Introduction
Atherosclerosis (AS) plaque cell apoptosis is the
main factor leading to plaque instability (1–3).
Cell apoptosis occurs in various periods of AS damage. Apoptotic
cells in the plaque are mainly vascular smooth muscle cells (VSMCs)
and macrophages. Excessive VSMC proliferation and apoptosis
constitute the main pathological basis of AS plaque formation,
which has a significant impact on the development and outcome of AS
(3,4). AS plaque formation depends on the
relative balance of apoptosis and proliferation of VSMCs. Cell
proliferation is dominant in the early period of AS. With
progression of AS, particularly in the late stage, VSMC apoptosis
directly affects the arterial shape and structure, and increases
the plaque instability. This is the major factor causing clinical
manifestations (5). Thus,
preventing apoptosis of VSMCs in late-stage AS would have a
positive impact in preventing the occurrence of serious clinical AS
complications.
As the knowledge of the importance of oxidative
stress and oxidized low density lipoprotein in the pathogenesis of
AS has increased, experimental reports on probucol inhibition of AS
progression, promotion of regression of the AS plaque, and
prevention of restenosis following interventional therapy have been
increasing (6–8). The correlation between probucol and
inflammatory mediators, endothelial function, cytokines,
antioxidant enzymes, particularly the correlation between
intracellular signaling proteins and transcription factor activity
on VSMC proliferation and apoptosis, and the role in the AS
pathophysiology, has become a hot topic of research in recent years
(7,9,10).
Previous studies confirmed that by maintaining intracellular redox
balance, probucol reduced intracellular GSH consumption and
inhibited H2O2-induced apoptosis in VSMCs
(11). In the process of
apoptosis, Trx-1 and ASK-1 associated with MAPK pathways also play
an important role. Whether the anti-apoptotic effects of probucol
are related to these factors has not been reported. In this study,
H2O2 was used as an apoptosis-inducing agent,
and we observed the effect of probucol on VSMC apoptosis and Trx-1
and ASK-1 expression, and explored its anti-apoptotic
mechanism.
Materials and methods
Animals
Healthy Wistar rats, male, clean and weighing 171±18
g, were provided by the Experimental Animal Center of Shandong
University. The study was approved by the College of Environmental
and Chemical Engineering, Nanchang University, Nanchang, Jiangxi,
P.R. China.
VSMC culture
VSMCs were cultured using the tissue attached
method. Rat thoracic aorta was obtained, the vascular adventitia
was stripped, the endometrium was removed, cut into lx1 mm tissue
slices and attached to the culture bottle, cultured at 37°C in 5%
CO2 conditions containing 20% fetal calf serum DMEM
medium, and the medium was changed once every 3–5 days. When the
cells grew to be 60–80% confluent, 0.25% trypsin was added for
digestion and cells were passaged at 4–6 days. Cells were
spindle-shaped, and the typical ‘peak-valley’-like structure
appeared when they grew into a dense layer. VSMCs of passage 3–8
were used in this experiment.
Flow cytometry
VSMCs were digested with 0.25% trypsin, adjusting
the cell concentration to 2×107 cells/l density and
seeded in 96-well culture plates. The cells were divided into a
control group, a model group (1 mmol/l
H2O2,), and probucol 1, 10, 100 μmol/l
groups. After 24 h of culture at 37°C in a 5% CO2
incubator, H2O2 and probucol were added and
the cells were cultured for 6 h. The medium was then discarded,
cells were washed with PBS and dispersed into single cells
following digestion with 0.25% trypsin protease. The cell
suspension (0.5–1×106/ml) was washed twice with PBS,
centrifuged at 2000 rpm/min for 5 min, the supernatant was
discarded and 100 μl binding buffer and 10 μl FITC-labeled Annexin
V (20 μg/ml) were added. The samples were kept in the dark at room
temperature for 30 min, then 5 μl PI (50 μg/ml) was added, the
samples were reacted in the dark for 5 min, 400 μl binding buffer
was added, and the cells were immediately detected by flow
cytometry using FACScan. Samples without Annexin V-FITC and PI were
used as the negative control. Cell apoptosis was analyzed using
WinMDI29 image processing software. The effects of different
concentrations of probucol treatment on the apoptosis rate of VSMCs
were observed.
TUNEL staining
The cell groups mentioned previously were cultured
in 6-well plates (built-in cover glass). The coverslips inoculated
with cells were taken and stained according to the TUNEL labeling
staining kit instructions and observed under the microscope. The
nuclei that appeared brown were positive. Four slides were
observed, each at ×200 magnification, in order to calculate the
apoptosis index (AI). AI represented the percentage of the number
of positive cells and the total cell number in five views selected
from each slide.
Hoechst 33258 staining
The cultured cells were taken, washed with PBS and
fixed for 10 min. Cells were washed with PBS, and stained with
Hoechst 33258 dye at room temperature for 10 min. After washing
with PBS, excess liquid was absorbed, cells were mounted under a
fluorescence microscope, and the morphological changes of the cells
were observed.
Western blot analysis
After the cells were collected and washed three
times with iced PBS, lysed by adding an appropriate amount of
lysate in ice bath conditions and centrifuged at 12000 rpm for 15
min, the supernatant was carefully drawn, and the protein
concentration was determined using the BCA method. Protein samples
(40 μg) were taken, ASK-l proteins were separately run on 10%
SDS-PAGE gels, and Trx-1 on 15% SDS-PAGE gels (laminated plastic 80
mV, gel 120 mV), then electrophoretically transferred (150 mA, 1.5
h) to a PVDF membrane, blocked using 5% non-fat dry milk for 1 h,
and incubated with rabbit anti-ASK-1 (1:100) and Trx-1 (1:100)
polyclonal antibodies overnight at 4°C. After washing with TBST
three times, l:1000 concentrations of second antibody were added
for l h. After washing with TBST three times, the membranes were
evenly coated with the same amount of chemiluminescence A and B
solution and underwent electrochemiluminescence immunoassay (ECL)
for 5 min. A GIS image processing system was used for analysis.
Statistical analysis
SPSS 15 statistical software was used for analysis,
and data were shown with the means ± SD. Sample means were compared
using analysis of variance and the LSD pairwise comparison
method.
Results
VSMC apoptosis
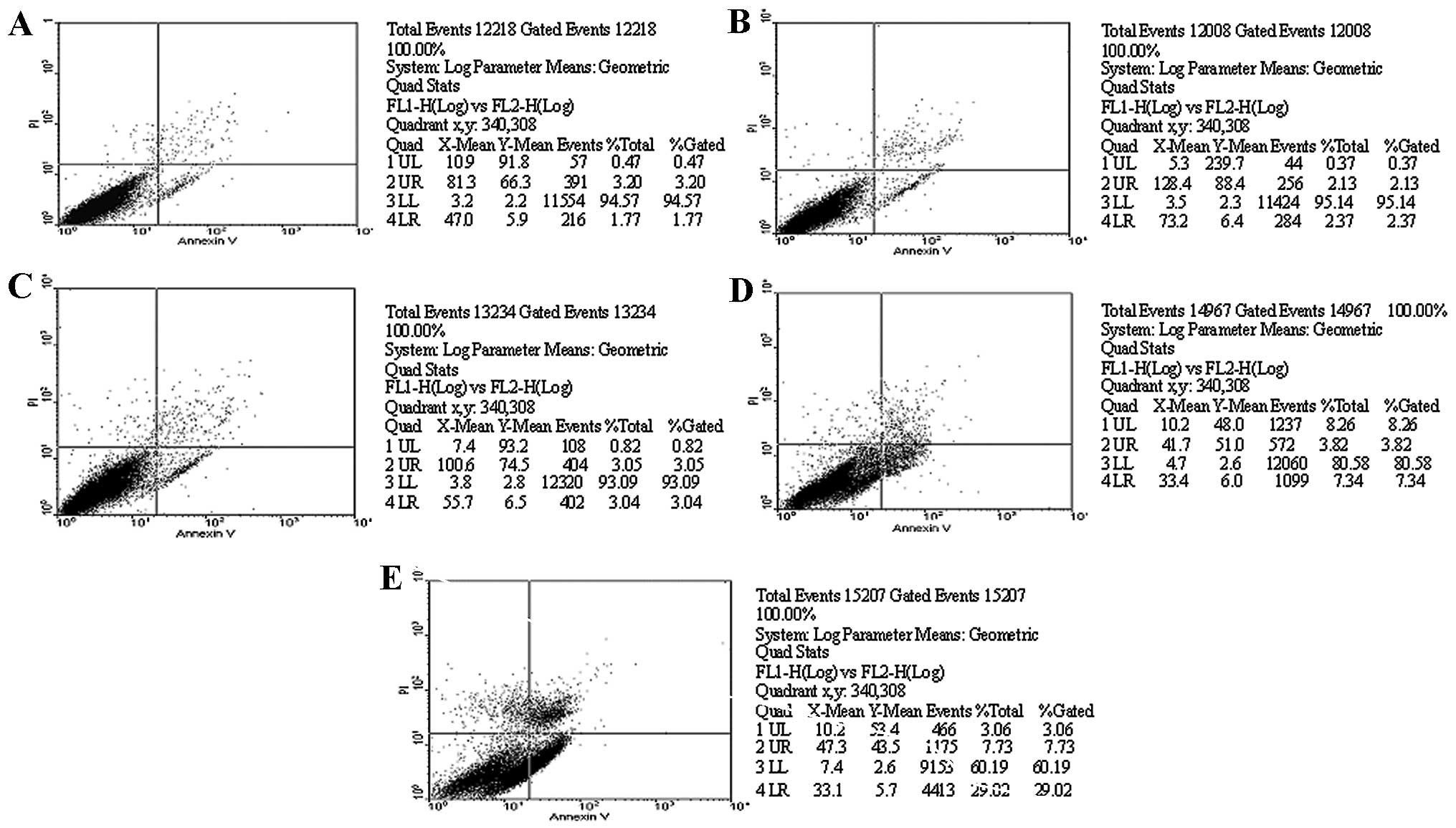
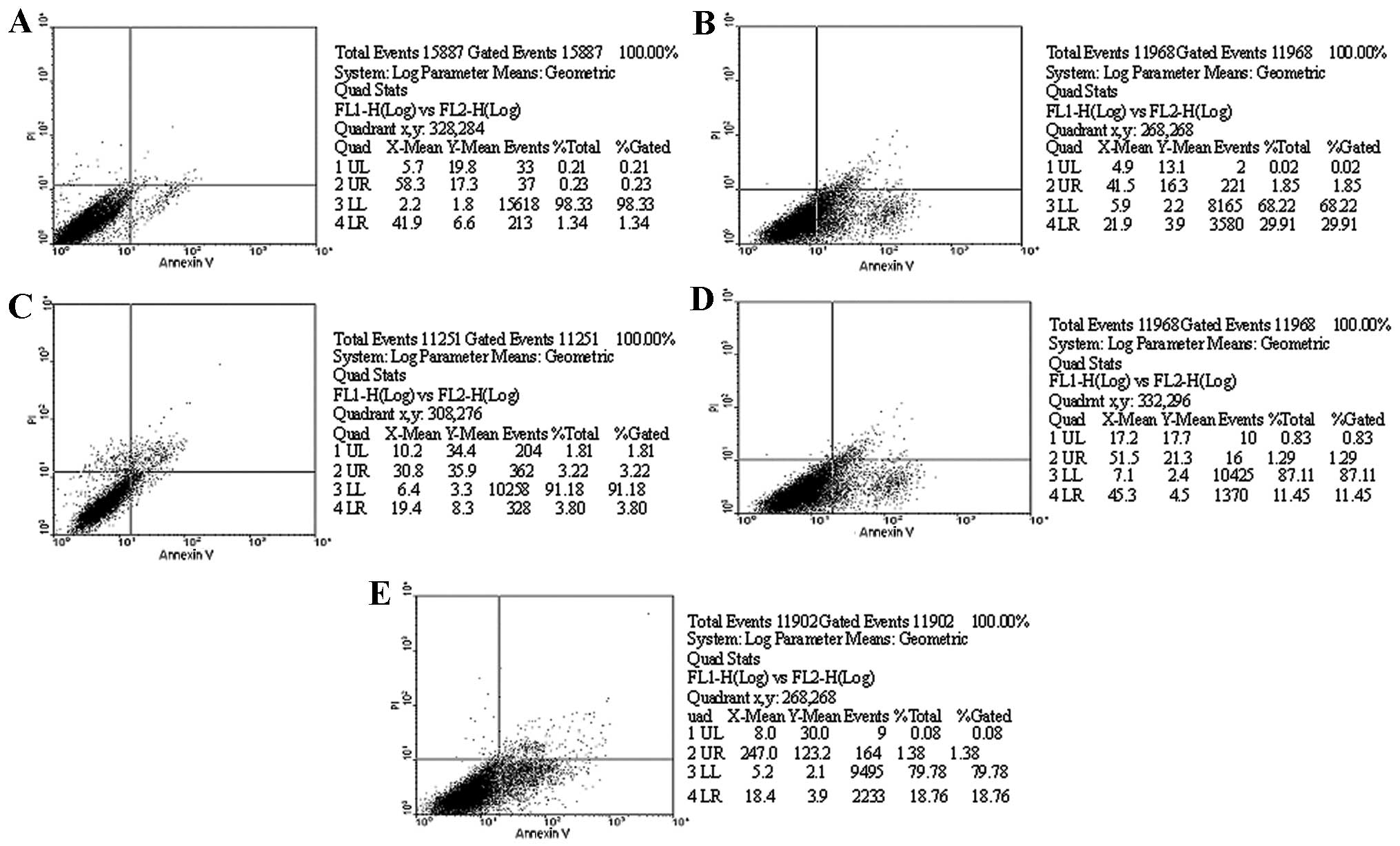
As shown in Table
I, H2O2 had marked effects on the VSMC apoptosis rate. Flow
cytometry analysis showed that VSMC apoptosis was significantly
increased by 23.9±5.8% after cells were treated with
H2O2 for 6 h. Compared with the
H2O2 group, the probucol group significantly
inhibited H2O2-induced apoptosis, and the
apoptotic rates were 3.8±2.8% in the 100 μmol/l group (P<0.01),
11.3±4.1% in the 10 μmol/l group (P<0.05) and 16.8±4.5% in the 1
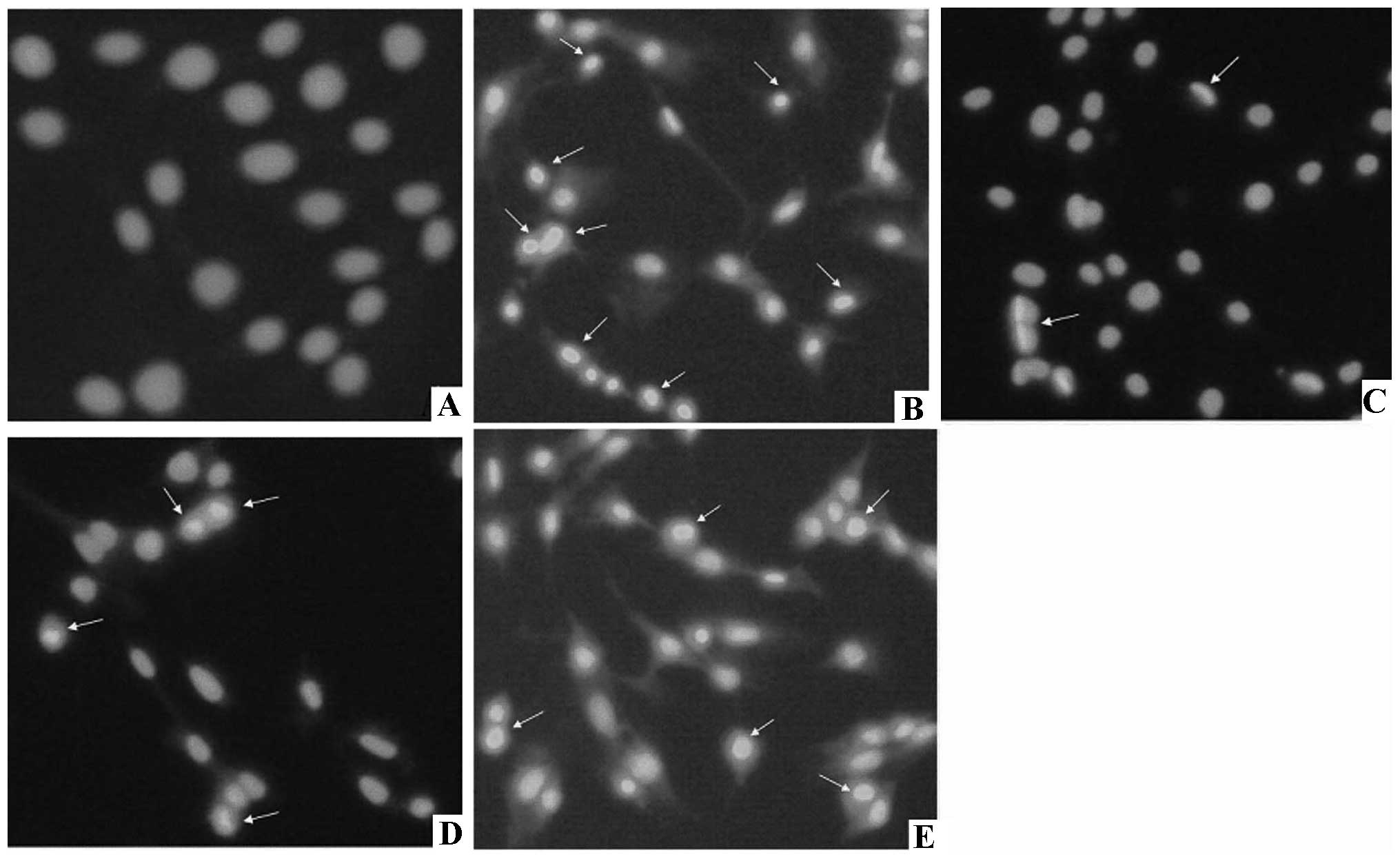
μmol/l, showing a concentration-dependent effect (Figs. 1 and 2). Hoechst 33258 staining showed that
nuclear condensation occurred in the H2O2
treatment group. A compact fluorescent stain was observed in the
nucleus and cytoplasm, with a deep and bright color. Hoechst 33258
staining showed that nuclear condensation occurred in the
H2O2 treatment group. A compact fluorescent
stain was observed in the nucleus and cytoplasm (Fig. 3). Compared to control (1.46±0.82%),
VSMC apoptosis rates for the H2O2 group, and
the 100, 10, 1 μmol/l probucol groups were 29.57±5.81, 3.50±1.54,
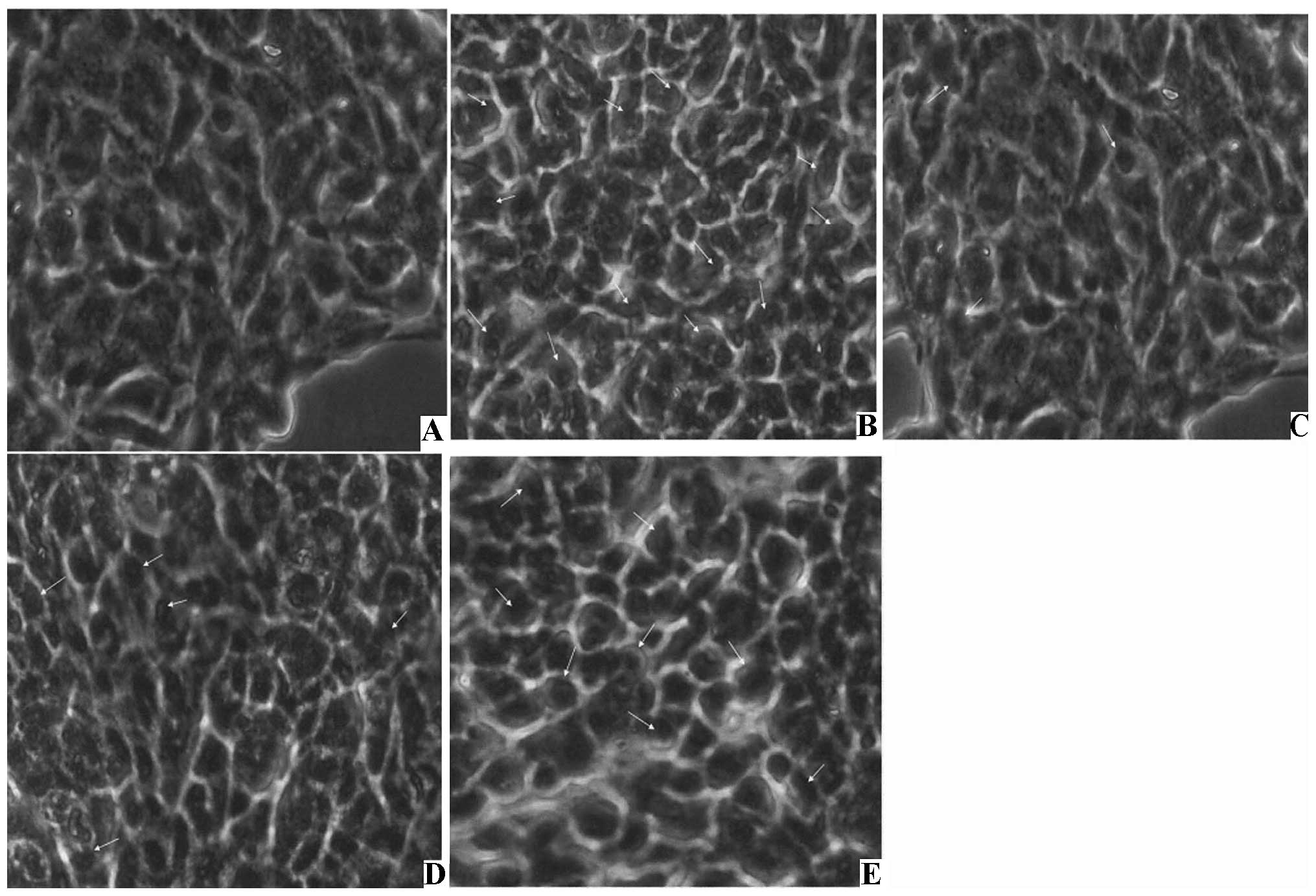
12.36±4.10 and 17.96±2.81%, respectively (Table II). The TUNEL assay revealed that
apoptotic nuclei were brown in color, had nuclear condensation, the
chromatin condensed into a cluster, and showed a strong positive
change (Fig. 4, Table III). The AI in the control group
was 3.22±1.12%, in the H2O2 group it was
28.28±5.24%, in the 100 μmol/l probucol group it was 4.58±1.27%
(P<0.01 compared with the H2O2 group), in
the 10 μmol/l it was 11.38±2.46% (P<0.05 compared with the
H2O2 group), and in the 1 μmol/l group it was
18.33±3.24%.
 | Table IEffect of H2O2
on VSMC apoptosis rate (mean ± SD, n=4). |
Table I
Effect of H2O2
on VSMC apoptosis rate (mean ± SD, n=4).
| Group | Concentration
(μmol/l) | Living cell rate
(%) | Apoptosis rate
(%) |
|---|
| Control | | 95.03±5.27 | 1.73±0.85 |
|
H2O2 | 100 | 94.52±6.43 | 2.35±0.81 |
| 250 | 93.13±8.82 | 3.10±1.26 |
| 500 | 80.49±3.16 | 7.39±1.04 |
| 1000 | 60.31±4.60 | 28.87±2.31a |
 | Table IIEffect of different concentrations of
probucol on H2O2-induced VSMC apoptosis
(Hoechst 33258 staining) (mean ± SD, n=4). |
Table II
Effect of different concentrations of
probucol on H2O2-induced VSMC apoptosis
(Hoechst 33258 staining) (mean ± SD, n=4).
| Group | Concentration
(μmol/l) | Living cell rate
(%) | Apoptosis rate
(%) |
|---|
| Control | | 97.86±5.27 | 1.46±0.82 |
|
H2O2 | 1000 | 68.54±1.43 | 29.57±5.81a |
| Probucol | 100 | 92.18±7.82 | 3.50±1.54b |
| 10 | 85.17±6.92 | 12.36±4.10c |
| 1 | 79.68±4.50 | 17.96±2.81 |
 | Table IIIEffect of different concentrations of
probucol on H2O2-induced VSMC apoptosis
(TUNEL method) (mean ± SD, n=4). |
Table III
Effect of different concentrations of
probucol on H2O2-induced VSMC apoptosis
(TUNEL method) (mean ± SD, n=4).
| Concentration
(μmol/l) | Apoptotic index
(%) |
|---|
| Control | | 3.22±1.12 |
|
H2O2 | 1000 | 28.28±5.24a |
| Probucol | 100 | 4.58±1.27c |
| 10 | 11.38±2.46b |
| 1 | 18.33±3.24 |
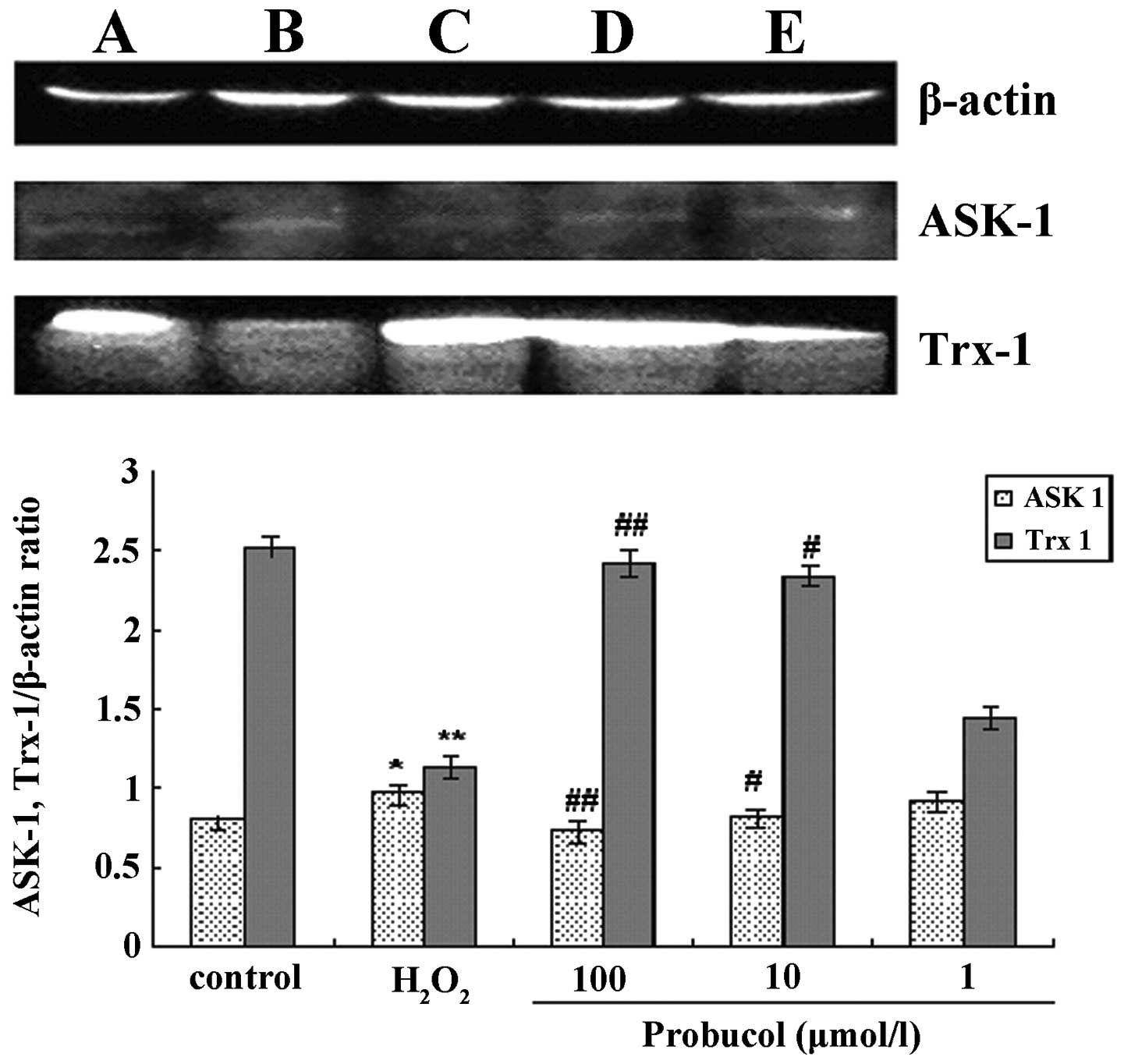
ASK-1 and Trx-1 protein expression
After VSMCs were treated with 1 mmol/l
H2O2 the ASK-1 protein expression was
significantly increased and Trx-1 protein expression decreased,
compared with the control group. Probucol treatment is capable of
reducing the ASK-1 protein expression and increasing Trx-1
expression (Fig. 5, Table IV).
 | Table IVEffects of different concentrations
of probucol on the expression of ASK-1 and Trx-1 in VSMCs
stimulated by H2O2 (mean ± SD, n=4). |
Table IV
Effects of different concentrations
of probucol on the expression of ASK-1 and Trx-1 in VSMCs
stimulated by H2O2 (mean ± SD, n=4).
| ASK-1 | Trx-1 |
|---|
| Control | 0.80±0.04 | 2.52±0.06 |
|
H2O2
(μmol·l−1) |
| 1000 | 0.97±0.05a | 1.13±0.07b |
| Probucol
(μmol·l−1) |
| 100 | 0.74±0.05d | 2.42±0.08d |
| 10 | 0.82±0.04c | 2.24±0.06d |
| 1 | 0.92±0.06 | 1.44±0.07 |
Discussion
Oxidative stress is considered to be a major factor
that causes VSMC damage. Reactive oxygen species (ROS) play an
important role in mediating the process of vascular wall apoptosis
(12,13). As they constitute the vessel wall
structure and are the major cellular components in the maintenance
of vascular tone, VSMCs are important in AS. Under many
pathological conditions, both oxidized low-density lipoprotein
(ox-LDL)-induced VSMC apoptosis and VSMC proliferation stimulated
by high glucose, ROS are important mediating factors (14–16).
ROS are capable of altering the intracellular redox balance,
activating the redox-sensitive signaling protein kinases and
transcription factors, and inducing apoptosis and proliferation
through different signal transduction pathways (15,17–19).
Therefore, the redox state of equilibrium formed by the molecular
oxidation and reduction reactions will have a decisive role in VSMC
proliferation or apoptosis, and thus affect vascular disease
development and prognosis. The intracellular thioredoxin and
glutathione (GSH) systems constitute the most two important
reduction systems. The thioredoxin system consists of NADPH,
thioredoxin reductase (TrxR) and Trx. Trx-1 is located in the
cytoplasm, which is characterized by the surface of the protein
molecules having a Trp-Cys-Gly-Pro-Cys-Lys structure, two of which
may be oxidized to form a Cys reversible disulfide bridge, and
re-reducted by the TrxR with NADPH participation. Trx activity was
capable of reducing the SS bridge exposed on the transcription
factor surface and keeping the reduction state and restoring the
transcription factor activity. Trx-1 has anti-apoptotic activity.
ASK-1 is activated by cytokines and stress stimuli factors. By
activating c-Jun NH2-terminal kinase (JNK) and p38MAPK, cell
apoptosis is induced (13,20,21).
Trx-1 is the main regulatory factor of ASK-1 that functions through
the oxidation of the Trx active site Trp-Cys-Gly-Pro-Cys-Lys
structure. ROS cause the two cysteines to form intramolecular
disulfide bonds, form the oxidized Trx, separate Trx and ASK-1, and
free ASK-1 induces apoptosis through the JNK/p38MAPK pathway
(21,22).
H2O2 is one of the most
important ROS signal transduction molecules. It can induce
apoptosis of many types of cells, and can also be used as an
extracellular second messenger of stimulating factors involved in
the process of intracellular signal transduction that induce
apoptosis (20,23). H2O2 may
induce apoptosis through a number of different signaling pathways.
H2O2 is capable of enhancing
mitogen-activated protein kinases (MAPKs), mainly through c-Jun
N-terminal kinase (JNK) and p38 MAPK. However, the
non-extracellular signal-regulated protein kinase (ERK) pathway
induced VMSC apoptosis (24).
H2O2 is capable of activating the
mitochondrial fusion protein 2 (Mitofusin 2, Mfn-2), which can
reduce the phosphorylation of Akt and inhibit its anti-apoptotic
activity, and increase the mitochondrial Bax/Bcl-2 ratio,
cytochrome C release, and activation of caspases-9 and caspase-3
signaling pathway-induced VSMC apoptosis (25). ASK-1 is a mitogen-activated protein
kinase kinase family member. ASK1 activity depends on the
interaction of the N-terminal homology (N-terminal homophilic
interaction). Studies have shown that after a reduction in Trx,
tumor necrosis factor receptor-associated factor (TRAF) combining
with ASK-1, the ASK-1 N-terminal coiled-coil domain could adjust
its N-terminal homology interactions (N-terminal homophilic
interaction) and thereby affect ASK-1 function (26). By inhibiting the interaction of the
N-terminal homology functional activity, Trx inhibits ASK-1, while
H2O2, TRAF2 and TRAF6 enhanced ASK-1 activity
through interactions between the N-terminal homology. However, at
present, H2O2-induced VSMC apoptosis and the
reports on the correlation of Trx-1 with ASK-1 have remained
relatively limited.
Probucol is a chain breaking antioxidant; its
structure consists of two butyl-hydroxy toluene parts, and it can
be used as a single electron donor and singlet oxygen scavenger to
remove oxygen free radicals within cells. In addition, probucol can
decrease oxidative lipid and cholesterol levels, and reduce the
lipid necrotic core area, causing AS plaque stabilization and
anti-restenosis (6–8,27).
Probucal is recognized to have antioxidant mechanisms, and acts on
ox-LDL and against ischemia-reperfusion injury-induced apoptosis
(28,29). The method by which probucol
inhibits apoptosis signal regulating mechanisms has not yet been
fully understood. Recent studies have shown that probucol
significantly reduced p53, Bax and caspase-3 mRNA expression, and
enhanced Bcl2 mRNA expression and resistance to apoptosis (30,31).
However, whether the effect of probucol against VSMC apoptosis is
related to ASK-1 and Trx-1 has not been reported. This study shows
that different concentrations of H2O2 are
capable of inducing VSMC apoptosis in a dose-dependent manner. High
concentrations (1000 μmol/l) of H2O2 led to a
large amount of VSMC apoptosis (apoptotic rate of 29.57%). Probucol
could cause significant resistance to
H2O2-induced VSMC apoptosis in a
dose-dependent manner; 100 μmol/l probucol is capable of reducing
the H2O2-induced VSMC apoptosis to
significantly near to the control group level (1.46±0.82 vs.
3.50±1.54). Probucol increased Trx-1 expression that was
significantly downregulated by the H2O2,
while ASK-1 expression was significantly reduced in a
dose-dependent manner. Thus, probucol inhibition of ROS-induced
apoptosis of VSMCs may be related to ASK-l and Trx-1, indicating
that increased Trx-1 levels and increased levels of Trx-1-ASK-1
complexes may be the mechanism of probucol inhibition of
ROS-induced apoptosis of VSMCs, but the detailed mechanism remains
to be studied.
References
|
1
|
Liu N and Liu JT: Anoikis in rapture of
atherosclerotic plaque. Chin Pharmacol Bull. 23:298–301. 2007.
|
|
2
|
Clarke MC, Littlewood TD, Figg N, et al:
Chronic apoptosis of vascular smooth muscle cells accelerates
atherosclerosis and promotes calcification and medial degeneration.
Circ Res. 102:1529–1538. 2008. View Article : Google Scholar
|
|
3
|
Rudijanto A: The role of vascular smooth
muscle cells on the pathogenesis of atherosclerosis. Acta Med
Indones. 39:86–93. 2007.PubMed/NCBI
|
|
4
|
Boyle JJ: Macrophage activation in
atherosclerosis: pathogenesis and pharmacology of plaque rupture.
Curr Vasc Pharmacol. 3:63–68. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stoneman VE and Bennelt MR: Role of
apoptosis in atherescleresis and its therapeutic implications. Clin
Sci (Lond). 107:343–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ko YG, Kim BK, Lee BK, et al: Study design
and rationale of "Synergistic effect of combination therapy with
cilostazol and ProbUcol on plaque stabilization and lesion
REgression (SECURE)" study: a double-blind randomised controlled
multicenter clinical trial. Trials. 12:102011. View Article : Google Scholar
|
|
7
|
Kaminnyi AI, Lankin VZ, Samko AN, et al:
Low daily dose of antioxidant probucol decreases incidence and
severity of restenosis after transluminal coronary balloon
angioplasty. Bull Exp Biol Med. 139:183–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu BJ, Kathir K, Witting PK, et al:
Antioxidants protect from atherosclerosis by a heme oxygenase-1
pathway that is independent of free radical scavenging. J Exp Med.
203:1117–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kyaw M, Yoshizumi M, Tsuchiya K, et al:
Atheroprotective effects of antioxidants through inhibition of
mitogen-activated protein kinases. Acta Pharmacol Sin. 25:977–985.
2004.PubMed/NCBI
|
|
10
|
Choy K, Beck K, Png FY, et al: Processes
involved in the site-specific effect of probucol on atherosclerosis
in apolipoprotein E gene knockout mice. Arterioscler Thromb Vasc
Biol. 25:1684–1690. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sheng L, Pan QX, Liu YJ, et al: Prevention
of probucol against H2O2 induced apoptosis in
rat vascular smooth muscle cells. Acts Acad Med Shandong.
39:370–372. 2001.
|
|
12
|
Irani K: Oxidant signaling in vascular
cell growth, death and survival. Circ Res. 87:179–187. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamawaki H, Haendeler J and Berk BC:
Thioredoxin: A Key Regulator of Cardiovascular Homeostasis. Circ
Res. 93:1029–1033. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsieh CC, Yen MH, Yen CH and Lau YT:
Oxidized low density lipoprotein induces apoptosis via generation
of reactive oxygen species in vascular smooth muscle cells.
Cardiovasc Res. 49:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schauer IE, Knaub LA, Lloyd M, et al: CREB
downregulation in vascular disease: a common response to
cardiovascular risk. Arterioscler Thromb Vasc Biol. 30:733–741.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sharpe PC, Yue KK, Catherwood MA, McMaster
D and Trimble ER: The effects of glucose-induced oxidative stress
on growth and extracellular matrix gene expression of vascular
smooth muscle cells. Diabetologia. 41:1210–1219. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Satoh K, Nigro P and Berk BC: Oxidative
stress and vascular smooth muscle cell growth: a mechanistic
linkage by cyclophilin A. Antioxid Redox Signal. 12:675–682. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Touyz RM: Reactive oxygen species and
angiotensin II signaling in vascular cells -- implications in
cardiovascular disease. Braz J Med Biol Res. 37:1263–1273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Paravicini TM and Touyz RM: Redox
signaling in hypertension. Cardiovasc Res. 71:247–258. 2006.
View Article : Google Scholar
|
|
20
|
Takeda K, Matsuzawa A, Nishitoh H and
Ichijo H: Roles of MAPKKK ASK1 in stress-induced cell death. Cell
Struct Funct. 28:23–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song JJ and Lee YJ: Differential role of
glutaredoxin and thioredoxin in metabolic oxidative stress-induced
activation of apoptosis signal-regulating kinase1. Biochem J.
373:845–853. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watson WH, Yang X, Choi YE, Jones DP and
Kehrer JP: Thioredoxin and its role in toxicology. Toxicol Sci.
78:3–14. 2004. View Article : Google Scholar
|
|
23
|
Qian ZQ, Zeng YY, Wang T, et al: VEGF
induces HUVECs to produce extracellular H2O2
and its proliferation role. Chin J Pathophysiology. 23:533–535.
2007.
|
|
24
|
Tsujimoto A, Takemura G, Mikami A, et al:
A therapeutic dose of the lipophilic statin pitavastatin enhances
oxidant-induced apoptosis in human vascular smooth muscle cells. J
Cardiovasc Pharmacol. 48:160–165. 2006. View Article : Google Scholar
|
|
25
|
Guo X, Chen KH, Guo Y, et al: Mitofusin 2
triggers vascular smooth muscle cell apoptosis via mitochondrial
death pathway. Circ Res. 101:1113–1122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujino G, Noguchi T, Matsuzawa A, et al:
Thioredoxin and TRAF family proteins regulate reactive oxygen
species-dependent activation of ASK1 through reciprocal modulation
of the N-terminal homophilic interaction of ASK1. Mol Cell Biol.
27:8152–8163. 2007. View Article : Google Scholar
|
|
27
|
Yang YB, Liao DF, Xie ZZ and Huang HL:
Probucol prevention against restenosis by regulating functional
vascular remodeling after percutaneous transluminal angioplasty in
rabbits. Chin Pharmacol Bull. 19:388–392. 2003.
|
|
28
|
Su B, He H, Luo QF, Zu BY and Liao DF:
Effects of probucol on ox-LDL induced apoptosis and CD36,
Caveolin-1 expression in THP-l macrophages. Chin Pharmacol Bull.
23:1167–1171. 2007.
|
|
29
|
Ruixing Y, Al-Ghazali R, Wenwu L and
Jinzhen W: Pretreatment with probucol attenuates cardiomyocyte
apoptosis in a rabbit model of ischemia/reperfusion. Scand J Clin
Lab Invest. 66:549–558. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Asiri YA: Probucol attenuates
cyclophosphamide-induced oxidative apoptosis, p53 and Bax signal
expression in rat cardiac tissues. Oxid Med Cell Longev. 3:308–316.
2010. View Article : Google Scholar
|
|
31
|
Tang X, Yang X, Peng Y and Lin J:
Protective effects of lycopene against
H2O2-induced oxidative injury and apoptosis
in human endothelial cells. Cardiovasc Drugs Ther. 23:439–448.
2009. View Article : Google Scholar : PubMed/NCBI
|