Introduction
Basic fibroblast growth factor (bFGF) is a
multifunctional growth factor involved in tumor development,
including cell differentiation, cell growth, migration,
angiogenesis and tumor formation (1–4). Its
biological effects have been reported to be exerted mainly through
interaction with its high-affinity receptor, fibroblast growth
factor receptor 1 (FGFR1) (5–8).
Narong and Leelawat (9) reported
that bFGF enhances the migration of cholangiocarcinoma cells by the
phosphorylation of MEK1/2. Results from previous studies have shown
that bFGF signaling plays a key role in the development of cancer,
including gastric, lung and endometrial cancer (10–12).
The cGMP-dependent protein kinases (PKGs) are
serine/threonine kinases and include two types of PKGs, PKG I and
PKG II (13,14). PKG I is widely distributed within
the body and its expression levels are lower in various tumor
tissues. PKG II is more tissue-restricted and is characterized by
reduced expression levels in many types of tumor cells (15). PKG I leads to decreased tumor
growth and invasiveness in many types of cells, including
cardiomyocytes, mesangial cells and neutrophils (16–19).
PKG I has been identified to be a tumor suppressor (20). Previous studies suggest that PKG II
has a role in the regulation of cell proliferation and apoptosis
(21–24). Swartling et al(25) reported that PKG II inhibits the
proliferation of human neuroglioma cells and that the inhibition
was related to reductions in transcription factor Sox9 expression
levels and Akt phosphorylation. We have prevously observed that the
expression and activity of PKG II in human gastric cancer cells
were significantly lower compared with those in normal cells
(26). Additionally, another study
conducted in our laboratory demonstrated an inhibitory effect of
PKG II on the proliferation of gastric cancer cells (27).
Previous studies have demonstrated the inhibitory
effect of PKG on cell proliferation and the stimulatory effect of
bFGF on cell proliferation and migration. However, whether PKG is
able to attenuate the bFGF-induced effects on U251 cells remains to
be elucidated. The aim of this study was to determine the
relationship between PKG and bFGF, and to investigate how PKG
exerts its inhibitory effects.
Materials and methods
Cell line
The human glioma cell line U251 was provided by the
Institute of Cell Biology (Shanghai, China).
Reagents
Antibodies against MEK and p-MEK (Ser217/221) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Antibodies against ERK, p-ERK1/2 and actin were from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies against p-ERK
(Thr202/Tyr204), p-FGFR (Y154), FGFR and β-actin were from Bioworld
Technology Co., Ltd. (St. Louis Park, MN, USA). Horseradish
peroxidase (HRP)-conjugated secondary antibodies were from Jackson
ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). The
cellular permeable cGMP analog 8-pCPT-cGMP and 8-Br-cGMP were from
Calbiochem (San Diego, CA, USA). Electrochemiluminescence (ECL)
reagent was from Millipore (Billerica, MA, USA). Dulbecco’s
modified Eagle’s medium (DMEM) and newborn calf serum (NBCS) were
from Gibco (Grand Island, NY, USA).
MTT assay
U251 cells (0.5–1×103) were plated on
96-well plates in 150 μl medium. The cells were infected with
Ad-Lacz, Ad-PKG I or Ad-PKG II for 24 h to establish Ad-Lacz+bFGF,
Ad-PKG I+bFGF and Ad-PKG II+bFGF groups. In the Ad-PKG I+bFGF and
Ad-PKG II+bFGF groups, 250 μM 8-Br-cGMP and 250 μM 8-pCPT-cGMP were
added to activate PKG I and PKG II, respectively. Then, the cells
were incubated with bFGF (100 ng/ml) for 12 h. The cultured cells
were washed with phosphate-buffered saline (PBS), treated with 20
μl MTT (0.5 mg/ml) and then incubated at 37°C for 1 h. The medium
was removed and 100 μl dimethylsulfoxide (DMSO) was added to each
well. The absorbance was determined at 570 nm using a microplate
reader. All the experiments were performed in triplicate.
Cell migration assay
The migration of the U251 human glioma cells was
investigated using a chamber with 8-μm pore filters (Transwell,
24-well cell culture; Coster, Boston, MA, USA). U251 cells were
infected with Ad-Lacz, Ad-PKG I or Ad-PKG II for 48 h to establish
Ad-Lacz+bFGF, Ad-PKG I+bFGF and Ad-PKG II+bFGF groups. The cells
were serum starved overnight and, in the Ad-PKG I+bFGF and Ad-PKG
II+bFGF groups, 250 μM 8-Br-cGMP and 250 μM 8-pCPT-cGMP were added
to activate PKG I and PKG II, respectively. The cells were then
incubated with bFGF (100 ng/ml) for 12 h at 37°C. Following
incubation, the filters were fixed and stained with hematoxylin and
the cells were counted in five random high-power fields under a
light microscope.
Nuclear protein preparation
According to the method described by Chen et
al(28), cells growing on
100-mm plates were harvested in HEM buffer (10 mM HEPES pH 7.5, 2
mM EDTA, 1 mM MgCl2) and homogenized with an ultrasonic
homogenizer. The homogenate was centrifuged at 500 × g at 4°C for 5
min to obtain the nuclei of the cells. Pre-heated SDS-PAGE loading
buffer was added to the pellet and boiled for 5 min to obtain the
nuclear proteins.
Western blot analysis
Sample proteins were separated on SDS-PAGE gels and
blotted onto polyvinyl difluoride (PVDF) membranes. The PVDF
membranes were blocked with 3% (w/v) bovine serum albumin (BSA) in
TBS-T for 1 h at room temperature. Incubation with the primary
antibody was conducted at 4°C overnight, and incubation with the
secondary antibody was conducted at room temperature for 1 h, with
three washes following each incubation. ECL reagents were used to
show the positive bands on the membrane. The bands were detected
using Typhoon 9400 (GE Healthcare, Piscataway, NJ, USA).
Statistical analysis
Values are expressed as the means ± SE (n=5;
*P<0.05). The Student’s t-test was used for
comparisons of two sample means. A P-value of <0.05 (P<0.05)
was considered to indicate a statistically significant
difference.
Results
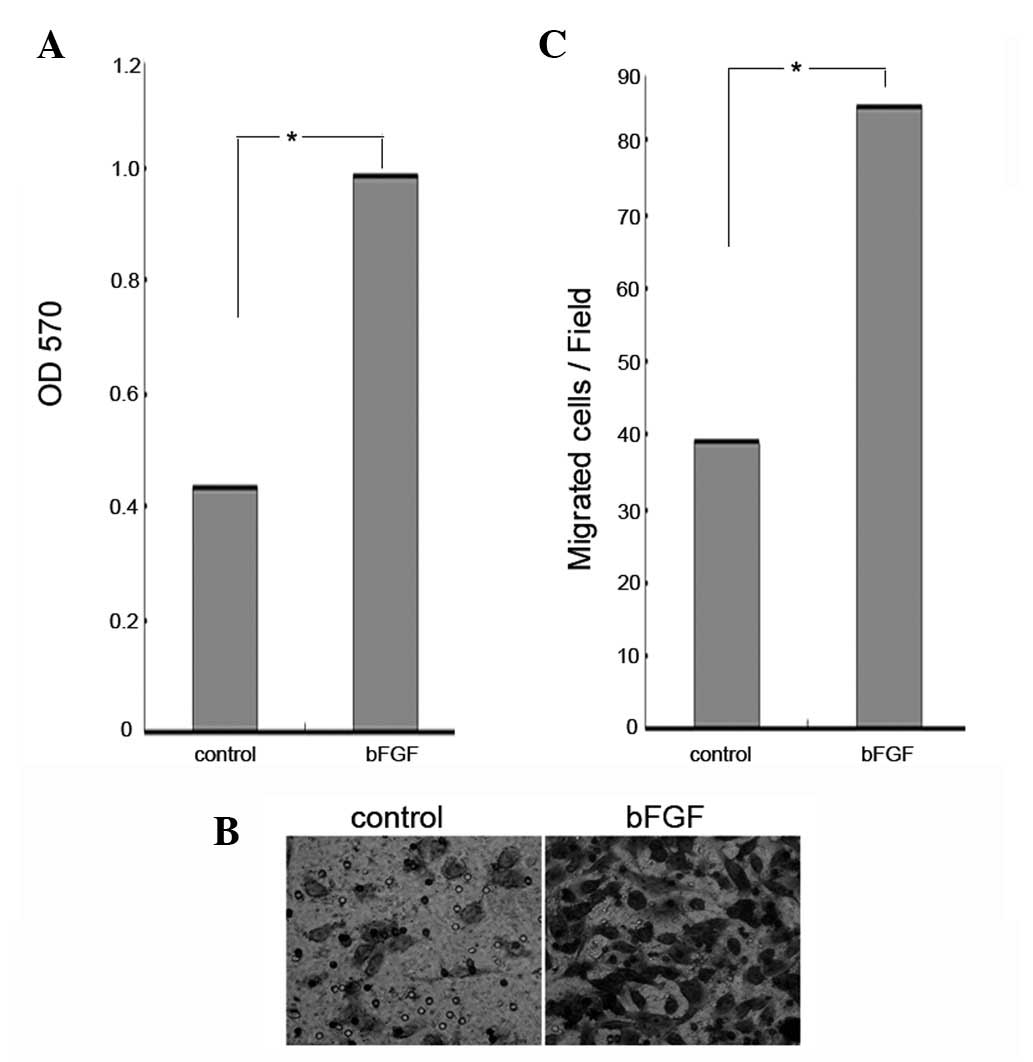
bFGF promotes the proliferation and
migration of U251 human glioma cells
bFGF has been observed to stimulate cancer cell
proliferation (29). In the
present study, an MTT assay was used to determine whether bFGF had
any effect on the proliferation of U251 human glioma cells. The
U251 cells were treated with bFGF at a concentration of 100 ng/ml
for 48 h. The results showed that there was a significant increase
in the proliferation of cells treated with bFGF (Fig. 1A). Recent findings have shown that
bFGF stimulates cancer cell migration (30). In order to determine the effects of
bFGF on the migration of U251 cells, the cells were treated with
bFGF at a concentration of 100 ng/ml for 12 h and then examined
using a cell migration assay. Compared with the control, the
percentage of U251 cell migration was significantly increased when
the cells were treated with 100 ng/ml of bFGF (P<0.0051)
(Fig. 1B). This demonstrates that
bFGF increases both the proliferation and migration of U251 human
glioma cells.
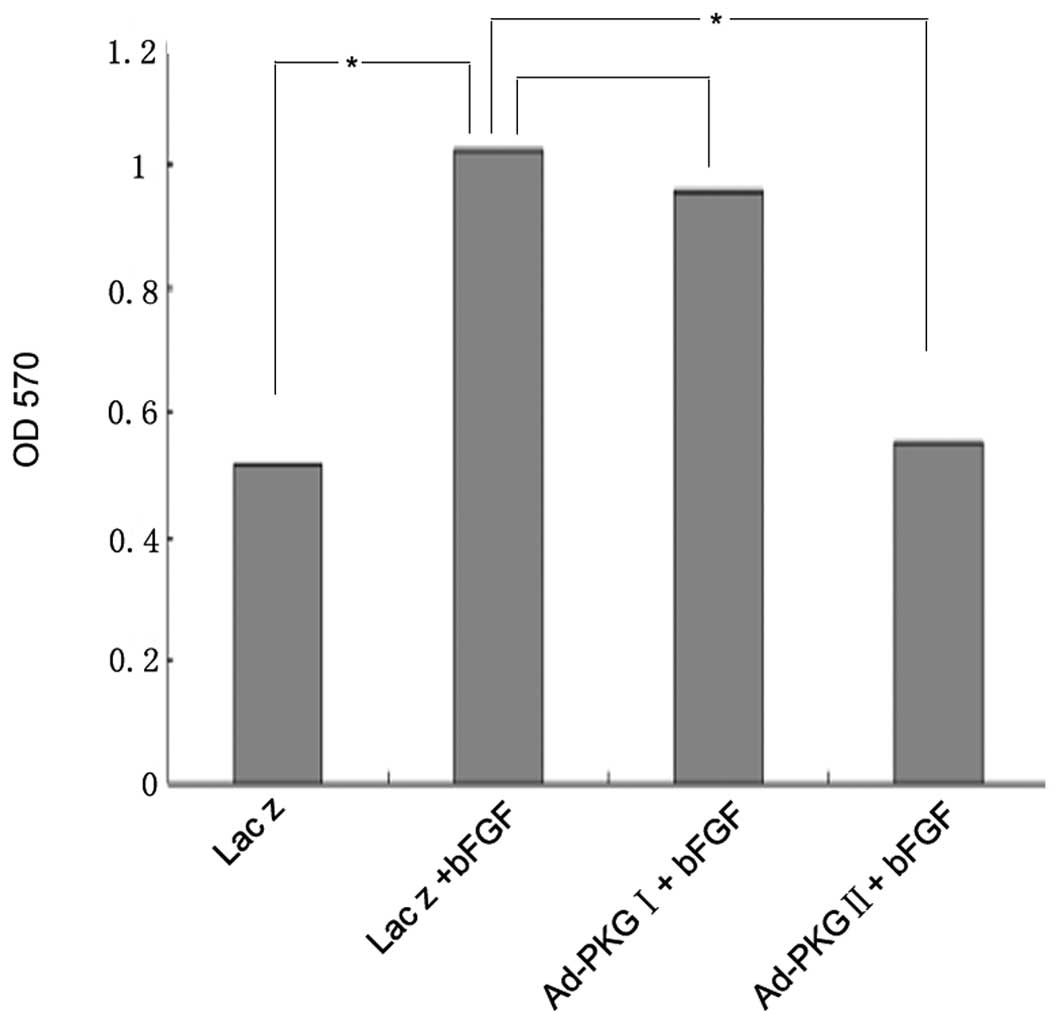
PKG II, but not PKG I, prevents the
bFGF-induced proliferation of U251 human glioma cells
In the present study, we demonstrated that bFGF
stimulates the proliferation of U251 human glioma cells. Since our
previous study demonstrated that PKG II inhibits the proliferation
of gastric cancer cells, the aim of the current study was to
investigate whether PKG II and PKG I are able to attenuate the
bFGF-induced proliferation of U251 cells. Compared with U251 cells
treated with bFGF at a concentration of 100 ng/ml alone, cells
infected with Ad-PKG II and stimulated with 8-pCPT-cGMP prior to
treatment with bFGF, showed a reduction in proliferation, while
there was no obvious change when the cells were infected with
Ad-PKG I and stimulated with 8-Br-cGMP (Fig. 2). This indicates that PKG II, but
not PKG I, inhibits the bFGF-induced proliferation of U251
cells.
PKG II, but not PKG I, prevents the
bFGF-induced migration of U251 human glioma cells
In the present study, it was demonstrated that bFGF
enhances the migration of U251 human glioma cells. There has been
no data demonstrating the effect of PKG on the migration of cancer
cells to date. In the present study, we investigated whether PKG
was able to prevent the bFGF-induced migration of U251 cells.
Compared with U251 cells treated with bFGF at a concentration of
100 ng/ml alone, cells infected with Ad-PKG II and stimulated with
8-pCPT-cGMP prior to treatment with bFGF, showed a decreased
migratory activity, while there was no clear change of the cells
infected with Ad-PKG I and stimulated with 8-Br-cGMP (Fig. 3). This indicates that PKG II, but
not PKG I, inhibits the bFGF-induced migration of U251 cells.
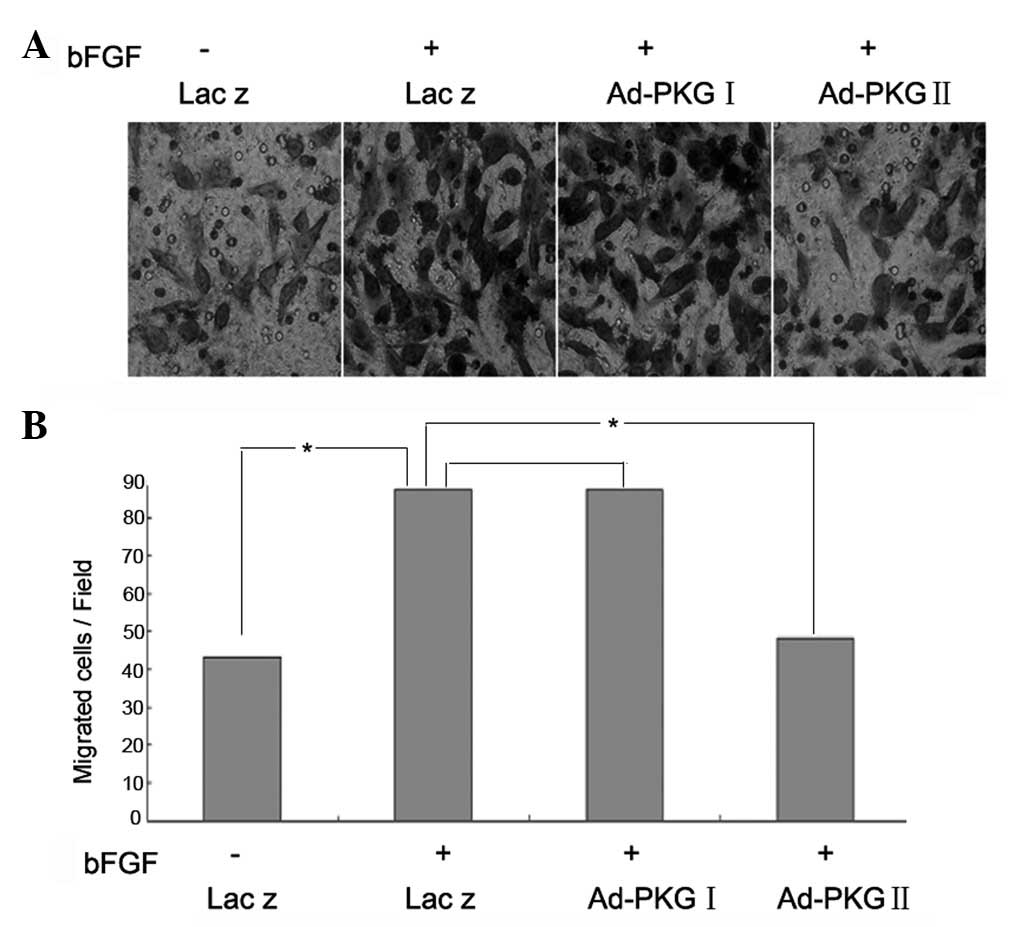 | Figure 3PKG II, but not PKG I, prevents
bFGF-induced migration of U251 human glioma cells. (A and B) A
Transwell migration assay was used to investigate the migration of
U251 cells. U251 cells were infected with Ad-Lacz, Ad-PKG I or
Ad-PKG II for 48 h to establish Ad-Lacz+bFGF, Ad-PKG I+bFGF and
Ad-PKG II+bFGF groups. The cells were serum starved overnight and,
in the Ad-PKG I+bFGF and Ad-PKG II+bFGF groups, 250 μM 8-Br-cGMP
and 250 μM 8-pCPT-cGMP were added to activate PKG I and PKG II,
respectively. Then, the cells were incubated with bFGF (100 ng/ml)
for 12 h. The means of five independent experiments ± standard
error are shown. *P<0.05. PKG, cGMP-dependent protein
kinase; bFGF, basic fibroblast growth factor. |
PKG II, but not PKG I, prevents the
bFGF-induced activation of the MAPK/ERK signaling pathway in U251
human glioma cells
FGF receptors activate several intracellular
signaling pathways, including the MAP kinase pathway (31–33).
Western blot analysis was used to detect FGFR phosphorylation. MEK1
and MEK2 are members of the dual specificity protein kinase family,
which act as MAPK or ERK kinases. Phosphorylation at both
Thr202/Tyr204 residues of ERK1 and Thr185/Tyr187 residues of ERK2
is required for full enzymatic activation. Western blot analysis
was used to detect MEK and ERK phosphorylation. The results
indicated that treatment with bFGF alone at a concentration of 100
ng/ml, increased the phosphorylation levels of FGFR, MEK and ERK.
The increased phosphorylation was inhibited by pre-infecting the
cells with Ad-PKG II and stimulating the enzyme with 8-pCPT-cGMP,
while no significant inhibitory effect was achieved by
pre-infecting the cells with Ad-PKG I and stimulating the enzyme
with 8-Br-cGMP. These results demonstrate that increased PKG II
activity prevents the bFGF-induced phosphorylation of FGFR, MEK and
ERK in U251 human glioma cells but increased PKG I activity does
not (Fig. 4). Furthermore, we
investigated the effect of PKG on the bFGF-induced nuclear
translocation of p-ERK. The results showed that bFGF stimulated the
nuclear distribution of p-ERK, and that the stimulatory effect was
inhibited by pre-infecting the cells with Ad-PKG II and stimulating
the enzyme with 8-pCPT-cGMP, while pre-infecting the cells with
Ad-PKG I and stimulating the enzyme with 8-Br-cGMP had no
inhibitory effect (Fig. 5). The
results indicate that increased PKG II activity attenuated the
bFGF-triggered p-ERK nuclear distribution whereas increased PKG I
activity did not.
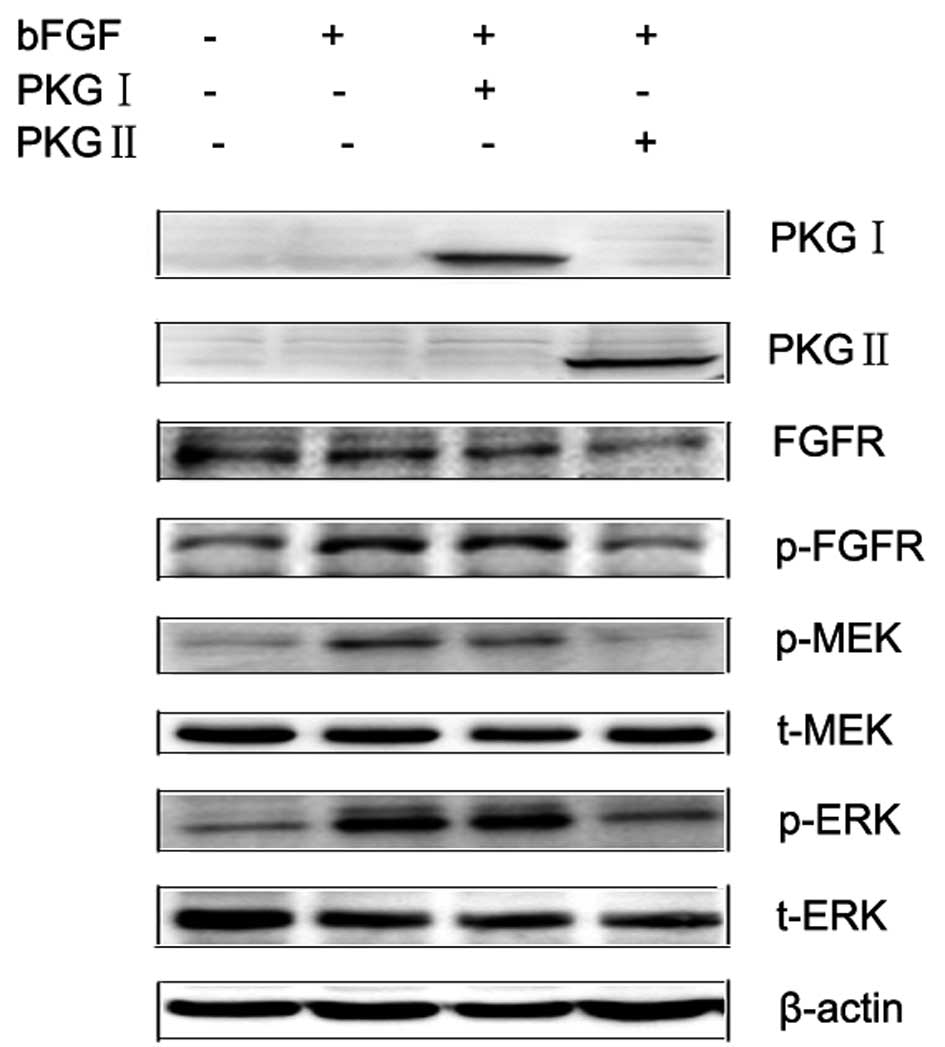 | Figure 4PKG II, but not PKG I, attenuated the
bFGF-induced activation of the MAPK/ERK pathway in U251 human
glioma cells. U251 cells were infected with Ad-Lacz, Ad-PKG I or
Ad-PKG II for 48 h to establish Ad-Lacz+bFGF, Ad-PKG I+bFGF and
Ad-PKG II+bFGF groups. The cells were serum starved overnight and,
in the Ad-PKG I+bFGF and Ad-PKG II+bFGF groups, 250 μM 8-Br-cGMP
and 250 μM 8-pCPT-cGMP were added to activate PKG I and PKG II,
respectively. Then, the cells were incubated with bFGF (100 ng/ml)
for 15 min. Whole cells were harvested and lysed as described in
Materials and methods and cell lysates were subjected to western
blot analysis. Results showed that infection with Ad-PKG I and
Ad-PKG II caused a marked increase of PKG I and PKG II expression
levels, respectively. bFGF treatment induced a significant increase
of FGFR, MEK and ERK phosphorylation. Infection with Ad-PKG II and
stimulation with 8-pCPT-cGMP, but not Ad-PKG I+8-Br-cGMP treatment,
efficiently inhibited the bFGF-induced phosphorylation of FGFR, MEK
and ERK. The means of five independent experiments ± standard error
are shown. PKG, cGMP-dependent protein kinase; bFGF, basic
fibroblast growth factor; FGFR, fibroblast growth factor
receptor. |
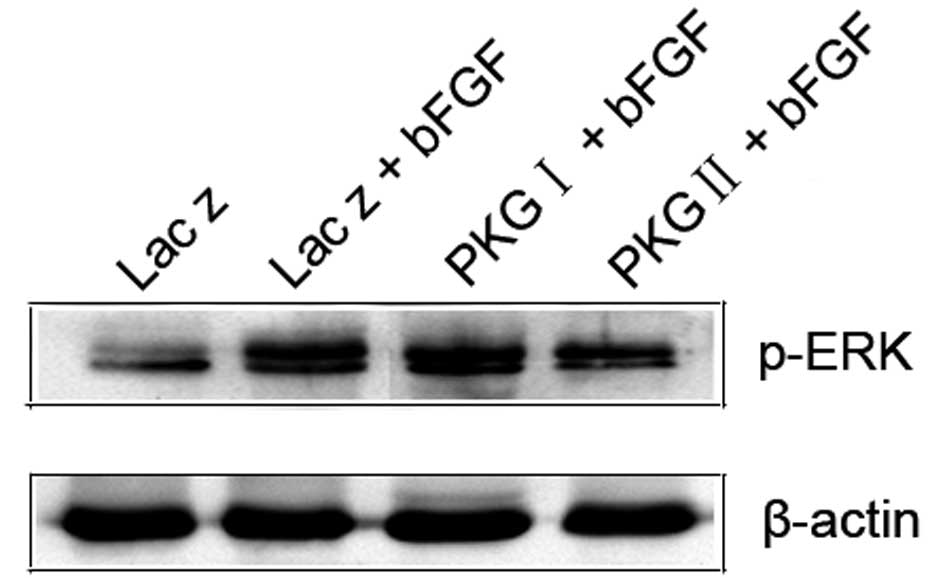 | Figure 5PKG II, but not PKG I, reverses the
bFGF-triggered nuclear distribution of p-ERK in U251 human glioma
cells. U251 cells were infected with Ad-Lacz, Ad-PKG I or Ad-PKG II
for 48 h to establish Ad-Lacz+bFGF, Ad-PKG I+bFGF and Ad-PKG
II+bFGF groups. The cells were serum starved overnight and, in the
Ad-PKG I+bFGF and Ad-PKG II+bFGF groups, 250 μM 8-Br-cGMP and 250
μM 8-pCPT-cGMP were added to activate PKG I and PKG II,
respectively. The cells were then incubated with bFGF (100 ng/ml)
for 30 min. Nuclear cell lysate was prepared as described in
Materials and methods and subjected to western blot analysis. The
results indicated that bFGF treatment induced a significant
increase in the expression of p-ERK in the nucleus. Infection with
Ad-PKG II and stimulation with 8-pCPT-cGMP, but not Ad-PKG
I+8-Br-cGMP treatment, efficiently inhibited the bFGF-induced
nuclear distribution of p-ERK. The means of five independent
experiments ± standard error are shown. PKG, cGMP-dependent protein
kinase; bFGF, basic fibroblast growth factor. |
Discussion
The growth of solid tumors depends on the occurrence
of neovascularization. bFGF is an important angiogenic factor,
widely distributed in neoplastic tissues (34). Numerous angiogenic peptides have
been identified and their effects on tumor vascularity have also
been identified (35–38). FGF receptors activate several
intracellular signaling pathways, including MAP kinase pathways.
MAP kinase pathways have been identified as the ERK/MAP kinase
pathway, the JNK/SAPK pathway and the p38 pathway (39,40).
These three pathways may be activated by different growth factors
and mediate several cellular events, including cell
differentiation, stress responses and growth. However, the
activation of each type of MAP kinase mainly depends on the type of
the stimulus and the cells.
PKG plays important regulatory roles in diverse
processes in many cell types (15,41,42).
Its expression is differently regulated in tumors and in normal
tissue (14,43,44).
In mammalian cells, two different genes encode type I and II PKGs
(45). PKG I includes two
isoforms, PKG Iα and PKG Iβ, which differ in the first ~100 amino
acids (46). PKG I has been
recognized as a tumor suppressor. PKG II is membrane-anchored and
is present at low levels in several types of human cancer cells
(47). Previous data have
indicated that PKG II is related to cell proliferation and
apoptosis (21,22). We have also found that PKG II
attenuates the EGF-induced proliferation and apoptosis of gastric
cancer cells (48,49). There has been no data showing the
relationship between PKG and migration. In the present study, the
exact stimulative effects of bFGF on the proliferation and
migration of U251 human glioma cells was confirmed. Consequently,
we performed further experiments to investigate whether PKG I or
PKG II exerted inhibitory effects on the bFGF-induced proliferation
and migration of human glioma cells, and the possible underlying
mechanism.
In the present study, the PKG I-selective cGMP
analog 8-Br-cGMP and the PKG II-selective cGMP analog 8-pCPT-cGMP
were applied to increase PKG I or PKG II activity when cells were
infected with Ad-PKG I or Ad-PKG II, respectively. After confirming
the effects of bFGF on the proliferation and migration of U251
human glioma cells, we analyzed the effects of PKG I and PKG II on
bFGF-stimulated cell proliferation and migration. Compared with
treatment with bFGF alone, increased PKG II activity clearly
attenuated bFGF-induced proliferation and migration, while
increased PKG I activity had no effect. Then, we investigated the
inhibitory effects of PKG I and PKG II on the bFGF-induced
phosphorylation of FGFR, MEK and ERK. It was found that increased
PKG II, but not PKG I, activity was able to attenuate bFGF-induced
phosphorylation. Furthermore, the inhibitory effects of PKG I and
PKG II on the bFGF-induced nuclear distribution of p-ERK were
detected. The results obtained showed that increased PKG II, but
not PKG I, activity was able to attenuate bFGF-induced p-ERK
nuclear distribution.
In this study it was shown that increased PKG II,
but not PKG I, activity inhibits bFGF-stimulated cell proliferation
and migration, bFGF-induced FGFR, MEK and ERK phosphorylation and
bFGF-induced p-ERK nuclear distribution in U251 human glioma cells.
In conclusion, the inhibitory effects of PKG II on bFGF-induced
cell proliferation and migration were mainly exerted by blocking
the MAPK/ERK signaling pathway.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 31100974 and 81001100) and the
Specialized Research Fund for Senior Personnel Program of Jiangsu
University (no. 11JDG032).
References
|
1
|
Ribatti D, Vacca A, Rusnati M and Presta
M: The discovery of basic fibroblast growth factor/fibroblast
growth factor-2 and its role in haematological malignancies.
Cytokine Growth Factor Rev. 18:327–334. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shi YH, Bingle L, Gong LH, Wang YX, Corke
KP and Fang WG: Basic FGF augments hypoxia induced HIF-1-alpha
expression and VEGF release in T47D breast cancer cells. Pathology.
39:396–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith JA, Madden T, Vijjeswarapu M and
Newman RA: Inhibition of export of fibroblast growth factor-2
(FGF-2) from the prostate cancer cell lines PC3 and DU145 by
Anvirzel and its cardiac glycoside component, oleandrin. Biochem
Pharmacol. 62:469–472. 2001. View Article : Google Scholar
|
|
4
|
Cronauer MV, Hittmair A, Eder IE, Hobisch
A, Culig Z, Ramoner R, Zhang J, Bartsch G, Reissigl A, Radmayr C,
Thurnher M and Klocker H: Basic fibroblast growth factor levels in
cancer cells and in sera of patients suffering from proliferative
disorders of the prostate. Prostate. 31:223–233. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giehl KA, Nägele U, Volkenandt M and
Berking C: Protein expression of melanocyte growth factors (bFGF,
SCF) and their receptors (FGFR-1, c-kit) in nevi and melanoma. J
Cutan Pathol. 34:7–14. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fortin D, Rom E, Sun H, Yayon A and Bansal
R: Distinct fibroblast growth factor (FGF)/FGF receptor signaling
pairs initiate diverse cellular responses in the oligodendrocyte
lineage. J Neurosci. 25:7470–7479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Udayakumar TS, Klein RD, Maliner MS, Nagle
RB and Bowden GT: Aberrant expression of fibroblast growth factor
receptor-1 in prostate epithelial cells allows induction of
promatrilysin expression by fibroblast growth factors. Int J
Cancer. 91:187–192. 2001. View Article : Google Scholar
|
|
8
|
Kamura S, Matsumoto Y, Fukushi JI,
Fujiwara T, Iida K, Okada Y and Iwamoto Y: Basic fibroblast growth
factor in the bone microenvironment enhances cell motility and
invasion of Ewing’s sarcoma family of tumours by activating the
FGFR1-PI3K-Rac1 pathway. Br J Cancer. 103:370–381. 2010.PubMed/NCBI
|
|
9
|
Narong S and Leelawat K: Basic fibroblast
growth factor induces cholangiocarcinoma cell migration via
activation of the MEK1/2 pathway. Oncol Lett. 2:821–825.
2011.PubMed/NCBI
|
|
10
|
Zhang W, Chu YQ, Ye ZY, Zhao ZS and Tao
HQ: Expression of hepatocyte growth factor and basic fibroblast
growth factor as prognostic indicators in gastric cancer. Anat Rec
(Hoboken). 292:1114–1121. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Massabeau C, Rouquette I, Lauwers-Cances
V, Mazières J, Bachaud JM, Armand JP, Delisle MB, Favre G, Toulas C
and Cohen-Jonathan-Moyal E: Basic fibroblast growth factor-2/beta3
integrin expression profile: signature of local progression after
chemoradiotherapy for patients with locally advanced non-small-cell
lung cancer. Int J Radiat Oncol Biol Phys. 75:696–702. 2009.
View Article : Google Scholar
|
|
12
|
Dai H, Zhao S, Xu L, Chen A and Dai S:
Expression of Efp, VEGF and bFGF in normal, hyperplastic and
malignant endo-metrial tissue. Oncol Rep. 23:795–799.
2010.PubMed/NCBI
|
|
13
|
Orstavik S, Natarajan V, Tasken K, Jahnsen
T and Sandberg M: Characterization of the human gene encoding the
type I alpha and type I beta cGMP-dependent protein kinase (PRKG1).
Genomics. 42:311–318. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Orstavik S, Solberg R, Tasken K, Nordahl
M, Altherr MR, Hansson V, Jahnsen T and Sandberg M: Molecular
cloning, cDNA structure, and chromosomal localization of the human
type II cGMP-dependent protein kinase. Biochem Biophys Res Commun.
220:759–765. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lincoln TM, Dey N and Sellak H: Invited
review: cGMP-dependent protein kinase signaling mechanisms in
smooth muscle: from the regulation of tone to gene expression. J
Appl Physiol. 91:1421–1430. 2001.PubMed/NCBI
|
|
16
|
Shimojo T, Hiroe M, Ishiyama S, Ito H,
Nishikawa T and Marumo F: Nitric oxide induces apoptotic death of
cardiomyocytes via a cyclic-GMP-dependent pathway. Exp Cell Res.
247:38–47. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Segawa K, Minami K, Shiga Y, Shiraishi M,
Sata T, Nakashima Y and Shigematsu A: Inhibitory effects of
nicorandil on rat mesangial cell proliferation via the protein
kinase G pathway. Nephron. 87:263–268. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loweth AC, Williams GT, Scarpello JH and
Morgan NG: Evidence for the involvement of cGMP and protein kinase
G in nitric oxide-induced apoptosis in the pancreatic B-cell line,
HIT-T15. FEBS Lett. 400:285–288. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brunetti M, Mascetra N, Manarini S,
Martelli N, Cerletti C, Musiani P, Aiello FB and Evangelista V:
Inhibition of cGMP-dependent protein kinases potently decreases
neutrophil spontaneous apoptosis. Biochem Biophys Res Commun.
297:498–501. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hou Y, Gupta N, Schoenlein P, Wong E,
Martindale R, Ganapathy V and Browning D: An anti-tumor role for
cGMP-dependent protein kinase. Cancer Lett. 240:60–68. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cook AL and Haynes JM: Protein kinase G
II-mediated proliferative effects in human cultured prostatic
stromal cells. Cell Signal. 16:253–261. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cook AL and Haynes JM: Phosphorylation of
the PKG substrate, vasodilator-stimulated phosphoprotein (VASP), in
human cultured prostatic stromal cells. Nitric Oxide. 16:10–17.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiche JD, Schlutsmeyer SM, Bloch DB, de
la Monte SM, Roberts JD Jr, Filippov G, Janssens SP, Rosenzweig A
and Bloch KD: Adenovirus-mediated gene transfer of cGMP-dependent
protein kinase increases the sensitivity of cultured vascular
smooth muscle cells to the antiproliferative and pro-apoptotic
effects of nitric oxide/cGMP. J Biol Chem. 273:34263–34271. 1998.
View Article : Google Scholar
|
|
24
|
Hood J and Granger HJ: Protein kinase G
mediates vascular endothelial growth factor-induced Raf-1
activation and proliferation in human endothelial cells. J Biol
Chem. 273:23504–23508. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Swartling FJ, Ferletta M, Kastemar M,
Weiss WA and Westermark B: Cyclic GMP-dependent protein kinase II
inhibits cell proliferation, Sox9 expression and Akt
phosphorylation in human glioma cell lines. Oncogene. 28:3121–3131.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang SQ, Chen YC, Wang Y and Tao Y:
Expression of cGMP dependent protein kinase II in cancer cell lines
was obviously decreased. J Jiangsu Univ. 18:1–5. 2008.(In
Chinese).
|
|
27
|
Chen YC, Ren F, Sang JR, Tao Y and Xu WR:
Type II cGMP-dependent protein kinase inhibits proliferation of the
gastric cancer cell line BGC-823. Mol Med Rep. 3:361–366.
2010.PubMed/NCBI
|
|
28
|
Chen JC, Zhuang S, Nguyen TH, Boss GR and
Pilz RB: Oncogenic Ras leads to Rho activation by activating the
mitogen-activated protein kinase pathway and decreasing
Rho-GTPase-activating protein activity. J Biol Chem. 278:2807–2818.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pardo OE, Latigo J, Jeffery RE, et al: The
fibroblast growth factor receptor inhibitor PD173074 blocks small
cell lung cancer growth in vitro and in vivo. Cancer Res.
69:8645–8651. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nomura S, Yoshitomi H, Takano S, Shida T,
Kobayashi S, Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A
and Miyazaki M: FGF10/FGFR2 signal induces cell migration and
invasion in pancreatic cancer. Br J Cancer. 99:305–313. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chikazu D, Hakeda Y, Ogata N, Nemoto K,
Itabashi A, Takato T, Kumegawa M, Nakamura K and Kawaguchi H:
Fibroblast growth factor (FGF)-2 directly stimulates mature
osteoclast function through activation of FGF receptor 1 and
p42/p44 MAP kinase. J Biol Chem. 275:31444–31450. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brauchle M, Gluck D, Di Padova F, Han J
and Gram H: Independent role of p38 and ERK1/2 mitogen-activated
kinases in the upregulation of matrix metalloproteinase-1. Exp Cell
Res. 258:135–144. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tokuda H, Kozawa O and Uematsu T: Basic
fibroblast growth factor stimulates vascular endothelial growth
factor release in osteoblasts: divergent regulation by p42/p44
mitogen-activated protein kinase and p38 mitogen-activated protein
kinase. J Bone Miner Res. 15:2371–2379. 2000. View Article : Google Scholar
|
|
34
|
Gonzalez AM, Buscaglia M, Ong M and Baird
A: Distribution of basic fibroblast growth factor in the 18-day rat
fetus: localization in the basement membranes of diverse tissues. J
Cell Biol. 110:753–765. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Folkman J and Klagsbrun M: Angiogenic
factors. Science. 235:442–447. 1987. View Article : Google Scholar
|
|
36
|
Leung DW, Cachianes G, Kuang WJ, Goeddel
DV and Ferrara N: Vascular endothelial growth factor is a secreted
angiogenic mitogen. Science. 246:1306–1309. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ferrara N, Houck K, Jakeman L and Leung
DW: Molecular and biological properties of the vascular endothelial
growth factor family of proteins. Endocr Rev. 13:18–32. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
New BA and Yeoman LC: Identifation of
basic firoblast growth factor sensitivity and receptor and ligand
expression in human colon tumor cell lines. Cell Physiol.
150:320–326. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hill CS and Treisman R: Transcriptional
regulation by extracellular signals: mechanisms and specificity.
Cell. 80:199–211. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alessi DR, Cuenda A, Cohen P, Dudley DT
and Saltiel AR: PD 098059 is a specific inhibitor of the activation
of mitogen-activated protein kinase kinase in vitro and in vivo. J
Biol Chem. 270:27489–27494. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Francis SH and Corbin JD: Cyclic
nucleotide-dependent protein kinases: intracellular receptors for
cAMP and cGMP action. Crit Rev Clin Lab Sci. 36:275–328. 1999.
View Article : Google Scholar
|
|
42
|
Ruth P: Cyclic GMP-dependent protein
kinases: understanding in vivo functions by gene targeting.
Pharmacol Ther. 82:355–372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boerth NJ, Dey NB, Cornwell TL and Lincoln
TM: Cyclic GMP-dependent protein kinase regulates vascular smooth
muscle cell phenotype. J Vasc Res. 34:245–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sellak H, Yang X, Cao X, Cornwell T, Soff
GA and Lincoln T: Sp1 transcription factor as a molecular target
for nitric oxide- and cyclic nucleotide-mediated suppression of
cGMP-dependent protein kinase-Ialpha expression in vascular smooth
muscle cells. Circ Res. 90:405–412. 2002. View Article : Google Scholar
|
|
45
|
Feil R, Hofmann F and Kleppisch T:
Function of cGMP-dependent protein kinases in the nervous system.
Rev Neurosci. 16:23–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Münzel T, Feil R, Mülsch A, Lohmann SM,
Hofmann F and Walter U: Physiology and pathophysiology of vascular
signaling controlled by guanosine 3′,5′-cyclic
monophosphate-dependent protein kinase [corrected]. Circulation.
108:2172–2183. 2003.
|
|
47
|
Schlossmann J, Feil R and Hofmann F:
Insights into cGMP signalling derived from cGMP kinase knockout
mice. Front Biosci. 10:1279–1289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu Y, Chen Y, Qu R, Lan T and Sang J: Type
II cGMP-dependent protein kinase inhibits EGF-triggered signal
transduction of the MAPK/ERK-mediated pathway in gastric cancer
cells. Oncol Rep. 27:553–558. 2012.PubMed/NCBI
|
|
49
|
Lan T, Chen Y, Sang J, Wu Y, Wang Y, Jiang
L and Tao Y: Type II cGMP-dependent protein kinase inhibits
EGF-induced MAPK/JNK signal transduction. Oncol Rep. 27:2039–2044.
2012.PubMed/NCBI
|