Introduction
Diabetes mellitus (DM) is a group of metabolic
disorders with different underlying etiologies. It is characterized
by absolute or relative deficiencies in insulin secretion and/or
insulin action associated with chronic hyperglycemia and
disturbances of carbohydrate, lipid and protein metabolism. As a
consequence of the metabolic derangements in diabetes, various
complications develop including macro- and microvascular
dysfunctions (1,2). The management of diabetes is
considered a global problem. Modern drugs, including insulin and
other hyperglycemic agents such as biguanides and sulphonylureas
control the blood glucose level only when they are regularly
administered, although these treatments are laborious and have
several disadvantages including hypoglycemia and obesity (3). Therefore the identification of
efficacious agents with less severe side-effects is crucial. Over
the past few decades, traditional Chinese medicine has played a key
role in the therapy of DM and its complications (4,5).
Based on a large number of chemical and pharmacological research
studies, numerous bioactive compounds have been found in Chinese
medicinal plants for the treatment of diabetes. These compounds
include polysaccharides, terpenoids, flavonoids, sterols and
alkanoids (6–10).
Licorice is the root and stolon of the
Glycyrrhiza plant, which belongs to the family Leguminosae.
This plant has been medicinally used for >4,000 years (11). It is a Chinese herb commonly used
as an expectorant and to arrest coughing, reduce fever, comfort the
stomach, alleviate urgency and potentiate the effects of various
other herbs (12). Licorice has
been reported to attenuate free radical-induced oxidative damage in
the kidney, prevent carcinogenesis induced by toxicants or hormones
and also has a significant hepatoprotective activity (13–15).
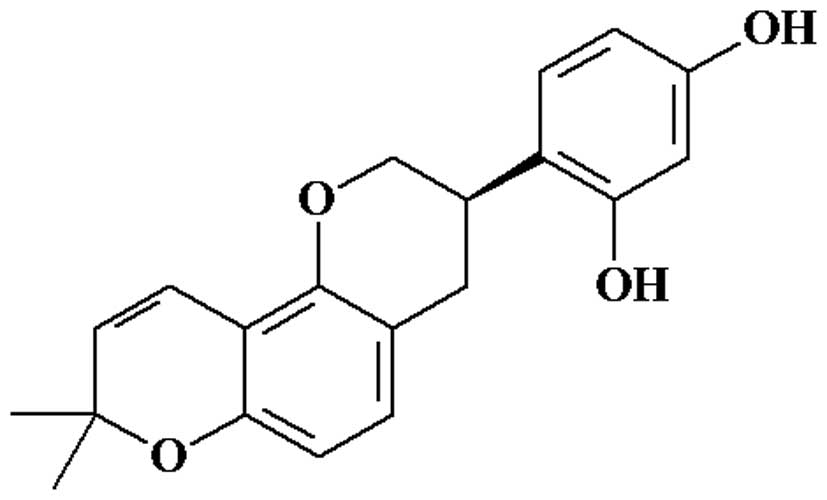
Licorice contains flavonoids and triterpenoids (15). Glabridin (Fig. 1), a polyphenolic flavonoid, is a
main active component in licorice, which has been reported to
exhibit multiple pharmacological activities, such as cytotoxic,
antimicrobial, anti-fatigue, estrogenic and anti-proliferative
activity against human breast cancer cells (16). It also affects melanogenesis,
inflammation, low-density lipoprotein oxidation and protection of
mitochondrial functions from oxidative stress (17). However, there is a limited number
of studies on the effect of glabridin on DM at present. Thus, the
aim of this study was to investigate the hypoglycemic effects of
glabridin from licorice in an animal model of DM.
Materials and methods
Reagents
Glabridin (purity >99% by HPLC analysis) was
purchased from Shaanxi Langrun Biotechnology Co., Ltd. (Xi’an,
China). Streptozotocin (STZ) was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Glyburide (glibenclamide) was purchased from
Shanxi Sanjin Pharmaceutical Co., Ltd. (Taiyuan, China). Glucose
kit was purchased from Biosino Biotechnology and Science, Inc.
(Beijing, China). Superoxide dismutase (SOD) and malondialdehyde
(MDA) kits were purchased from Jiancheng Bioengineering Institute
(Nanjing, China). Other chemicals were obtained from local sources
and were of analytical grade.
Animals
Male Kunming mice (2 months old, 18–22-g body
weight) were obtained from the Shanghai Center of Experimental
Animals (Shanghai, China). The animals were housed under standard
environmental conditions (22–25°C, humidity 60–70%, 12-h light/dark
cycle) with free access to standard diet and water ad
libitum. The mice used in this study were processed in
accordance with the UK Animals (Scientific Procedures) Act 1986 and
associated guidelines. The experimental protocol was approved by
the Shanghai Jiao Tong University Animal Care and Use Committee
(Shanghai, China).
Induction of diabetes mellitus
The mice were fasted for 16 h prior to the induction
of DM. STZ was freshly prepared in 0.1-mol/l citrate buffer
solution (pH 4.5) and was intraperitoneally injected into mice with
a single dose of 60 mg/kg. DM was confirmed by the measurement of
blood glucose from the tail vein 72 h after injection of STZ. Mice
with a blood glucose level >11.0 mmol/l, as well as polydipsia,
polyuria and polyphagia were selected for the experiment (18).
Experimental design
Following confirmation of the diabetic state, the
mice were randomized into six groups of 10 animals each: i) normal
control group (NC), non diabetic mice administered 0.5 ml of 0.5%
Tween-80 solution; ii) diabetic control group (DC), diabetic mice
administered 0.5 ml of 0.5% Tween-80 solution; iii) diabetic +
low-dose glabridin treatment group (DLG), diabetic mice
administered glabridin (10 mg/kg) in 0.5 ml 0.5% Tween-80 solution;
iv) diabetic + medium-dose glabridin treatment group (DMG),
diabetic mice administered glabridin (20 mg/kg) in 0.5 ml 0.5%
Tween-80 solution; v) diabetic + high-dose glabridin treatment
group (DHG), diabetic mice administered glabridin (40 mg/kg) in 0.5
ml 0.5% Tween-80 solution and vi) diabetic + glyburide treatment
group (DG), diabetic mice administered 0.5 ml of 0.5% Tween-80
solution containing glyburide (4 mg/kg).
Each treatment was continued daily for 28 days.
Fasting blood glucose (FBG) levels were measured once every week.
Blood was collected from the tip of the tail vein (starting from
9:00 a.m.) after a 12- to 14-h overnight fast. At the same time,
body weights of mice were measured. An oral glucose tolerance test
(OGTT) was performed on the last day of treatment after overnight
fasting. Blood was collected at 0, 30, 60 and 120 min after an oral
glucose load of 3.0 g/kg of body weight. Following completion of
the experiment, the mice were sacrificed by cervical decapitation.
The liver, kidney and pancreas were dissected out, washed in
ice-cold saline, and homogenized in Tris-HCl buffer. Supernatant
fractions of liver homogenate were used to measure SOD activity and
MDA content.
Statistical analysis
Results were presented as the means ± standard
deviation (SD). The data were evaluated by means of an analysis of
variance (ANOVA:MANOVA), using the Newman-Keuls test. Differences
with a value of P<0.05 were considered to indicate a
statistically significant difference.
Results
Effect of glabridin on the body weight of
mice
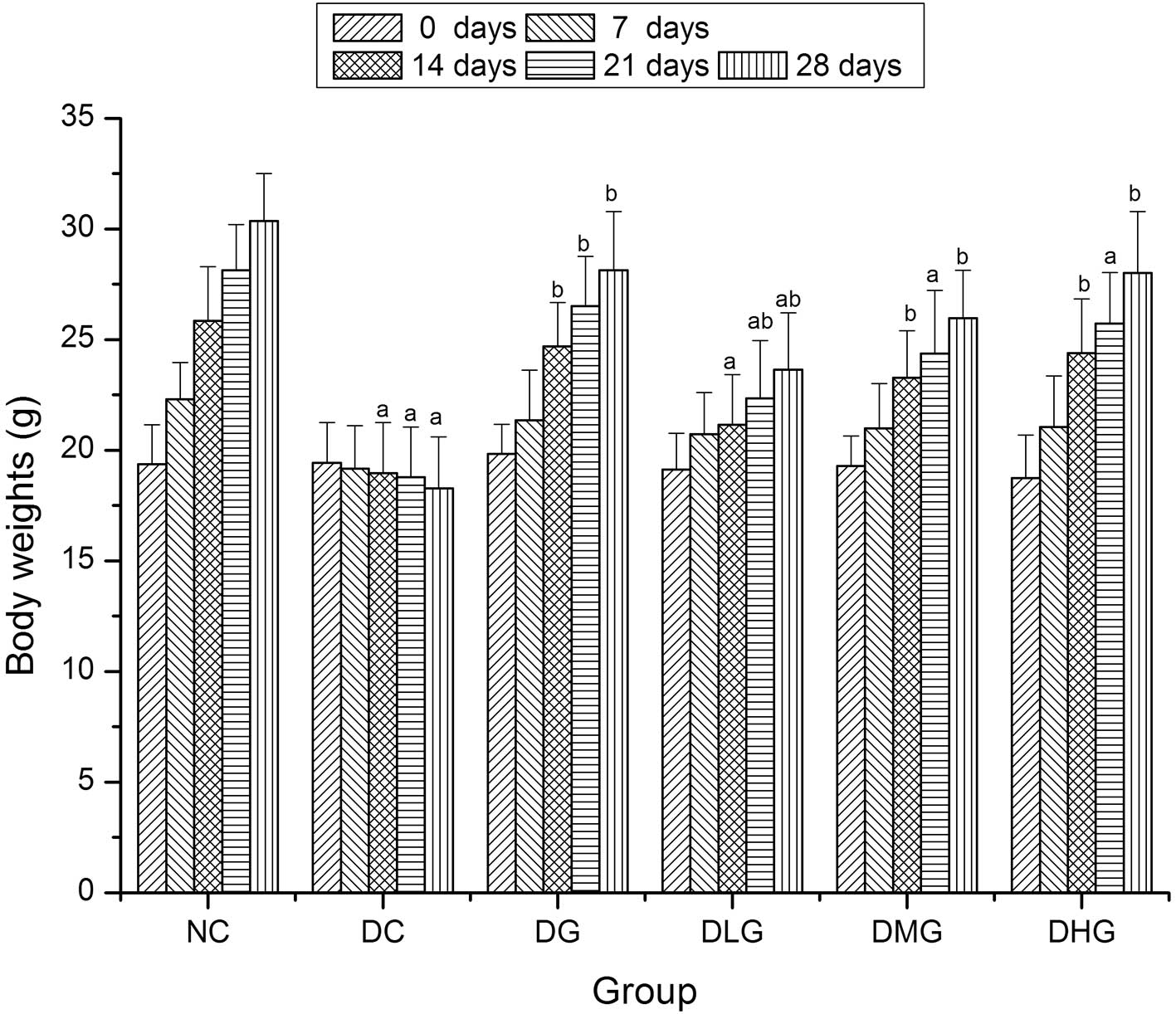
Prior to the experiment, the body weights were not
significantly different among all the groups (P>0.05) (Fig. 2). After 14 days, the body weights
of the mice in the DG, DMG and DHG groups were significantly
increased when compared with the DC group (P<0.05), while the
body weights of mice in the DHG group were increased, although not
significantly (P>0.05). After 28 days, the body weights of the
mice in the DG, DLG, DMG and DHG groups were significantly
increased when compared with the DC group (P<0.05), while the
body weights of the mice in the DLG group remained significantly
decreased when compared with the NC group (P<0.05).
Effect of glabridin on FBG levels of
mice
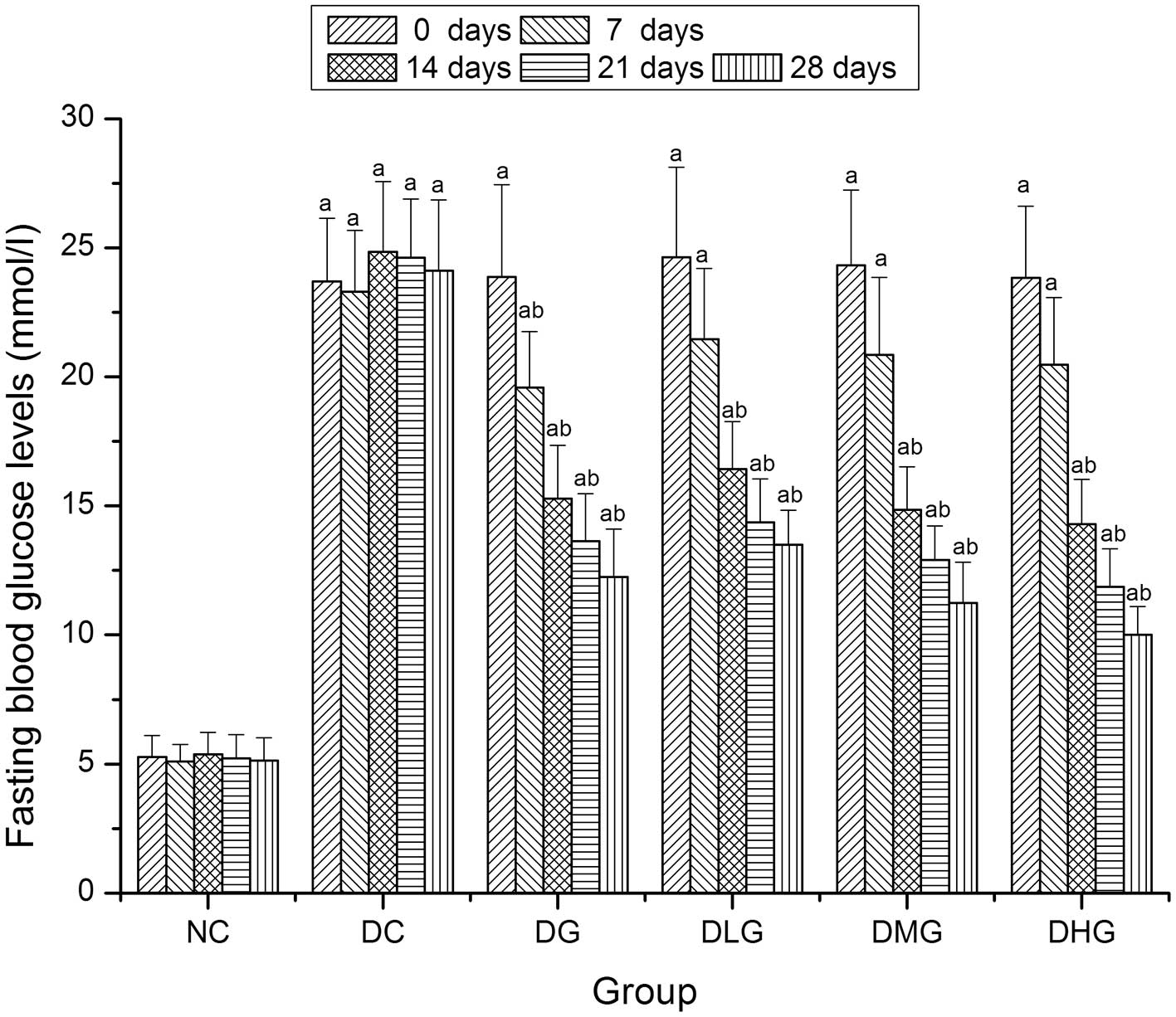
As shown in Fig. 3,
FBG levels in the NC group remained constant and were significantly
decreased as compared with the diabetic groups (DC, DG, DLG, DMG
and DHG) during the experimental period (P<0.05). After 7 days,
FBG levels in the diabetic treatment groups (DG, DLG, DMG and DHG)
showed a decreasing trend. After 28 days, FBG levels in the DG,
DLG, DMG and DHG groups were significantly decreased as compared
with the DC group (P<0.05), being 196.9, 178.8, 214.6 and 240.9%
lower, respectively.
Effect of glabridin on glucose tolerance
of mice
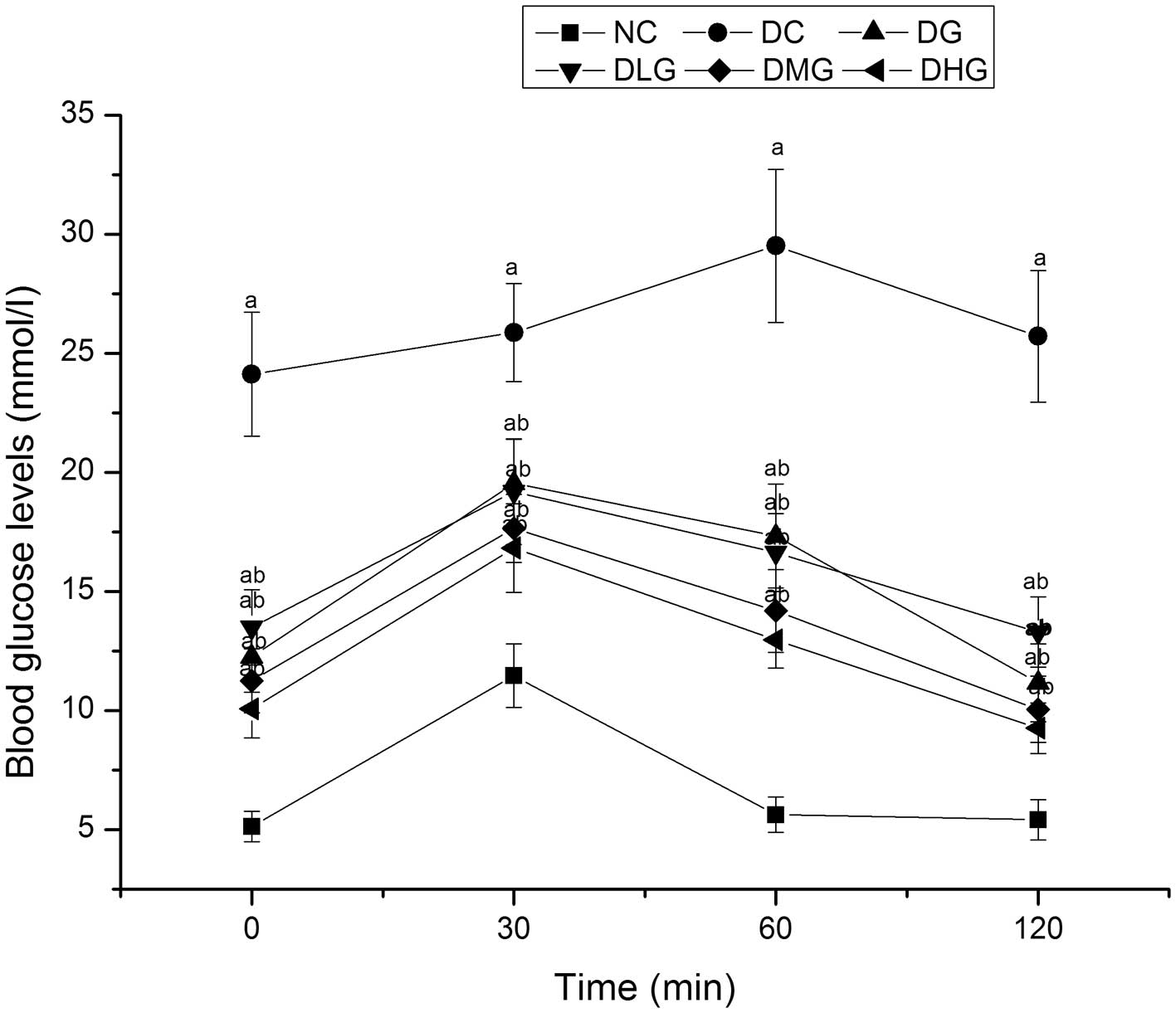
Blood glucose levels in the DG, DLG, DMG and DHG
groups were significantly decreased when compared with the DC
groups at different time intervals (0, 30, 60 and 120 min)
(P<0.05), while they remained significantly increased when
compared with the NC group (P<0.05) (Fig. 4).
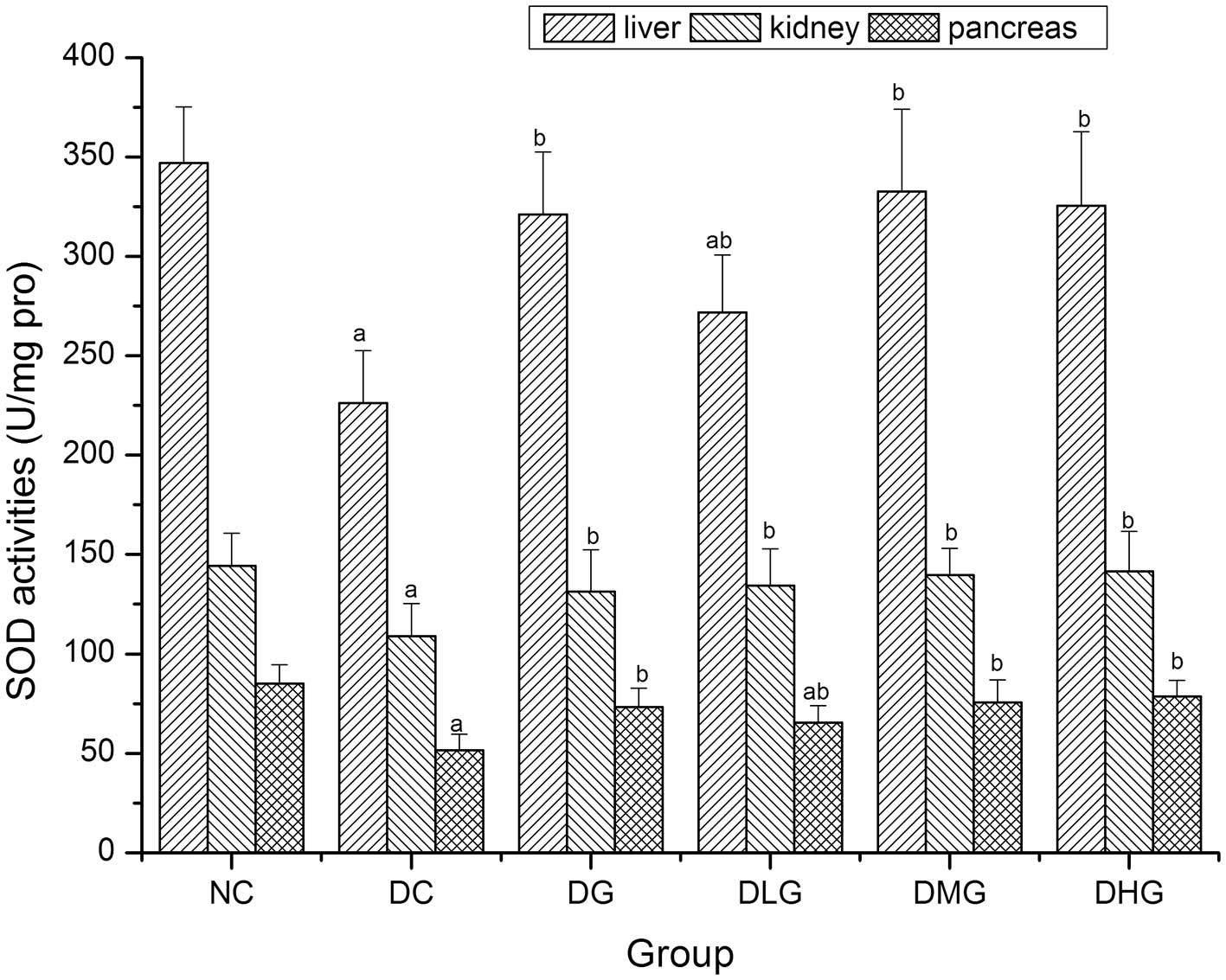
Effect of glabridin on SOD activities in
the liver, kidney and pancreas of mice
As shown in Fig. 5,
when compared with the DC group, SOD activities in the liver,
kidney and pancreas were significantly increased in the DG, DLG,
DMG and DHG groups (P<0.05), while SOD activities in the liver
and pancreas in the DLG group were still significantly decreased
when compared with the NC group (P<0.05).
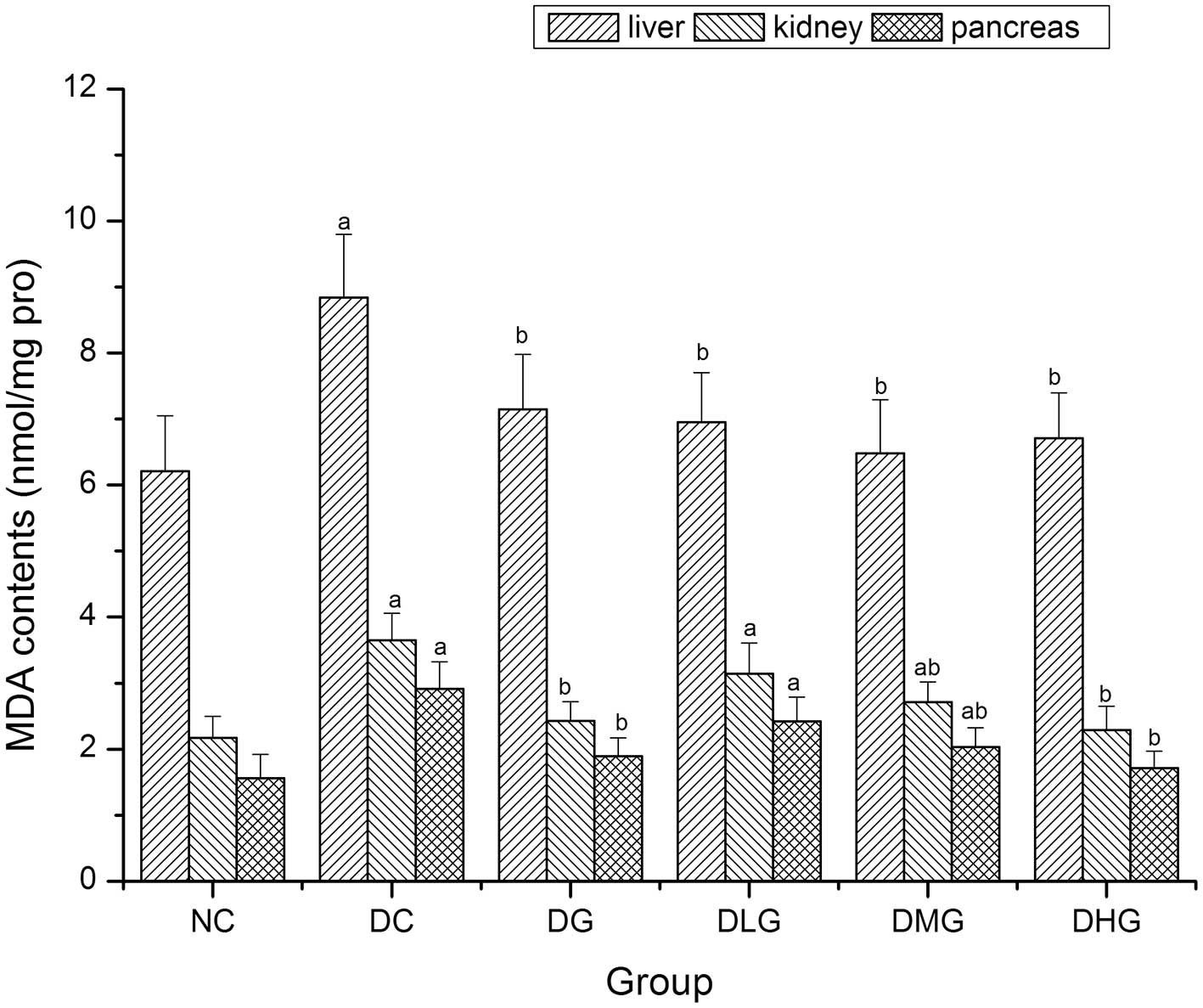
Effect of glabridin on MDA contents in
the liver, kidney and pancreas of mice
MDA contents in the liver, kidney and pancreas were
significantly decreased in the DG, DMG and DHG groups when compared
with the DC group (P<0.05) (Fig.
6). In the DLG group, MDA content in the liver was
significantly decreased (P<0.05) when compared with the DC
group, and MDA contents in the kidney and pancreas were decreased,
although not significantly (P<0.05).
Discussion
STZ (N-nitroso derivative of glucosamine) is a
broadspectrum antibiotic extracted from Streptomyces
achromogenes. It is a pancreatic β-cell toxin that induces
rapid and irreversible necrosis of β-cells and is widely used to
induce DM in experimental animal models (19,20).
STZ-induced DM is characterized by severe loss in body weight,
which may be due to degradation of structural proteins since they
are known to contribute to body weight (21). In this study, a significant body
weight loss was observed in the DC group and significant
improvement of body weight was observed in the glabridin treatment
groups (DLP, DMP and DHP). This finding may be due to the ability
of glabridin to reduce hyperglycemia.
DM is a serious chronic disease. Effective blood
glucose control is the key to preventing or reversing diabetic
complications and improving quality of life in patients with
diabetes (22,23). In the present study, STZ-induced
diabetic mice presented obvious hyperglycemic symptoms, while
glabridin produced a significant decrease in FBG levels in diabetic
mice. In addition, glucose tolerance also improved significantly
after glabridin treatment. These results indicated that glabridin
possesses hypoglycemic effects and that the 40-mg/kg dose of the
glabridin exerted a better effect when compared to doses of 10 or
20 mg.
Previous studies have shown that reactive oxygen
species (ROS) and lipid peroxidation are important in the
pathogenesis of DM and its complications (24–26).
An imbalance between ROS generation and the reduced activity of
antioxidant defenses or both of these phenomena lead to oxidative
stress. Hyperglycemia is a cause of oxidative stress in diabetic
patients and reduces the capacity of the endogenous antioxidant
defense system via the production of several reducing sugars
(through glycolysis and the polyol pathway) (26). In the present study, glabridin
significantly increased SOD activities, while decreasing MDA
contents in the liver, kidney and pancreas. Therefore, it may be
concluded that the antioxidative activities of glabridin in
STZ-induced diabetic mice, at least in part, may be related to
hypoglycemic effects.
In summary, the present study has demonstrated that
glabridin possesses hypoglycemic effects. The 40-mg/kg dose of
glabridin exerted a better effect when compared to doses of 10 or
20 mg. Further pharmacological and biochemical investigations would
clearly elucidate the mechanism of action and would be beneficial
in investigating the role of glabridin as a therapeutic target in
diabetes treatment research.
Acknowledgements
This study was supported by grants from the Research
Project of Science and Technology of Shanghai Jiao Tong University
(Shanghai, China).
References
|
1
|
El-Alfy AT, Ahmed AA and Fatani SAJ:
Protective effect of red grape seeds proanthocyanidins against
induction of diabetes by alloxan in rats. Pharmacol Res.
52:264–270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li F, Tang H, Xiao F, et al: Protective
effect of salidroside from Rhodiolae radix on
diabetes-induced oxidative stress in mice. Molecules. 16:9912–9924.
2011.
|
|
3
|
Zhang ZF, Lv GY, Pan HJ, et al:
Antidiabetic activities of ethanol extract of dry matters of
culture broth of Coriolus versiolor in submerged culture.
Braz Arch Biol Technol. 54:701–708. 2011. View Article : Google Scholar
|
|
4
|
Li WL, Zheng HC, Bukuru J and De Kimpe N:
Natural medicines used in the traditional Chinese medical system
for therapy of diabetes mellitus. J Ethnopharmacol. 92:1–21. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang LQ, Wei W, Chen LM and Liu S: Effects
of berberine on diabetes induced by alloxan and a
high-fat/high-cholesterol diet in rats. J Ethnopharmacol.
108:109–115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo Q, Cai Y, Yan J, et al: Hypoglycemic
and hypolipidemic effects and antioxidant activity of fruit
extracts from Lycium barbarum. Life Sci. 76:137–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lü H, Chen J, Li WL, et al: Hypoglycemic
effect of the total flavonoid fraction from folium
Eriobotryae. Phytomedicine. 16:967–971. 2009.PubMed/NCBI
|
|
8
|
Liu Z, Wang LJ, Li X, et al: Hypoglycemic
effects of malonyl-ginsenosides extracted from roots of Panax
ginseng on streptozotocin-induced diabetic mice. Phytother Res.
23:1426–1430. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi LW, Liu EH, Chu C, et al: Anti-diabetic
agents from natural products - an update from 2004 to 2009. Curr
Top Med Chem. 10:434–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Patel MB and Mishra S: Hypoglycemic
activity of alkaloidal fraction of Tinospora cordifolia.
Phytomedicine. 18:1045–1052. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian ML, Yan HY and Row KH: Simultaneous
extraction and separation of liquiritin, glycyrrhizic acid, and
glabridin from licorice root with analytical and preparative
chromatography. Biotechnol Bioprocess Eng. 13:671–676. 2008.
View Article : Google Scholar
|
|
12
|
Sabbioni C, Mandrioli R, Ferranti A, et
al: Separation and analysis of glycyrrhizin, 18beta-glycyrrhetic
acid and 18alpha-glycyrrhetic acid in liquorice roots by means of
capillary zone electrophoresis. J Chromatogr A. 1081:65–71. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mori H, Niwa K, Zheng Q, et al: Cell
proliferation in cancer prevention; effects of preventive agents on
estrogen-related endometrial carcinogenesis model and on an in
vitro model in human colorectal cells. Mutat Res. 480–481:201–207.
2001.
|
|
14
|
Yokozawa T, Cho EJ, Rhyu DY, et al:
Glycyrrhizae Radix attenuates peroxynitrite-induced renal
oxidative damage through inhibition of protein nitration. Free
Radic Res. 39:203–211. 2005. View Article : Google Scholar
|
|
15
|
Lee JY, Lee JH, Park JH, et al:
Liquiritigenin, a licorice flavonoid, helps mice resist
disseminated candidiasis due to Candida albicans by Th1
immune response, whereas liquiritin, its glycoside form, does not.
Int Immunopharmacol. 9:632–638. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shang H, Cao S, Wang J, et al: Glabridin
from Chinese herb licorice inhibits fatigue in mice. Afr J Tradit
Complement Altern Med. 7:17–23. 2009.PubMed/NCBI
|
|
17
|
Choi EM: The licorice root derived
isoflavan glabridin increases the function of osteoblastic MC3T3-E1
cells. Biochem Pharmacol. 70:363–368. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma S, Kulkarni SK, Agrewala JN and
Chopra K: Curcumin attenuates thermal hyperalgesia in a diabetic
mouse model of neuropathic pain. Eur J Pharmacol. 536:256–261.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang HJ, Kim SW, Lim JM, et al:
Hypoglycemic effect of crude exopolysaccharides produced by a
medicinal mushroom Phellinus baumii in
streptozotocin-induced diabetic rats. Life Sci. 76:3069–3080. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashi K, Kojima R and Ito M: Strain
differences in the diabetogenic activity of streptozotocin in mice.
Biol Pharm Bull. 29:1110–1119. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sarkhail P, Rahmanipour S, Fadyevatan S,
et al: Antidiabetic effect of Phlomis anisodonta: effects on
hepatic cells lipid peroxidation and antioxidant enzymes in
experimental diabetes. Pharmacol Res. 56:261–266. 2007.
|
|
22
|
Li F, Li Q, Gao D and Peng Y: The optimal
extraction parameters and anti-diabetic activity of flavonoids from
Ipomoea batatas leaf. Afr J Tradit Complement Altern Med.
6:195–202. 2009.PubMed/NCBI
|
|
23
|
Li F, Zhang Y and Zhong Z:
Antihyperglycemic effect of ganoderma lucidum polysaccharides on
streptozotocin-induced diabetic mice. Int J Mol Sci. 12:6135–6145.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Raghavan B and Kumari SK: Effect of
Terminalia arjuna stem bark on antioxidant status in liver
and kidney of alloxan diabetic rats. Indian J Physiol Pharmacol.
50:133–142. 2006.
|
|
25
|
Ha H, Hwang IA, Park JH and Lee HB: Role
of reactive oxygen species in the pathogenesis of diabetic
nephropathy. Diabetes Res Clin Pract. 82(Suppl 1): S42–S45. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cemek M, Kağa S, Simşek N, et al:
Antihyperglycemic and antioxidative potential of Matricaria
chamomilla L. in streptozotocin-induced diabetic rats. J Nat
Med. 62:284–293. 2008.
|