Introduction
Interventional cardiology with drug-eluting stents
(DES) has significantly improved the treatment of coronary
atherosclerotic disease, and it has currently become the gold
standard. However, with the advent of using DES to treat complex
lesions, the risk of stent thrombosis has also increased (1,2), and
late stent thrombosis has been reported following DES implantation
(3). Long-term treatment with dual
anti-platelet therapy following stent implantation has provided a
solution to counteract the risk of stent thrombosis. However, this
treatment has risks and is expensive. An additional problem of DES
is delayed endothelialization, which may be a contributing factor
to the prolonged period of thrombotic risk, as shown by
pathological findings from autopsies following DES implantation.
These investigations have shown that even after 16 months, intimal
healing is still incomplete with ~20% of stent struts uncovered
(4). These problems of late
thrombosis and delayed endothelialization of stent struts with DES
may be caused by the permanent polymer used to bond the
anti-restenosis compounds to the stents. To confirm this
speculation, intravascular ultrasonography and angiography have
been previously used to gain information on DES (5). These techniques provide 2- and
3-dimensional views of the vessel.
However, with intravascular ultrasonography,
shadowing occurs around and behind the stent struts since they are
reflectors of sonic waves. Intravascular ultrasonography is also
characterized by limitations in detecting the malapposition of the
struts to the vessel wall, especially when the area between the
vessel wall and the strut is small. Optical coherence tomography
(OCT) is a novel imaging modality that visualizes intracoronary
features with an axial resolution of 3–20 μm, which is
substantially higher resolution compared with that of intravascular
ultrasonography (100–150 μm). OCT is able to detect intimal
coverage upon the follow-up of DES more clearly and accurately
(6,7). The present study aimed to assess and
compare the presence and morphology of intimal hyperplasia
following the implantation of DES with a biodegradable polymer vs.
the DES with a permanent polymer using OCT.
Materials and methods
Study design
This was a prospective, randomized, open-label,
single-center study designed to assess and compare intimal
hyperplasia using OCT following implantation of either a stent with
a biodegradable polymer (Excel; JW Medical Systems, Weihai, China)
or a stent with a permanent polymer (Cypher®; Cordis,
Fremont, CA, USA). One hundred patients with de novo
coronary artery stenosis were included in this study. Half of the
participants received each type of stent. A member of the research
team approached each patient to obtain written informed consent
before percutaneous coronary intervention or any study-related
procedure, and each patient signed the consent form prior to
enrollment. All the procedures were approved by the Local Ethics
Committee.
Inclusion and exclusion criteria
The inclusion criteria included age, 30–75 years;
binary stenosis, ≥70% in a de novo lesion in the native
coronary artery; reference lumen diameter proximal to the target
lesion, 2.5 and ≤4.0 mm; lesion length ≤30 mm; and signed, written
informed consent.
The exclusion criteria included pregnancy or
breastfeeding, intolerance to aspirin or clopidogrel, acute
myocardial infarction, congestive heart failure (left ventricular
ejection fraction <40%), renal insufficiency (serum creatinine
>1.8 mg/dl), left main coronary artery disease, chronic total
occlusion, ostium lesion, severely tortuous arteries, history of
revascularisation and no signed informed consent.
Stent implantation
The patients were administered aspirin (100 mg/day),
clopidogrel (300 mg loading dosage, 75 mg/day) and heparin at a
dose of 100 U/kg before the procedure. Percutaneous coronary
intervention procedure was performed according to the standard
technique. Stent implantation was conducted following the
manufacturer’s instructions of each stent.
Clinical and coronary angiography
follow-up
The patients underwent follow-up evaluations 1 year
after stent implantation. Major adverse cardiac events were noted
and stored in our database. Such events included cardiac death,
heart failure, myocardial infarction (Q and non-Q waves), as well
as ischemia-driven target lesion revascularization. The patients
underwent coronary angiography at 1 year of follow-up after stent
implantation.
OCT image acquisition and analysis
The patients underwent 3 OCT examinations, which
were performed before and after stent implantation, and at the
first-year follow-up. The M3 OCT system (LightLab Imaging, Inc.,
Westford, MA, USA) was used in the current study. The speed of the
automatic pullback was 1.5 mm/sec. The image included the entire
length of the stent and a ≥5-mm segment beyond the stent edges. The
OCT images were analyzed offline by 2 independent blinded doctors.
The software used was provided by LightLab Imaging, Inc. A single
OCT cross-sectional still frame was selected for quantitative
analysis from each 1-mm segment throughout the entire length of the
stent. The still frames were selected based on the appearance of
stent struts, and the lack of OCT motion artifacts or other image
artifacts. The coronary plaque was classified as fibrous,
lipid-rich or mixed using previously validated criteria (8–11).
OCT evaluation of stent malapposition, tissue prolapse, in-stent
thrombosis and stent edge dissection followed stent implantation.
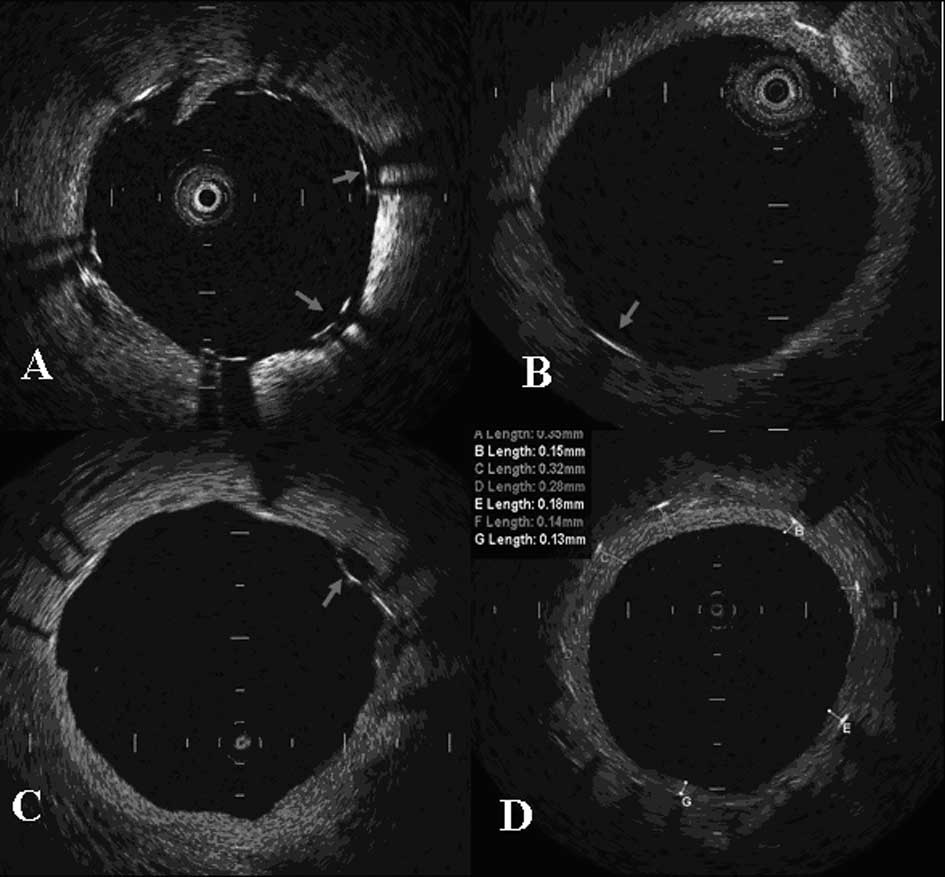
For the OCT imaging at 1 year follow-up, each stent strut in the
still frame was examined regarding whether the strut was in
malapposition and whether there was complete coverage. The average
thickness was measured when intimal coverage on the strut was
observed (Fig. 1).
Statistical analysis
Continuous variables are expressed as the means ±
standard deviation (SD) and were compared using the t-test on
independent samples. Categorical variables were expressed as
absolute numbers and percentages compared with χ2
statistics or Fisher’s exact test. A multivariable logistic
regression analysis was performed to assess independent predictors
for coronary positive remodeling. The independent variables
included in the model were age, history of diabetes mellitus,
hypertension, hypercholesterolemia and smoking, as well as stent
category, number, diameter and length. P<0.05 was considered to
indicate a statistically significant difference. All the
statistical analyses were performed using SPSS 11.5 for Windows
(SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
One hundred patients were included in this study.
Percutaneous coronary intervention and OCT procedures were
successful and without complications in the 2 groups. The baseline
characteristics of the 2 groups are shown in Table I. Demographic baseline
characteristics including age, gender and risk factors of coronary
heart disease were not significantly different. Similarly, the
characteristics of the target vessels and lesions were not
significantly different among the 2 groups. The average number of
stents placed was higher in the group with biodegradable polymer
stents (1.40±0.70 vs. 1.21±0.50, P=0.039). The stent dilatation
pressure (13.83±1.86 vs. 14.19±1.49 atm, P=0.417) and the rate of
post-dilatation (24 vs. 32%, P=0.373) were not significantly
different between the groups.
 | Table IBaseline clinical and procedural
characteristics. |
Table I
Baseline clinical and procedural
characteristics.
| Characteristics | Biodegradable polymer
group (n=50) | Permanent polymer
group (n=50) | P-value |
|---|
| Age (years), mean ±
SD | 62.05±9.65 | 60.41±9.46 | 0.228 |
| Male, n (%) | 42 (84) | 37 (74) | 0.220 |
| Smoking, n (%) | 13 (26) | 14 (28) | 0.822 |
| Hypertension, n
(%) | 27 (54) | 29 (58) | 0.687 |
| Hypercholesterolemia,
n (%) | 5 (10) | 7 (14) | 0.538 |
| Diabetes mellitus, n
(%) | 17 (34) | 12 (24) | 0.271 |
| Statin therapy, n
(%) | 46 (92) | 48 (96) | 0.674 |
| Blood glucose
(mmol/l) | 6.31±2.46 | 6.53±2.16 | 0.710 |
| CHO (mmol/l), mean ±
SD | 4.38±1.20 | 4.20±1.03 | 0.533 |
| LDL-C (mmol/l), mean
± SD | 2.60±1.04 | 2.47±0.82 | 0.563 |
| HDL-C (mmol/l), mean
± SD | 1.16±0.25 | 1.12±0.27 | 0.482 |
| TG (mmol/l), mean ±
SD | 1.67±0.95 | 1.58±0.92 | 0.705 |
| Target vessel, n
(%) | | | 0.112 |
| LAD | 31 (62) | 37 (74) | |
| LCX | 13 (26) | 5 (10) | |
| RCA | 6 (12) | 8 (16) | |
| Lesion length (mm),
mean ± SD | 20.01±6.04 | 21.23±5.07 | 0.125 |
| Diameter stenosis
(%), mean ± SD | 87.16±4.75 | 88.05±5.76 | 0.230 |
| Stent (n), mean ±
SD | 1.40±0.70 | 1.21±0.50 | 0.039 |
| Stent diameter (mm),
mean ± SD | 3.10±0.45 | 3.15±0.34 | 0.377 |
| Stent length (mm),
mean ± SD | 23.65±5.83 | 24.95±5.21 | 0.099 |
| Stent dilatation
pressure (atm), mean ± SD | 13.83±1.86 | 14.19±1.49 | 0.417 |
| Post dilatation, n
(%) | 12 (24) | 16 (32) | 0.373 |
Clinical and coronary angiography
follow-up
The patients were followed up clinically 1 year
after stent implantation. There was no major acute coronary event
in the group with biodegradable polymer stents. A total of 46
patients (92%) in the group with biodegradable polymer stents and
45 patients (90%) with permanent polymer stents had a coronary
angiography follow-up at 1 year after stent implantation (P=0.727).
In the group with permanent polymer stents, 2 patients had angina
pectoris. Coronary angiography showed that 1 of the patients had
in-segment restenosis. This patient underwent target lesion
revascularization. The remaining patients underwent coronary
positive remodeling at the proximal stent segment.
OCT analysis
Vessel response after stent
implantation
OCT examinations were performed on all the patients
after stent implantation. Table
II shows the plaque characteristics and vessel responses after
stent implantation. The frequencies of lipid-rich (48 vs. 46%),
fibrous-lipid (42 vs. 38%) and mixed (10 vs. 16%) plaques of the 2
groups were not significantly different (P=0.66). The frequencies
of prolapse (38 vs. 52%, P=0.159), stent strut malapposition (10
vs. 12%, P=0.749), edge dissection (2 vs. 4%, P=0.558) and small
thrombosis (6 vs. 10%, P=0.461) of the stents in the 2 groups were
also not significantly different.
 | Table IIPlaque characterisistics and vessel
response after stent implantation. |
Table II
Plaque characterisistics and vessel
response after stent implantation.
| Characteristics | Biodegradable polymer
group (n=50) | Permanent polymer
group (n=50) | P-value |
|---|
| Plaque
characteristics | | | 0.66 |
| Lipid-rich plaque, n
(%) | 24 (48) | 23 (46) | - |
| Fibrous-lipid
plaque, n (%) | 21 (42) | 19 (38) | - |
| Mixed plaque, n
(%) | 5 (10) | 8 (16) | - |
| TIMI grade III after
stenting, n (%) | 50 (100) | 50 (100) | - |
| Stent struts
malapposition, n (%) | 5 (10) | 6 (12) | 0.749 |
| Tissue prolapse, n
(%) | 19 (38) | 26 (52) | 0.159 |
| Stent edge
dissection, n (%) | 1 (2) | 2 (4) | 0.558 |
| Thrombosis, n
(%) | 3 (6) | 5 (10) | 0.461 |
OCT results at follow-up
OCT examinations were performed upon the first-year
follow-up on 43 patients in the biodegradable polymer group and 41
patients in the permanent polymer group. The frequencies of
follow-up in the 2 groups were not significantly different (86 vs.
82%, P=0.585). Table III shows
the OCT analysis at follow-up. A total of 4,575 stent struts in the
biodegradable polymer group and 5,829 in the permanent polymer
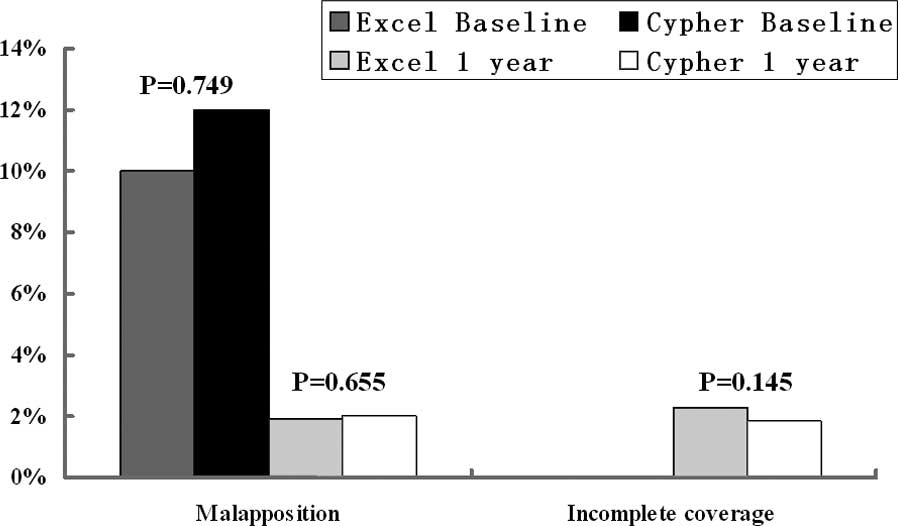
group were analyzed. The uncovered stent struts (2.27 vs. 1.87%,
P=0.145) and stent strut malapposition (1.9 vs. 2.02%, P=0.655) at
1 year follow-up were not significantly different (Fig. 2), although the frequencies of
uncovered stent struts were higher in the biodegradable compared
with the permanent polymer group. The average neointimal thickness
was lower in the biodegradable compared with the permanent polymer
group, and the difference was statistically significant
(106.12±80.65 vs. 181.20±146.96 μm, P<0.001). The neointimal
thickness was divided into 4 zones with 100-μm intervals, namely,
zone A with an intimal thickness of ≤100 μm, zone B with a
neointimal thickness of 101–200 μm, zone C with a neointimal
thickness of 201–300 μm and zone D with a neointimal thickness ≥301
μm. Table IV and Fig. 3 show the distribution of neointimal
thickness in the 2 groups. The frequencies of neointimal thickness
<100 μm were significantly higher in the biodegradable compared
with the permanent polymer group (62.1 vs. 35.9%, P<0.001). The
average intimal thickness was also lower in the biodegradable
compared with the permanent polymer group (57.7±24.6 vs. 67.6±22.4
μm, P<0.001). The distributions of intimal thickness in zones C
(7.3 vs. 16.3%, P=0.046) and D (3.3 vs. 14.3%, P<0.005) were
also lower in the biodegradable compared with the permanent polymer
group. No significant difference was found in zone B (27.3 vs.
33.5%, P=0.304).
 | Table IIIOCT analysis at follow-up. |
Table III
OCT analysis at follow-up.
| Variables | Biodegradable
polymer group | Permanent polymer
group | P-value |
|---|
| Patients, n
(%) | 43 (86) | 41 (82) | 0.585 |
| Months after
stenting (n) | 12.4±1.8 | 11.8±2.1 | 0.647 |
| Total stent struts
(n) | 4,575 | 5,829 | - |
| Uncovered stent
struts, n (%) | 104 (2.27) | 109 (1.87) | 0.145 |
| Stent struts
malapposition, n (%) | 87 (1.9) | 118 (2.02) | 0.655 |
| Average intimal
thickness (μm), mean ± SD | 106.12±80.65 | 181.20±146.96 | <0.001 |
 | Table IVDistribution of neointimal
thickness. |
Table IV
Distribution of neointimal
thickness.
| Zones (%) | Biodegradable
polymer group | Permanent polymer
group | P-value |
|---|
| A, ≤100 μm | 62.1 | 35.9 | <0.001 |
| B, 101–200 μm | 27.3 | 33.5 | 0.304 |
| C, 201–300 μm | 7.3 | 16.3 | 0.046 |
| D, ≥301 μm | 3.3 | 14.3 | 0.005 |
Discussion
The present study was a prospective, randomized,
open-label study that used OCT to assess intimal hyperplasia
following the implantation of the DES with either a biodegradable
or permanent polymer. The DES with a biodegradable polymer was
found to significantly decrease intimal hyperplasia and to have a
well-proportioned coverage compared with DES with a permanent
polymer. This finding was associated with an insignificantly higher
rate of uncovered stent struts in biodegradable polymer DES at
1-year follow-up after stent implantation.
Stent strut coverage is an important potential
surrogate for stent safety. Incomplete stent coverage reported
after DES implantation is a risk factor of late stent thrombosis
(12). The polymer coating system
of drug delivery has been shown to activate chronic arterial wall
inflammation, retarding healing and intimal coverage (13). Biodegradable polymers are composed
of polylactic acid (PLA), polyglycolide and polymer, which are
completely metabolized into water and carbon dioxide after
fulfilling their drug delivery function. The safety and
effectiveness of biodegradable polymer DES have been demonstrated
in previous clinical trials (14–16).
However, such studies have not indicated whether biodegradable
polymer coatings increase healing and intimal coverage since the
included patients were not evaluated with either intravascular
sonography or OCT. OCT is a novel imaging modality with a higher
resolution compared to travascular sonography used to assess the
intimal coverage upon DES follow-up (7). The LEADERS study is a multi-center,
head-to-head randomized trial which assessed the safety and
effectiveness of biolimus-eluting stents (BES) with a biodegradable
polymer and DES with a permanent polymer. Tissue coverage of the
stents at the nine-month follow-up was evaluated using OCT in the
subgroups of the LEADERS trial. Stent strut coverage appeared to be
more complete in patients who were implanted with biodegradable
compared with permanent polymer (Cypher®) DES (17). However, in the OCTDESI trial, 60
patients with de novo lesions were examined with OCT at the
6-month follow-up; the patients were implanted with
paclitaxel-eluting stent (PES) with either permanent polymer
(TAXUS® Liberté®, Boston Scientific, Natick,
MA, USA) or with JACTAX PES (Boston Scientific) with an ultrathin
microdot biodegradable abluminal polymer at 2 levels of drug
(18). Results showed that JACTAX
PES did not improve strut coverage at 6 months compared with PES
(5.3±14.7% for TAXUS®, 7.0±12.2% for JACTAX HD and
4.6±7.3% for JACTAX LD, P=0.81).
In the current study, there was no significant
difference in the number of uncovered stent struts in the
biodegradable and permanent polymer groups (2.27 vs. 1.87%,
P=0.145) at the first-year follow-up. These discrepancies in strut
coverage in the various trials may be attributed to several
factors.
Firstly, different types of DES with a biodegradable
polymer have been used in various clinical trials in which both DES
designs and drugs were different. A BES with the biodegradable PLA
polymer was used in the LEADERS trial (17); a PES with the biodegradable
abluminal polymer was used in the OCTDESI trial (18); and a sirolimus-eluting stent with
the biodegradable PLA polymer was used in the current study. A
meta-analysis has indicated that different DES with a biodegradable
polymer yield different clinical results (19).
Secondly, the OCT follow-up examination periods were
not the same [9 months in the LEADERS trial subgroup (17), 6 months in the OCTDESI trial
(18) and 1 year in the current
study]. Previous studies have indicated that the frequency of
incomplete stent coverage gradually decreases with a prolonged
follow-up period (7,20). The small number of patients and
different inclusion criteria for OCT examination also affected the
results of the current study. The uncovered stent struts in the
biodegradable polymer DES also indicated that dual antiplatelet
treatments for <1 year were not safe enough. However, Han et
al(21) reported the
satisfactory safety and efficacy of DES with a biodegradable
polymer when used with 6 months of dual antiplatelet therapy in a
real-world setting.
Previous studies have reported that DES has a higher
frequency of stent malapposition than occurs with bare metal
stents, as assessed by an OCT follow-up (22,23).
This finding could be attributed to the fact that the polymer
coating may induce vessel wall inflammation and positive
remodeling. Similar frequencies of malapposition after the stenting
procedure and upon follow-up were detected in the 2 groups in the
current study.
Intimal hyperplasia after stent implantation
increased late lumen loss and was used to assess stent efficacy.
The thickness of intimal coverage was significantly lower in the
biodegradable compared with the permanent polymer group
(106.12±80.65 vs. 181.20±146.96 μm, P<0.001), and the
distribution of intimal thickness <100 μm was significantly
higher in the biodegradable compared with the permanent polymer
group (62.1 vs. 35.9%, P<0.001). The distribution of intimal
thickness in the group with biodegradable polymer DES was similar
to the result of the LEADERS subgroup trial (17). Hence, the biodegradable polymer DES
was more efficient in preventing stent restenosis due to its
biodegradable polymer stent design. The biodegradable polymer had
the advantage of completely eluting drugs and decreasing chronic
arterial wall inflammation. Distinctive inflammatory responses
(e.g., giant cell infiltration, progressive granulomatous and
eosinophilic reaction) around the stent struts with a permanent
polymer have been detected in previous studies (24,25).
Chronic inflammation stimulates intimal hyperplasia and decreases
stenting efficacy.
The present study has limitations with regard to its
single-center source and the small sample population. Larger
patient populations from more centers are needed to confirm the
results. The biodegradable and permanent polymer DES have different
metal platforms, and the metal of the biodegradable polymer DES is
thinner compared with that of the permanent polymer DES. The stent
structure also results in different intimal hyperplasia and stent
malapposition. Although OCT is presently the highest resolution
technique, it could not detect a thin intimal coverage (<10 μm).
This limitation may have increased the frequency of uncovered stent
struts during the OCT imaging analysis. In conclusion,
biodegradable polymer DES resulted in significantly lower intimal
hyperplasia and well-proportioned intimal coverage compared with
permanent polymer DES.
Acknowledgements
This study was supported by a grant from the
National High-Technology Research and Development Program (‘863’
Program) of China (no. 2009AA02Z420).
References
|
1
|
Iakovou I, Schmidt T, Bonizzoni E, et al:
Incidence, predictors, and outcome of thrombosis after successful
implantation of drug-eluting stents. JAMA. 293:2126–2130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuchulakanti PK, Chu WW, Torguson R, et
al: Correlates and long-term outcomes of angiographically proven
stent thrombosis with sirolimus- and paclitaxel-eluting stents.
Circulation. 113:1108–1113. 2006. View Article : Google Scholar
|
|
3
|
McFadden EP, Stabile E, Regar E, et al:
Late thrombosis in drug-eluting coronary stents after
discontinuation of antiplatelet therapy. Lancet. 364:1519–1521.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guagliumi G, Farb A, Musumeci G, Valsecchi
O, Tespili M, Motta T and Virmani R: Images in cardiovascular
medicine. Sirolimus-eluting stent implanted in human coronary
artery for 16 months: pathological findings. Circulation.
107:1340–1341. 2003. View Article : Google Scholar
|
|
5
|
Byrne RA, Joner M and Kastrati A: Polymer
coatings and delayed arterial healing following drug-eluting stent
implantation. Minerva Cardioangiol. 57:567–584. 2009.PubMed/NCBI
|
|
6
|
Takano M, Inami S, Jang IK, Yamamoto M,
Murakami D, Seimiya K, Ohba T and Mizuno K: Evaluation by optical
coherence tomography of neointimal coverage of sirolimus-eluting
stent three months after implantation. Am J Cardiol. 99:1033–1038.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto D, Shite J, Shinke T, Otake H,
Tanino Y, Ogasawara D, Sawada T, Paredes OL, Hirata K and Yokoyama
M: Neointimal coverage of sirolimus-eluting stents at 6-month
follow-up: evaluated by optical coherence tomography. Eur Heart J.
28:961–967. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yabushita H, Bouma BE, Houser SL, Aretz
HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH,
Halpern EF and Tearney GJ: Characterization of human
atherosclerosis by optical coherence tomography. Circulation.
106:1640–1645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jang IK, Tearney GJ, MacNeill B, Takano M,
Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF
and Bouma BE: In vivo characterization of coronary atherosclerotic
plaque by use of optical coherence tomography. Circulation.
111:1551–1555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Manfrini O, Mont E, Leone O, Arbustini E,
Eusebi V, Virmani R and Bugiardini R: Sources of error and
interpretation of plaque morphology by optical coherence
tomography. Am J Cardiol. 98:156–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kume T, Akasaka T, Kawamoto T, Ogasawara
Y, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y and
Yoshida K: Assessment of coronary arterial thrombus by optical
coherence tomography. Am J Cardiol. 97:1713–1717. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotani J, Awata M, Nanto S, Uematsu M,
Oshima F, Minamiguchi H, Mintz GS and Nagata S: Incomplete
neointimal coverage of sirolimus-eluting stents: angioscopic
findings. J Am Coll Cardiol. 47:2108–2111. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Joner M, Finn AV, Farb A, Mont EK,
Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK and Virmani R:
Pathology of drug-eluting stents in humans: delayed healing and
late thrombotic risk. J Am Coll Cardiol. 48:193–202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Onuma Y, Serruys P, den Heijer P, et al:
MAHOROBA, first-in-man study: 6-month results of a biodegradable
polymer sustained release tacrolimus-eluting stent in de novo
coronary stenoses. Eur Heart J. 30:1477–1485. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrne RA, Kastrati A, Massberg S, et al:
Biodegradable polymer versus permanent polymer drug-eluting stents
and everolimus- versus sirolimus-eluting stents in patients with
coronary artery disease: 3-year outcomes from a randomized clinical
trial. J Am Coll Cardiol. 58:1325–1331. 2011.
|
|
16
|
Windecker S, Serruys PW, Wandel S, et al:
Biolimus-eluting stent with biodegradable polymer versus
sirolimus-eluting stent with durable polymer for coronary
revascularisation (LEADERS): a randomised non-inferiority trial.
Lancet. 372:1163–1173. 2008. View Article : Google Scholar
|
|
17
|
Barlis P, Regar E, Serruys PW, et al: An
optical coherence tomography study of a biodegradable vs. durable
polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur
Heart J. 31:165–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guagliumi G, Sirbu V, Musumeci G, et al:
Strut coverage and vessel wall response to a new-generation
paclitaxel-eluting stent with an ultrathin biodegradable abluminal
polymer: Optical Coherence Tomography Drug-Eluting Stent
Investigation (OCTDESI). Circ Cardiovasc Interv. 3:367–375. 2010.
View Article : Google Scholar
|
|
19
|
Ahmed TA, Bergheanu SC, Stijnen T, Plevier
JW, Quax PH and Jukema JW: Clinical performance of drug-eluting
stents with biodegradable polymeric coating: a meta-analysis and
systematic review. EuroIntervention. 7:505–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim JS, Jang IK, Kim JS, Kim TH, Takano M,
Kume T, Hur NW, Ko YG, Choi D, Hong MK and Jang Y: Optical
coherence tomography evaluation of zotarolimus-eluting stents at
9-month follow-up: comparison with sirolimus-eluting stents. Heart.
95:1907–1912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Y, Jing Q, Xu B, et al: Safety and
efficacy of biodegradable polymer-coated sirolimus-eluting stents
in ‘real-world’ practice: 18-month clinical and 9-month
angiographic outcomes. JACC Cardiovasc Interv. 2:303–309. 2009.
|
|
22
|
Gonzalo N, Barlis P, Serruys PW,
Garcia-Garcia HM, Onuma Y, Ligthart J and Regar E: Incomplete stent
apposition and delayed tissue coverage are more frequent in
drug-eluting stents implanted during primary percutaneous coronary
intervention for ST-segment elevation myocardial infarction than in
drug-eluting stents implanted for stable/unstable angina: insights
from optical coherence tomography. JACC Cardiovasc Interv.
2:445–452. 2009.
|
|
23
|
Guagliumi G, Costa MA, Sirbu V, et al:
Strut coverage and late malapposition with paclitaxel-eluting
stents compared with bare metal stents in acute myocardial
infarction: optical coherence tomography substudy of the
Harmonizing Outcomes with Revascularization and Stents in Acute
Myocardial Infarction (HORIZONS-AMI) Trial. Circulation.
123:274–281. 2011.
|
|
24
|
Finn AV, Nakazawa G, Kolodgie FD and
Virmani R: Temporal course of neointimal formation after
drug-eluting stent placement: is our understanding of restenosis
changing? JACC Cardiovasc Interv. 2:300–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Finn AV, Kolodgie FD, Harnek J, Guerrero
LJ, Acampado E, Tefera K, Skorija K, Weber DK, Gold HK and Virmani
R: Differential response of delayed healing and persistent
inflammation at sites of overlapping sirolimus- or
paclitaxel-eluting stents. Circulation. 112:270–278. 2005.
View Article : Google Scholar : PubMed/NCBI
|