiopoietin-2 and Tie-2 was induced
within the venous limbs of the AVF. The results showed that protein
expression of angiopoietin-2 and Tie-2 was upregulated while
angiopoietin-1 was downregulated in the model. Following sulodexide
treatment, angiopoietin-2 expression was downregulated and
angiogenesis was decreased in the AVF. Angiopoietin-2 participates
in flow-dependent vascular adaptation (22) and its expression may be increased
by activation of the AMP-activated protein kinase induced by shear
stress (23). The upregulation of
angiopoietin-2 may be a result of angiogenesis responding to the
unusual increased blood flow. In models characterized by vascular
injury, another study has also demonstrated that sulodexide is able
to inhibit intima proliferation in the carotid artery (24).
The present study extends these observations by
demonstrating that upregulation of vWF also occurs in the venous
circulation when it is subjected to an increased blood flow.
Angiopoietin-2 and vWF antibody were also used for
immunofluorescent analysis to determine the phenotype of these
angiopoietin-2-positive cells using laser confocal microscopy. A
colabeling technique was employed that also probed for the presence
of a marker of the endothelial cells (vWF). Angiopoietin-2 and vWF
were identified as colocalized in the cytoplasm of the endothelial
cells. This showed that sulodexide was able to decrease
angiopoietin-2 expression which was increased in the AVF.
Endothelial cell proliferation was clearly observed in the venous
limbs of the AVF. It is possible that sulodexide decreases the
angiopoietin-2 expression by inhibiting endothelial cell
proliferation to a certain extent.
In the present study, sulodexide decreased intimal
hyperplasia, possibly through regulation of the angiopoietin/Tie
system, which is dysregulated in the process of intimal hyperplasia
induced by venous hypertension. Further study of the details of
this mechanism are required. To a certain extent, this study may
provide therapeutic prospects for intimal hyperplasia of AVF in the
future.
Acknowledgements
The authors would like to thank Dr Liu from the
Department of Vascular Surgery for his assistance in making the AVF
model and also Professor Huang Xi from the Department of
Immunology, Sun Yat-sen University, for his assistance with the
experiment. This study was financially supported by the Natural
Science Foundation of Guangdong Province, P.R. China
(2012B031800448).
References
|
1
|
Manca O, Pisano GL, Carta P, et al: The
management of hemodialysis arteriovenous fistulas in well
functioning renal transplanted patients: many doubts, few
certainties. J Vasc Access. 6:182–186. 2005.PubMed/NCBI
|
|
2
|
Lee T and Roy-Chaudhury P: Advances and
new frontiers in the pathophysiology of venous neointimal
hyperplasia and dialysis access stenosis. Adv Chronic Kidney Dis.
16:329–338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Langer S, Heiss C, Paulus N, et al:
Functional and structural response of arterialized femoral veins in
a rodent AV fistula model. Nephrol Dial Transplant. 24:2201–2206.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kokubo T, Ishikawa N, Uchida H, et al: CKD
accelerates development of neointimal hyperplasia in arteriovenous
fistulas. J Am Soc Nephrol. 20:1236–1245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Langer S, Kokozidou M, Heiss C, et al:
Chronic kidney disease aggravates arteriovenous fistula damage in
rats. Kidney Int. 78:1312–1321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rodríguez-Pla A, Bosch-Gil JA,
Rosselló-Urgell J, Huguet-Redecilla P, Stone JH and
Vilardell-Tarres M: Metalloproteinase-2 and -9 in giant cell
arteritis: involvement in vascular remodeling. Circulation.
112:264–269. 2005.PubMed/NCBI
|
|
7
|
Caplice NM, Wang S, Tracz M, et al:
Neoangiogenesis and the presence of progenitor cells in the venous
limb of an arteriovenous fistula in the rat. Am J Physiol Renal
Physiol. 293:F470–F475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kanwar YS: Functional duality of
progenitor cells influxing into arteriovenous fistula during its
neoangiogenesis. Am J Physiol Renal Physiol. 293:F468–F469. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maisonpierre PC, Suri C, Jones PF, et al:
Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo
angiogenesis. Science. 277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
David S, Kümpers P, Hellpap J, et al:
Angiopoietin 2 and cardiovascular disease in dialysis and kidney
transplantation. Am J Kidney Dis. 53:770–778. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vandenbunder B: Angiopoietin-2, a new
molecular actor involved in vascular tree morphogenesis. Bull
Cancer. 84:1079–1080. 1997.(In French).
|
|
12
|
Augustin HG, Koh GY, Thurston G and
Alitalo K: Control of vascular morphogenesis and homeostasis
through the angiopoietin-Tie system. Nat Rev Mol Cell Biol.
10:165–177. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chong AY, Caine GJ, Freestone B, Blann AD
and Lip GY: Plasma angiopoietin-1, angiopoietin-2, and angiopoietin
receptor tie-2 levels in congestive heart failure. J Am Coll
Cardiol. 43:423–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel JV, Lim HS, Varughese GI, Hughes EA
and Lip GY: Angiopoietin-2 levels as a biomarker of cardiovascular
risk in patients with hypertension. Ann Med. 40:215–222. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Westra J, de Groot L, Plaxton SL, et al:
Angiopoietin-2 is highly correlated with inflammation and disease
activity in recent-onset rheumatoid arthritis and could be
predictive for cardiovascular disease. Rheumatology (Oxford).
50:665–673. 2011. View Article : Google Scholar
|
|
16
|
Lauver DA, Booth EA, White AJ, Poradosu E
and Lucchesi BR: Sulodexide attenuates myocardial
ischemia/reperfusion injury and the deposition of C-reactive
protein in areas of infarction without affecting hemostasis. J
Pharmacol Exp Ther. 312:794–800. 2005. View Article : Google Scholar
|
|
17
|
Kim SB, Kim SH, Lee MS, Chang JW, Lee SK
and Park JS: Effects of sulodexide on hemostatic factors, lipid
profile, and inflammation in chronic peritoneal dialysis patients.
Perit Dial Int. 27:456–460. 2007.PubMed/NCBI
|
|
18
|
Shustov SB: Controlled clinical trial on
the efficacy and safety of oral sulodexide in patients with
peripheral occlusive arterial disease. Curr Med Res Opin.
13:573–582. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lunetta M and Salanitri T: Lowering of
plasma viscosity by the oral administration of the
glycosaminoglycan sulodexide in patients with peripheral vascular
disease. J Int Med Res. 20:45–53. 1992.PubMed/NCBI
|
|
20
|
Rossini M, Naito T, Yang H, et al:
Sulodexide ameliorates early but not late kidney disease in models
of radiation nephropathy and diabetic nephropathy. Nephrol Dial
Transplant. 25:1803–1810. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin T, Horsfield C and Robson MG:
Arteriovenous fistula in the rat tail: a new model of hemodialysis
access dysfunction. Kidney Int. 74:528–531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bongrazio M, Baumann C, Zakrzewicz A,
Pries AR and Gaehtgens P: Evidence for modulation of genes involved
in vascular adaptation by prolonged exposure of endothelial cells
to shear stress. Cardiovasc Res. 47:384–393. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dixit M, Bess E, Fisslthaler B, et al:
Shear stress-induced activation of the AMP-activated protein kinase
regulates FoxO1a and angiopoietin-2 in endothelial cells.
Cardiovasc Res. 77:160–168. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park HY, Kang S, Kim GY, et al: Inhibition
of neointimal proliferation of rat carotid artery by sulodexide. J
Korean Med Sci. 12:210–214. 1997. View Article : Google Scholar : PubMed/NCBI
|