Introduction
Adipose tissue is characterized by the infiltration
of immune cells, such as macrophages and T cells, during obesity
(1). Macrophages release
cytokines, such as IL-1β, IL-6 and TNF-α, leading to a
proinflammatory environment. Furthermore, the immune cells in
adipose tissues, including activated macrophages, T cells and
dendritic cells (DCs), produce a pleiotropic cytokine, osteopontin
(OPN), which is upregulated during inflammation (2). The obesity-driven inflammation and
macro- phage accumulation is blocked by OPN deficiency (3). OPN expression is significantly
upregulated in the adipose tissue of high fat diet-induced and
genetically obese mice, while it remains largely unaltered in the
liver (4,5). OPN acts as a chemokine and an
inflammatory cytokine through a variety of different receptors,
including CD44 and integrins. Thus, it is important in various
inflammatory disorders, such as rheumatoid arthritis (6), experimental autoimmune
encephalomyelitis (EAE), multiple sclerosis (MS) (7), allergic disease (8) and cardiovascular disease (9). It is also involved in
non-inflammatory pathophysiological processes, including bone
remodeling, neoplastic transformation, progression of metastases,
promotion of cell survival and wound healing (10,11).
Therefore, OPN has been investigated as a therapeutic target for
certain diseases (12,13).
Serum OPN levels are positively correlated with body
fat percentage and may be reduced by fat mass loss. Thus,
diet-induced weight loss has been demonstrated to significantly
decrease plasma OPN concentrations (5). This reduction of OPN was hypothesized
to be secondary to the loss of adipose tissue. However, bariatric
surgery, the most effective treatment to achieve weight loss in
morbidly obese humans, has been identified to gradually increase
plasma OPN levels, although it significantly reduced the body
weight, body mass index (BMI), waist circumference, homeostasis
model of assessment-insulin resistance and blood concentrations of
C-reactive protein (14,15). An elevated OPN concentration
following bariatric surgery and weight loss is hypothesized to
reflect the increased bone turnover, which is secondary to the
reduced weight load on bone. In addition, the plasma OPN
concentration remained unchanged in murine models of obesity,
regardless of an elevated expression of OPN in adipose tissue.
Furthermore, OPN levels are only moderately altered in morbidly
obese patients (4). Thus, whether
serum OPN levels are associated with body fat percentage has not
been elucidated in mice and humans. OPN is also produced by
multiple tissues, including epithelia, kidney, thyroid, breast,
uterus, placenta and testes (16).
Furthermore, OPN is highly expressed in bone matrix (17) and is important in bone turnover,
anchoring osteoclasts to bone and activating the resorption cascade
(18). This may indicate that
serum OPN levels are not only regulated by fat mass but also by
other tissues. In the present study, to evaluate how significantly
fat mass contributes to serum OPN concentrations, we investigated
whether serum OPN levels are decreased by exercise-induced fat mass
loss and are associated with body fat percentage in obese humans
(excluding those that were morbidly obese).
Subjects and methods
Study subjects
Study subjects (23 female college students aged
19–23 years) were recruited from Inha University (Incheon, Korea).
All 23 subjects submitted written informed consent to participate
in an 8-week body weight control program. The study protocol was
reviewed and approved by the Institutional Review Board at Kyung
Hee University Hospital (Seoul, Korea). To investigate whether
serum OPN levels are predominantly dependant on fat mass, only
obese subjects (n=23) (>30% body fat percentage), based on body
fat percentage and not on BMI, were recruited. Out of the 23
subjects recruited, 18 completed the 8-week body weight control
program. Morbidly obese females were excluded, as excessive fat
mass loss may overwhelm the effect of other tissues or factors on
serum OPN concentration. Moreover, the low frequency of morbidly
obese individuals suggests that subjects would be difficult to find
and recruit.
The subjects were free-living and were allowed a
self-selected diet. No medication or other nutritional supplements
were taken. The study was conducted from May to July, 2010. The
analysis of the results was conducted for the 18 students who
completed the 8-week program (drop out rate, ~21.7%). The subjects
did not present with any chronic diseases, and did not take any
medication.
Body weight control program
The 8-week body weight control program consisted of
diet therapy, exercise and behavioral change. The subjects were
recommended by a dietitian at an introductory class to consume an
individualized low-calorie diet. The subjects were required to
perform treadmill exercise three times a week during the 8-week
program, reaching 70% of the anaerobic threshold (AT), in order to
consume 200 kcal during exercise. To implement behavioral changes,
subjects were provided with an online lecture and were asked to
submit a weekly self-monitored diet and exercise diary to the
researcher. In addition, subjects were counseled at weekly
face-to-face meetings and via e-mail.
Body composition assessment
Anthropometric measurements were obtained from each
subject. Individual height was measured with an anthropometer and
body composition (body weight, soft lean mass, body fat mass, body
fat percentage, waist-hip ratio and BMI) was assessed at least once
per week using bioelectrical impedance (InBody 3.0, Biospace Co.,
Ltd., Seoul, Korea) (Table I).
 | Table IChanges in the anthropometric
parameters of the subjects before and after the 8-week body weight
control program. |
Table I
Changes in the anthropometric
parameters of the subjects before and after the 8-week body weight
control program.
| Variables | Before | After | Difference |
|---|
| Age (years) | 20.7±0.41 | | |
| Height (cm) | 161.1±1.3 | | |
| Body weight (kg) | 62.5±1.7 | 60.5±1.7 | −2.1±0.3a |
| Body mass index
(kg/m2) | 24.1±0.7 | 23.3±0.7 | −0.8±0.1a |
| Soft lean mass
(kg) | 38.2±1.0 | 39.3±1.0 | 1.1±0.2a |
| Body fat mass
(kg) | 22.0±0.8 | 18.8±0.9 | −3.2±0.3a |
| Percentage body fat
(%) | 35.1±0.7 | 30.9±0.9 | −4.0±0.4a |
Serum lipid profiles
Following overnight fasting, blood was collected at
a specific time in the morning prior to and following the program.
The collected blood was centrifuged at 1650 × g for 15 min. The
supernatant serum was separated in microtubes and stored at −70ºC
until it was analyzed for the serum lipid concentrations. Serum
total cholesterol (TC), high-density lipoprotein (HDL) cholesterol
and triglyceride (TG) levels were determined using an automatic
clinical analyzer (BPC BioSed Srl, Rome, Italy). Serum low-density
lipoprotein (LDL) cholesterol levels were calculated from the serum
TC, HDL and TG levels (19)
(Table II).
 | Table IIChanges in the serum lipid profiles of
the subjects before and after the 8-week body weight control
program. |
Table II
Changes in the serum lipid profiles of
the subjects before and after the 8-week body weight control
program.
| Serum lipid level
(mg/dl) | Before | After | Difference |
|---|
| Total
cholesterol | 188.0±6.3 | 178.0±4.5 | −9.7±3.5a |
| HDL cholesterol | 43.7±3.1 | 41.5±2.7 | −1.3±1.2 |
| LDL
cholesterol | 125.9±5.6 | 120.7±4.9 | −4.61±2.9 |
| Triglyceride | 97.4±12.0 | 68.9±8.6 | −28.2±4.7b |
Serum osteopontin, adiponectin and leptin
levels
The collected sera were analyzed for osteopontin,
adiponectin and leptin with an enzyme-linked immunosorbent assay
(ELISA) kit according to the manufacturer's instructions (R&D
Systems, Inc., Minneapolis, MN, USA).
Statistical analysis
Experimental data are expressed as the mean ±
standard error of the mean (SEM). The levels of fat and muscle mass
and serum adiponectin, leptin and osteopontin prior to and
following the 8-week body weight control program were compared
using a Wilcoxon signed-rank test (two-tailed). To determine the
degree of linearity between two variables, the data were compared
using the Spearman's correlation test (two-tailed). Prism software,
version 5.02 (Graphpad Software Inc., La Jolla, CA, USA) was used
for statistical analysis and graphing. P<0.05 was considered to
indicate a statistically significant difference.
Results
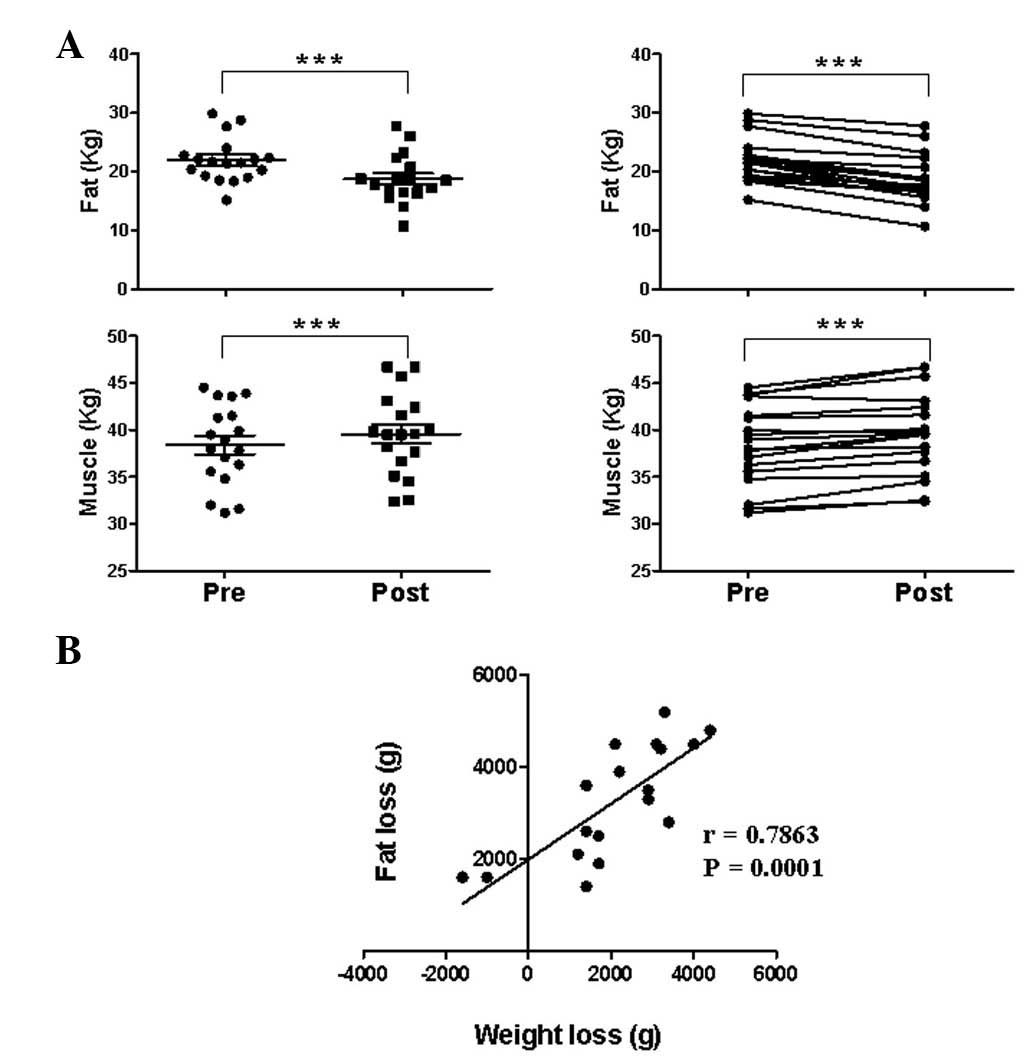
Effect of the 8-week exercise program on
fat and muscle mass
Following the program, the average BMI decreased
significantly from 24.1±0.7 to 23.3±0.7
kg/m2, as did the average body fat
percentage, from 35.1±0.7 to 30.9±0.9%, respectively (Table I). Consistent with the BMI and body
fat percentage reductions, the fat and muscle masses were
significantly decreased and increased, respectively (Fig. 1A). Weight loss was positively
correlated with fat loss, indicating that exercise-induced weight
loss is mainly due to fat mass loss (Fig. 1B).
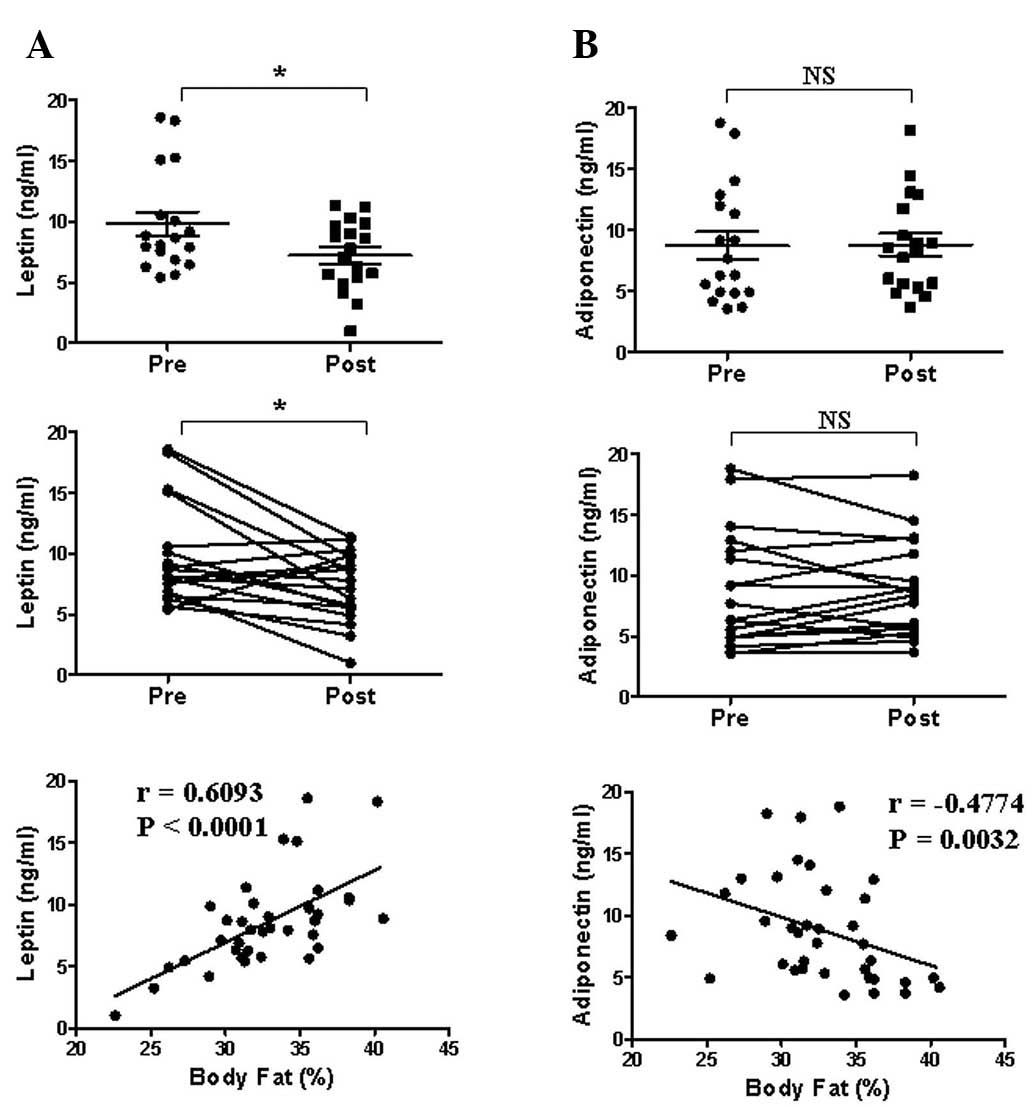
Effect of the 8-week exercise program on
serum leptin and adiponectin levels
To assess whether fat mass loss reduced the serum
levels of adipokine, which is predominantly produced in adipose
tissue, the leptin and adiponectin levels were measured prior to
and following the program (Fig.
2). Serum leptin levels (mean ± SEM) were significantly
decreased following the program (from 9.82±0.98 to 7.23±0.67
ng/ml), and were significantly correlated with body fat percentage
(r=0.6093, P<0.0001). Serum adiponectin levels were unchanged
and were significantly negatively correlated with body fat
percentage (r=−0.4774, P=0.0032). The results demonstrated that
serum leptin levels were more strongly correlated with body fat
percentage than with serum adiponectin levels, which is also
controlled by other physiological factors (Fig. 2).
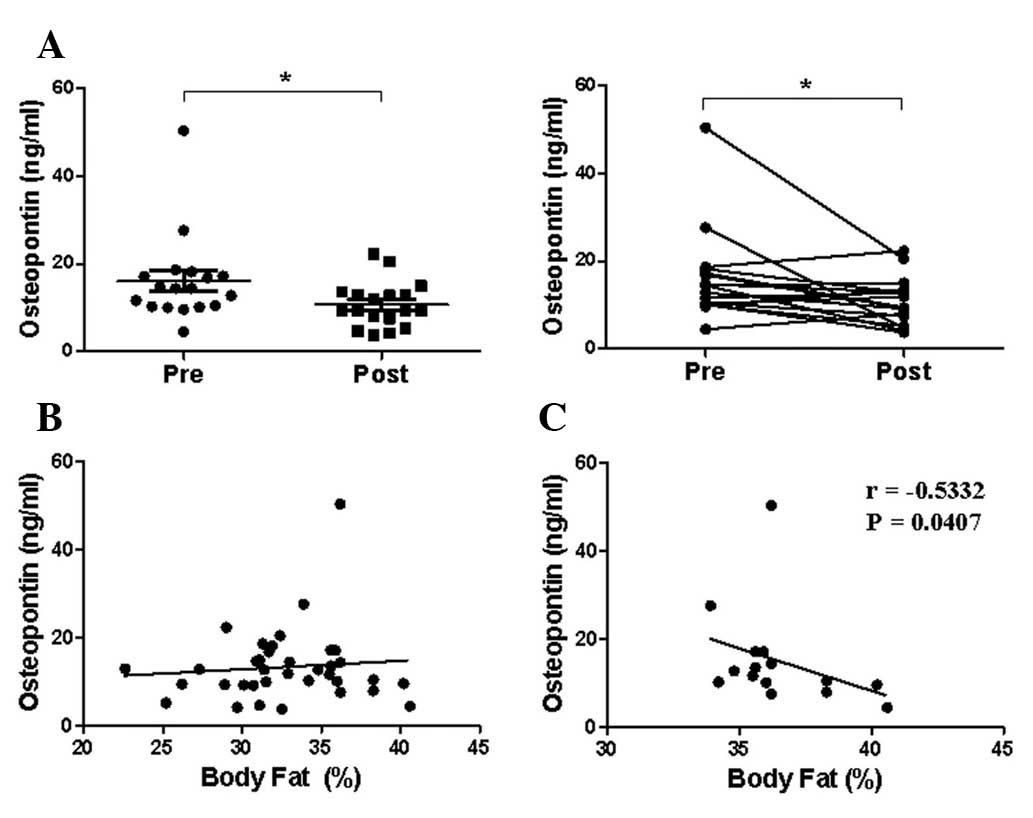
Effect of the 8-week exercise program on
serum osteopontin (OPN) levels
To determine whether serum OPN levels were
significantly correlated with body fat percentage, the levels were
measured prior to and following the 8-week body weight control
program (Fig. 3A). Serum OPN
levels (mean ± SEM) were significantly decreased following the
program (from 16.03±2.34 to 10.65±1.22 ng/ml). However, serum OPN
levels were not correlated with body fat percentage in the 18
subjects who completed the 8-week program, suggesting that serum
OPN levels are controlled by other factors in humans (Fig. 3B). In addition, serum OPN levels of
subjects with a body fat percentage of >33% were negatively
correlated with body fat percentage (r=−0.5332, P<0.05)
(Fig. 3C).
Discussion
We hypothesized that serum OPN levels are decreased
by exercise-induced fat mass loss and are associated with body fat
percentage. As additional and indirect tests of the hypothesis, the
association of body fat percentage with adipokines, serum leptin
and adiponectin, which are mainly produced in adipose tissues, were
also determined. Thus, it was demonstrated that serum leptin levels
were positively correlated with body fat percentage, while serum
adiponectin levels were negatively correlated with body fat
percentage. By contrast, serum OPN levels were not correlated with
body fat percentage, regardless of the fact that serum OPN levels
decreased following 8 weeks of exercise-induced weight loss.
Furthermore, serum OPN levels were negatively associated with body
fat percentage in subjects with a body fat percentage of >33%
(Fig. 3C). This indicated that
serum OPN levels were not correlated with body fat percentage and
may be affected by other tissues and physiological conditions.
Obesity is regarded as a state of systemic, chronic,
low-grade inflammation. It is considered to be a risk factor
associated with the genesis or development of various diseases,
including coronary heart disease, hypertension, type 2 diabetes
mellitus, cancer, respiratory complications and osteoarthritis
(20–22). Exercise-induced weight loss is
regarded as the safest method to prevent obesity-related diseases.
Thus, there has been interest in clarifying how physical activity
and exercise modulate obesity-mediated inflammation (23). The anti-inflammatory effects of
regular exercise may be mediated via a reduction in the visceral
fat mass (with a subsequent decreased release of adipokines) and
the induction of an anti-inflammatory environment with each
exercise session (24).
Exercise-induced weight loss resulted in a decrease
in serum leptin levels and no change in serum adiponectin levels in
this study. Similarly, even mild weight loss induced by calorie
restriction has been suggested to have beneficial effects on serum
leptin levels in humans; however, it has no clear impact on serum
adiponectin levels (25). This
observation suggested that leptin is mainly produced in adipose
tissues; the serum leptin concentration was increased in obese
patients and was correlated with body fat percentage in the present
study, as previously demonstrated (26). However, serum adiponectin levels
were not altered following weight loss in the present study, but
were negatively correlated with body fat percentage, although
adiponectin is also highly expressed in adipose tissues (27). Unlike leptin, serum adiponectin
levels appear to be regulated by unknown physiological factors.
Adiponectin levels are inversely proportional to obesity, diabetes
and other insulin-resistant states (28). Therefore, in obesity, reducing
chronic adipose tissue inflammation and macrophage infiltration may
be beneficial for reversing the downregulation of adiponectin gene
expression by pro-inflammatory cytokines (29). Serum OPN levels also appear to be
associated with fat mass. OPN is extensively upregulated in the
adipose tissue of obese humans, as well as in that of diet-induced
and genetically (db/db) obese mice. However, there is controversy
concerning the correlation between serum OPN concentrations and fat
mass in humans and mice. Serum OPN concentrations remained
unchanged in murine models of obesity, although OPN was highly
upregulated in the adipose tissue of high-fat diet-induced and
genetically obese mice (4). By
contrast, in another study, obese and overweight patients exhibited
significantly increased circulating OPN concentrations as compared
with lean subjects (obese, 72.6±28.5 ng/ml; overweight, 68.2±20.8
ng/ml; lean, 42.7±27.9 ng/ml; P<0.001) (5). The results of the present study
showed that serum OPN levels may also be affected by other
physiological factors as opposed to body fat percentage alone. For
example, serum OPN levels appear to be affected by age. Riedl et
al demonstrated that there is a weak, but significant, negative
correlation between OPN levels and age (30). A possible correlation between OPN
and age-related changes in bone mineral density (BMD) was
hypothesized. Thus, only college students (mean age, 20.7±0.4
years) were recruited in the present study to exclude the age
factor. No significant difference was identified in serum OPN
levels between males and females (30). In another study, whole body
vibration was demonstrated to decrease serum OPN levels, an effect
that appears to be associated with the change in bone metabolism
(31). Thus, in our study, the
reduction of serum OPN levels may have resulted from body
vibrations which may be triggered by excercise, as opposed to
exercise-induced fat loss. This observation is due to the fact that
OPN is a component of bone matrix and is important in bone
turnover, serving as an anchor for osteoclasts and thus activating
the resorption cascade (18).
Exercise may also be correlated with the change in bone metabolism
(32), as physical activity has
the potential to reduce the risk of osteoporotic fractures. Extreme
inactivity may cause rapid bone loss of ≤40%, while athletic
activity results in bone hypertrophy of ≤40% (32). The mechanisms for the beneficial
effect of exercise on bone mass appear to be due to a cell response
to hormonal and mechanical load stimuli.
Furthermore, dietary components are known to affect
the gene expression and plasma concentration of adiponectin in
humans and animals (33). Animal
models have demonstrated that the consumption of hyperlipidemic
diets, rich in saturated fat, reduces the levels of adiponectin,
while diets rich in polyunsaturated fatty acids supplemented with
Ω3 and eicosapentaenoic acid increase its gene expression and
plasma levels. In humans, the consumption of a healthy and
Mediterranean diet is positively correlated with adiponectin
levels, although the mechanisms have not been elucidated (33). Exercise and dietary components may
also have affected serum OPN levels in the present study, as a
specific/calorie-restricted diet was not implemented. It has been
demonstrated that OPN expression in cardiomyocytes was
significantly correlated with the impaired function of the left
ventricle, which was the main source of circulating OPN plasma
levels (34). Furthermore,
adipokines is involved either directly or indirectly in the
regulation of bone remodeling (35); the change in serum leptin level
induced by exercise may induce the change of bone remodeling and
the change of bone metabolism may affect the serum OPN levels. In
addition, calorie restriction-induced weight loss appears to be a
risk factor for rapid bone loss. However, physical activity-induced
weight loss preserves BMD (36).
In conclusion, serum OPN levels may be regulated by various
physiological factors. Thus, the elevated expression of OPN in
adipose tissues may not be correlated with serum OPN levels.
Instead, other tissues or physiological factors may have a greater
contribution to serum OPN levels as compared to fat mass. Thus, the
correlation between serum OPN levels and body fat loss remains to
be elucidated.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) and funded by the Ministry of Education, Science and
Technology (Korea) (grant nos. 2012-0002659 and 2011-0026939).
References
|
1
|
Harford KA, Reynolds CM, McGillicuddy FC
and Roche HM: Fats, inflammation and insulin resistance: insights
to the role of macrophage and T-cell accumulation in adipose
tissue. Proc Nutr Soc. 70:408–417. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Morimoto J, Kon S, Matsui Y and Uede T:
Osteopontin; as a target molecule for the treatment of inflammatory
diseases. Curr Drug Targets. 11:494–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nomiyama T, Perez-Tilve D, Ogawa D, et al:
Osteopontin mediates obesity-induced adipose tissue macrophage
infiltration and insulin resistance in mice. J Clin Invest.
117:2877–2888. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kiefer FW, Zeyda M, Todoric J, et al:
Osteopontin expression in human and murine obesity: extensive local
up-regulation in adipose tissue but minimal systemic alterations.
Endocrinology. 149:1350–1357. 2008. View Article : Google Scholar
|
|
5
|
Gómez-Ambrosi J, Catalán V, Ramírez B, et
al: Plasma osteopontin levels and expression in adipose tissue are
increased in obesity. J Clin Endocrinol Metab. 92:3719–3727.
2007.PubMed/NCBI
|
|
6
|
Xu G, Sun W, He D, et al: Overexpression
of osteopontin in rheumatoid synovial mononuclear cells is
associated with joint inflammation, not with genetic polymorphism.
J Rheumatol. 32:410–416. 2005.PubMed/NCBI
|
|
7
|
Braitch M and Constantinescu CS: The role
of osteopontin in experimental autoimmune encephalomyelitis (EAE)
and multiple sclerosis (MS). Inflamm Allergy Drug Targets.
9:249–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frenzel DF and Weiss JM: Osteopontin and
allergic disease: pathophysiology and implications for diagnostics
and therapy. Expert Rev Clin Immunol. 7:93–109. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Waller AH, Sanchez-Ross M, Kaluski E and
Klapholz M: Osteopontin in cardiovascular disease: a potential
therapeutic target. Cardiol Rev. 18:125–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Naldini A, Leali D, Pucci A, et al:
Cutting edge: IL-1beta mediates the proangiogenic activity of
osteopontin-activated human monocytes. J Immunol. 177:4267–4270.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rangaswami H, Bulbule A and Kundu GC:
Osteopontin: role in cell signaling and cancer progression. Trends
Cell Biol. 16:79–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kiefer FW, Neschen S, Pfau B, et al:
Osteopontin deficiency protects against obesity-induced hepatic
steatosis and attenuates glucose production in mice. Diabetologia.
54:2132–2142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zeyda M, Gollinger K, Todoric J, et al:
Osteopontin is an activator of human adipose tissue macrophages and
directly affects adipocyte function. Endocrinology. 152:2219–2227.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Komorowski J, Jankiewicz-Wika J, Kolomecki
K, et al: Systemic blood osteopontin, endostatin, and E-selectin
concentrations after vertical banding surgery in severely obese
adults. Cytokine. 55:56–61. 2011. View Article : Google Scholar
|
|
15
|
Schaller G, Aso Y, Schernthaner GH, et al:
Increase of osteopontin plasma concentrations after bariatric
surgery independent from inflammation and insulin resistance. Obes
Surg. 19:351–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shevde LA, Das S, Clark DW and Samant RS:
Osteopontin: an effector and an effect of tumor metastasis. Curr
Mol Med. 10:71–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giachelli CM and Steitz S: Osteopontin: a
versatile regulator of inflammation and biomineralization. Matrix
Biol. 19:615–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reinholt FP, Hultenby K, Oldberg A and
Heinegård D: Osteopontin - a possible anchor of osteoclasts to
bone. Proc Natl Acad Sci USA. 87:4473–4475. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friedewald WT, Levy RI and Fredrickson DS:
Estimation of the concentration of low-density lipoprotein
cholesterol in plasma, without use of the preparative
ultracentrifuge. Clin Chem. 18:499–502. 1972.PubMed/NCBI
|
|
20
|
Sowers MR and Karvonen-Gutierrez CA: The
evolving role of obesity in knee osteoarthritis. Curr Opin
Rheumatol. 22:533–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shehzad A, Ha T, Subhan F and Lee YS: New
mechanisms and the anti-inflammatory role of curcumin in obesity
and obesity-related metabolic diseases. Eur J Nutr. 50:151–161.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trayhurn P and Wood IS: Adipokines:
inflammation and the pleiotropic role of white adipose tissue. Br J
Nutr. 92:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wärnberg J, Cunningham K, Romeo J and
Marcos A: Physical activity, exercise and low-grade systemic
inflammation. Proc Nutr Soc. 69:400–406. 2010.PubMed/NCBI
|
|
24
|
Gleeson M, Bishop NC, Stensel DJ, et al:
The anti-inflammatory effects of exercise: mechanisms and
implications for the prevention and treatment of disease. Nat Rev
Immunol. 11:607–615. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Klempel MC and Varady KA: Reliability of
leptin, but not adiponectin, as a biomarker for diet-induced weight
loss in humans. Nutr Rev. 69:145–154. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ
and Burrell MA: The adipocyte: a model for integration of endocrine
and metabolic signaling in energy metabolism regulation. Am J
Physiol Endocrinol Metab. 280:E827–E847. 2001.PubMed/NCBI
|
|
27
|
Arita Y, Kihara S, Ouchi N, et al:
Paradoxical decrease of an adipose-specific protein, adiponectin,
in obesity 1999. Biochem Biophys Res Commun. 31:560–564.
2012.PubMed/NCBI
|
|
28
|
Shehzad A, Iqbal W, Shehzad O and Lee YS:
Adiponectin: regulation of its production and its role in human
diseases. Hormones (Athens). 11:8–20. 2012.PubMed/NCBI
|
|
29
|
Guerre-Millo M: Adiponectin: an update.
Diabetes Metab. 34:12–18. 2008. View Article : Google Scholar
|
|
30
|
Riedl M, Vila G, Maier C, et al: Plasma
osteopontin increases after bariatric surgery and correlates with
markers of bone turnover but not with insulin resistance. J Clin
Endocrinol Metab. 93:2307–2312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Humphries B, Fenning A, Dugan E, et al:
Whole-body vibration effects on bone mineral density in women with
or without resistance training. Aviat Space Environ Med.
80:1025–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith EL and Gilligan C: Physical activity
effects on bone metabolism. Calcif Tissue Int. 49(Suppl): S50–S54.
1991. View Article : Google Scholar
|
|
33
|
Reis CE, Bressan J and Alfenas RC: Effect
of the diet components on adiponectin levels. Nutr Hosp.
25:881–888. 2010.PubMed/NCBI
|
|
34
|
Tamura A, Shingai M, Aso N, et al:
Osteopontin is released from the heart into the coronary
circulation in patients with a previous anterior wall myocardial
infarction. Circ J. 67:742–744. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oh KW, Lee WY, Rhee EJ, et al: The
relationship between serum resistin, leptin, adiponectin, ghrelin
levels and bone mineral density in middle-aged men. Clin Endocrinol
(Oxf). 63:131–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Villareal DT, Fontana L, Weiss EP, et al:
Bone mineral density response to caloric restriction-induced weight
loss or exercise-induced weight loss: a randomized controlled
trial. Arch Intern Med. 166:2502–2510. 2006. View Article : Google Scholar : PubMed/NCBI
|