Introduction
Osteoarthritis (OA) is a chronic joint disorder
characterized by the chronic progressive degeneration of the
articular cartilage, subchondral bone alteration and variable
secondary synovial inflammation (1). In response to macrophage-derived
proinflammatory cytokines, such as interleukin (IL)-1β, OA synovial
fibroblasts produce chemokines that promote inflammation,
neovascularization and cartilage degradation via the activation of
matrix-degrading enzymes, such as matrix metalloproteinases (MMPs)
(2–3). Although the pathogenesis of the
disease remains unknown, an increasing number of studies indicate
that mononuclear cell migration is important in the perpetuation of
inflammation in the synovium (4–5). In
addition, the adhesion and infiltration of these cells to
inflammatory sites are regulated by chemokines (6–7).
However, the molecular mechanisms of human OA remain to be
elucidated.
Connective tissue growth factor (CTGF; also known as
CCN2) is a member of a family of secreted multifunctional proteins
that contain high levels of cysteine (8). Previous studies have demonstrated
that CTGF promotes the inflammatory response (9). CTGF mRNA has been observed to be
upregulated adjacent to areas of cartilage surface damage and to be
present in chondro-osteophytes (10). In an animal model, CTGF
overexpression in mouse knee joints induced cartilage damage, which
may have been a direct effect of CTGF overexpression or a result of
the factors excreted by the CTGF-induced fibrotic synovial tissue
(11). Therefore, CTGF may
contribute to the pathogenesis of OA. Nevertheless, the
proinflammatory activity of CTGF in fibroblast-like synoviocytes
(FLS) and its participation in synovitis during OA remain to be
observed. In the present study, the role of CTGF in OA synovial
inflammation was investigated.
Materials and methods
Patient samples
Human synovial membranes were obtained from patients
with knee OA (n=15 males, aged 65.7±1.8 years) undergoing total
knee arthroplasty. One knee per patient was obtained. All patients
fulfilled the American College of Rheumatology criteria for OA of
the knee (12). Normal knees (n=3
males, aged 75±2.5 years) were obtained within 10 h of death. The
tissues were examined macroscopically and microscopically to ensure
that only normal tissue was used. This study was approved by the
Ethics Committee of PLA General Hospital, Beijing, China and was
conducted in compliance with all ethical standards, and informed
consent was obtained from each pateint according to the Declaration
of Helsinki (2000).
Cell culture and treatment
Synovial specimens were finely minced and isolated
by enzymatic digestion with collagenase type 1A in Dulbecco’s
Modified Eagle’s Medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA)
at 37°C in a 5% CO2 atmosphere for 16 h. The digested
tissue was filtered through a 70 mm nylon mesh, washed and
centrifuged. Cell viability was >95% according to the Trypan
blue exclusion test. Collected cells were resuspended in DMEM
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Rockford, IL, USA) and cultured at 37°C in a 5% CO2
atmosphere until the third passage (95% fibroblasts, detected by
immunocytochemistry with anti-collagen I antibody; Millipore,
Billerica, MA, USA). FLS were allowed to grow to ~100% confluence
and were incubated with recombinant human CTGF (BioVendor R&D,
Asheville, NC, USA) at 15 and 25 ng/ml, with IL-1β (10 ng/ml;
Peprotech Inc., Rocky Hill, NJ, USA) or culture media. Viability
studies were performed for all the experimental conditions. None of
the treatments significantly affected cell viability, which was
>90%, as tested by the Trypan blue exclusion test.
Western blot analysis
Following stimulation for 5 min with IL-1β (10
ng/ml), CTGF (15 and 25 ng/ml) or a combination of IL-1β and CTGF,
FLSs were lysed in 100 μl Cell Lysis Buffer (Cell Signaling
Technology, Inc., Beverly, MA, USA) and centrifuged at 4°C for 20
min at 12,000 × g. Proteins (25 μg) in cell lysates were separated
by 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
and transferred onto polyvinylidene difluoride membranes. Membranes
were blocked with 3% bovine serum albumin and incubated with
specific antibodies against phosphorylated extracellular
signal-related kinase (ERK; dilution 1:1000), phosphorylated or
total Akt and ERK (dilution 1:500), and phosphorylated or total
c-Jun N-terminal kinase (JNK; dilution 1:250) and p38MAPK (dilution
1:250; Cell Signaling Technology, Inc.) overnight at 4°C. Finally,
membranes were incubated with peroxidase-conjugated goat
anti-rabbit IgG (Dako, Carpinteria, CA, USA) and the immunoreactive
bands were visualized by enhanced chemiluminescence using the
AutoChemi image analyzer (GE Healthcare, Pittsburgh, PA, USA).
Determination of MMP activity
Cells were stimulated with IL-1β (10 ng/ml), CTGF
(15 and 25 ng/ml) or a combination of IL-1β and CTGF for 24 h.
Supernatants were harvested, centrifuged and incubated with
p-aminophenylmercuric acetate for 6 h at 37°C to activate MMPs.
Aliquots of the supernatants were transferred to a 96-well plate
and, following the addition of the 5-carboxyfluorescein (5-FAM)
peptide substrate (AnaSpec Inc. Fremont, CA, USA), the fluorescence
was measured at various time points at 490 nm (excitation)/520 nm
(emission) in a VICTOR™ X3 Multilabel Plate Reader (PerkinElmer,
Shelton, CT, USA).
Enzyme-linked immunosorbent assay
(ELISA)
FLSs were stimulated with IL-1β (10 ng/ml) for 24 h,
in the presence or absence of CTGF at 15 and 25 ng/ml. Supernatants
were harvested, centrifuged and frozen at −80°C until analyzed.
IL-6, IL-8 and chemokine C-C motif ligand 2 (CCL2) protein levels
were determined by specific IL-6, IL-8 and CCL2 ELISA kits from
eBioscience (San Diego, CA, USA) with sensitivities of 2, 4 and 7
pg/ml, respectively. CCL20, MMP-1, MMP-3 and MMP-13 protein
expression levels were determined with specific ELISA kits (all
from R&D Systems, Minneapolis, MN, USA).
qPCR
Following incubation for 24 h, total RNA was
extracted with TRIzol reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to the manufacturer’s instructions.
Reverse transcription was accomplished on 1 μg total RNA using
random primers (TaqMan reverse transcription reagents; Invitrogen
Life Technologies). PCR assays were performed in duplicate on an
iCycler Real-Time PCR Detection system using SYBR-Green PCR Master
mix (Bio-Rad, Hercules, CA, USA). The sequences of the primers used
in this study were reported in previous studies (13–14).
For each sample, differences in the threshold cycle (Ct) values
were calculated by normalizing the Ct of the target gene to that of
the reference gene glyceraldehyde 3-phosphate dehydrogenase.
Activation of nuclear factor (NF)-κB
Cells were seeded into 6-well plates and grown to
60% confluence. Transient transfection was performed for 45 min
with 2 μg reporter construct, NF-κB-luc (Stratagene, La Jolla, CA,
USA) and 1 μg internal control, pRL-TK (Promega Corporation,
Madison, WI, USA) by the Magnetofection™ system (OZ Biosciences,
Marseille, France), according to the manufacturer’s instructions.
The medium was then replaced and the cells were treated for 24 h
with CTGF at 25 ng/ml, in the absence or presence of IL-1β (10
ng/ml). Following lysis and centrifugation, aliquots of the
supernatants were used to assay firefly and Renilla luciferase
activity using the Dual-Luciferase Reporter Assay System kit
(Promega Corporation). Luminescence was measured using a Bio-Tek
Synergy HT spectrophotometer (Bio-Tek, Winooski, VT, USA).
Statistical analysis
Results are presented as the mean ± standard error
of the mean. Statistical analyses were performed using one-way
analysis of variance followed by a Dunnett’s t-test for multiple
comparisons or a two-tailed unpaired Student’s t-test for dual
comparisons. P<0.05 was considered to indicate a statistically
significant result.
Results
Effect of CTGF on the production of
cytokines and chemokines induced by IL-1β
To determine whether extracellular CTGF modulated
cytokine and chemokine production in human FLSs, cells were
incubated with CTGF in the presence or absence of IL-1β (10 ng/ml).
Stimulation with IL-1β resulted in the enhanced production of
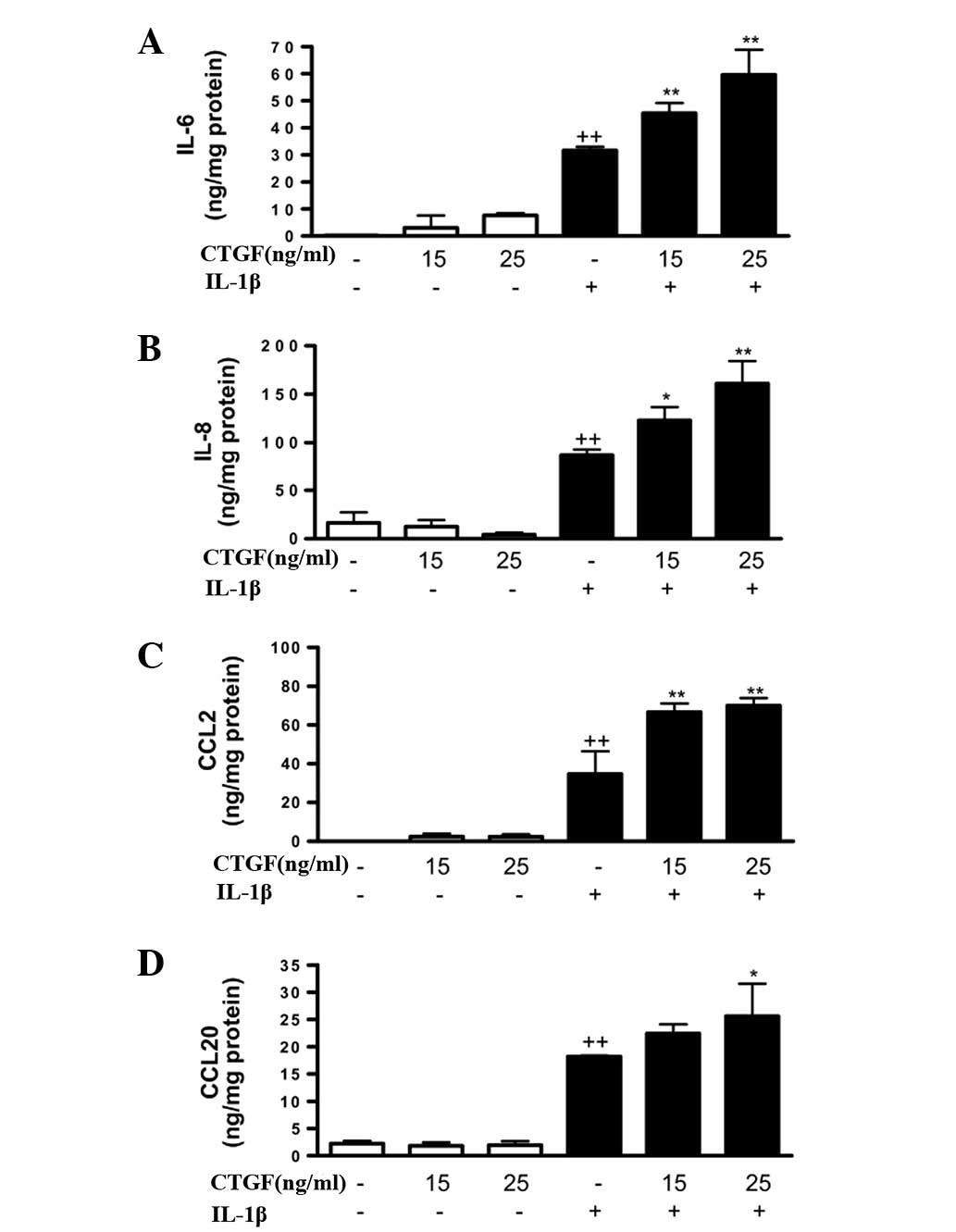
proinflammatory cytokines and chemokines. As shown in Fig. 1, although CTGF alone (at
concentrations of 15 or 25 ng/ml) did not affect the production of
IL-6, IL-8, CCL2 or CCL20, a significant increase in these
proinflammatory mediators was observed in the presence of IL-1β.
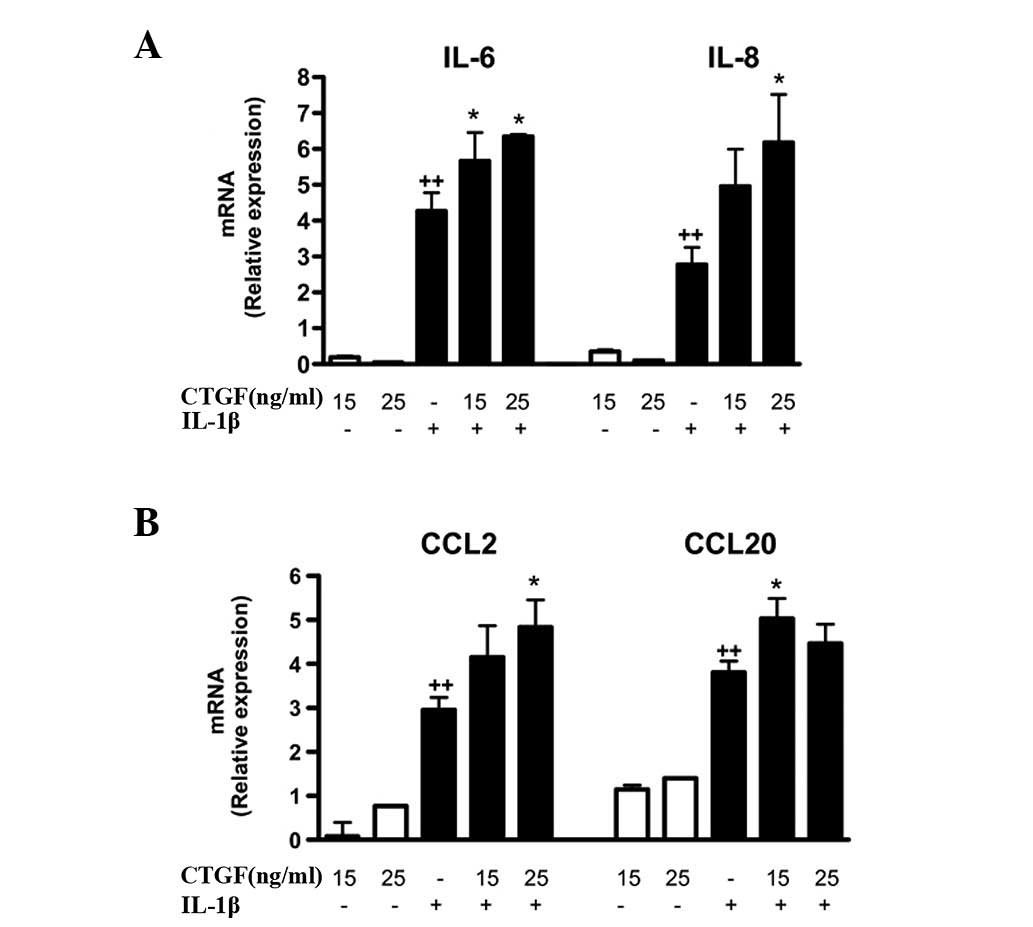
These effects were confirmed at the mRNA level, with an enhancement
of IL-6, IL-8, CCL2 and CCL20 mRNA expression in cells stimulated
with IL-1β and CTGF (Fig. 2).
Effect of CTGF on MMPs induced by
IL-1β
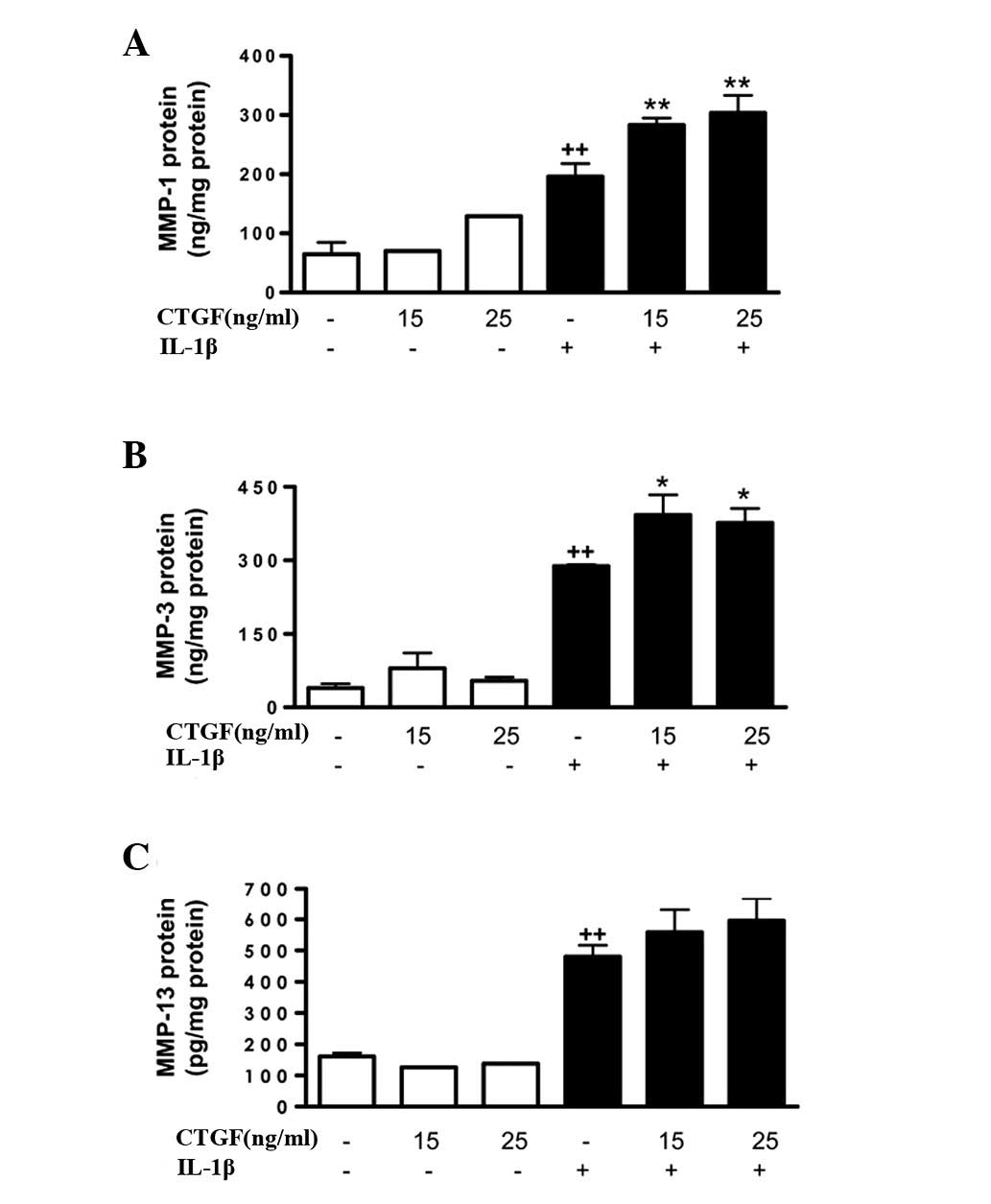
Cell activation by IL-1β (10 ng/ml) potently induced
MMP gene expression, as well as MMP protein expression and
activity. mRNA expression was measured by qPCR and in the cell
supernatants, protein levels were measured by ELISA and MMP
activity was measured by a fluorometric procedure. As shown in
Fig. 3, CTGF alone did not induce
significant changes in the MMP-1, MMP-3 or MMP-13 protein levels in
the cell supernatants; however, it potentiated the effect of IL-1β
(stimulating the expression of MMP-1 and MMP-3 proteins). In
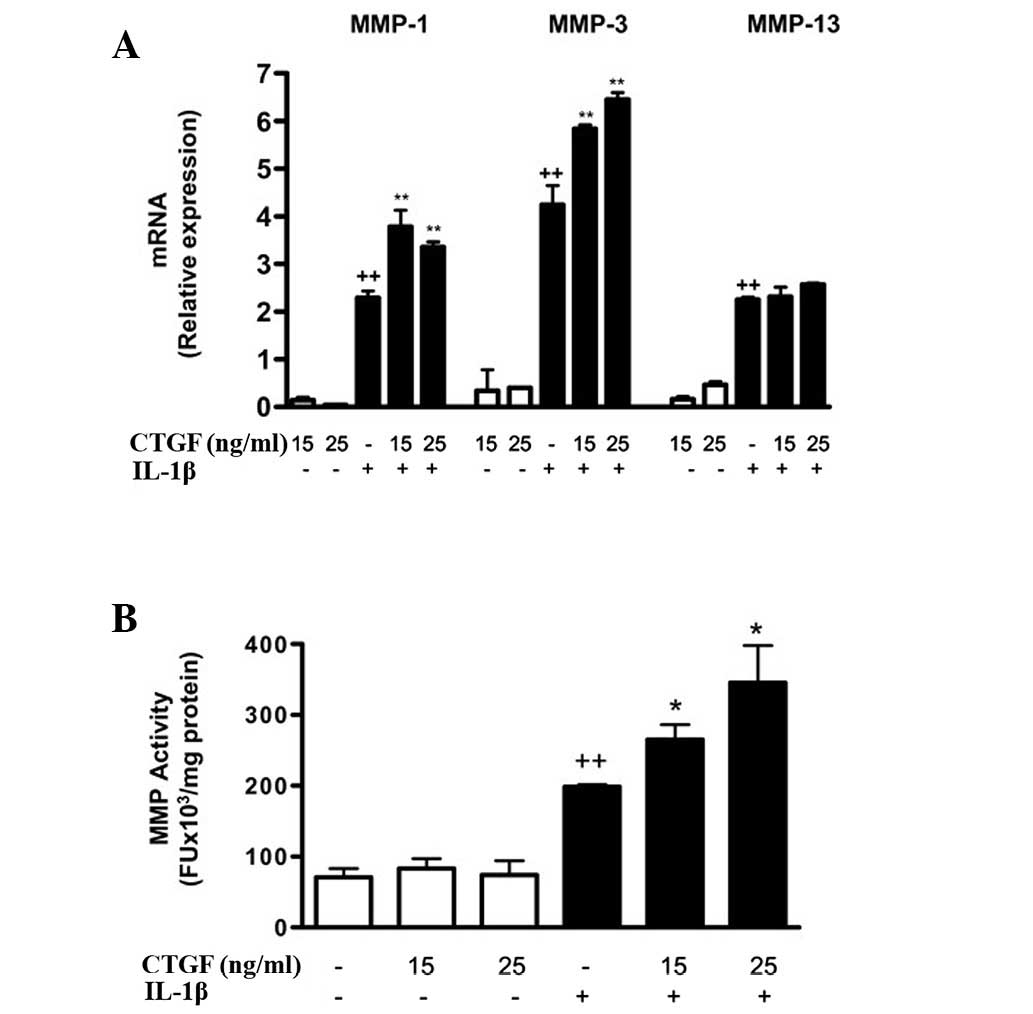
addition, CTGF significantly increased MMP-1 and MMP-3 mRNA
expression in the presence of IL-1β (Fig. 4a). The levels of MMP activity in
the medium were also significantly increased by CTGF following
IL-1β stimulation.
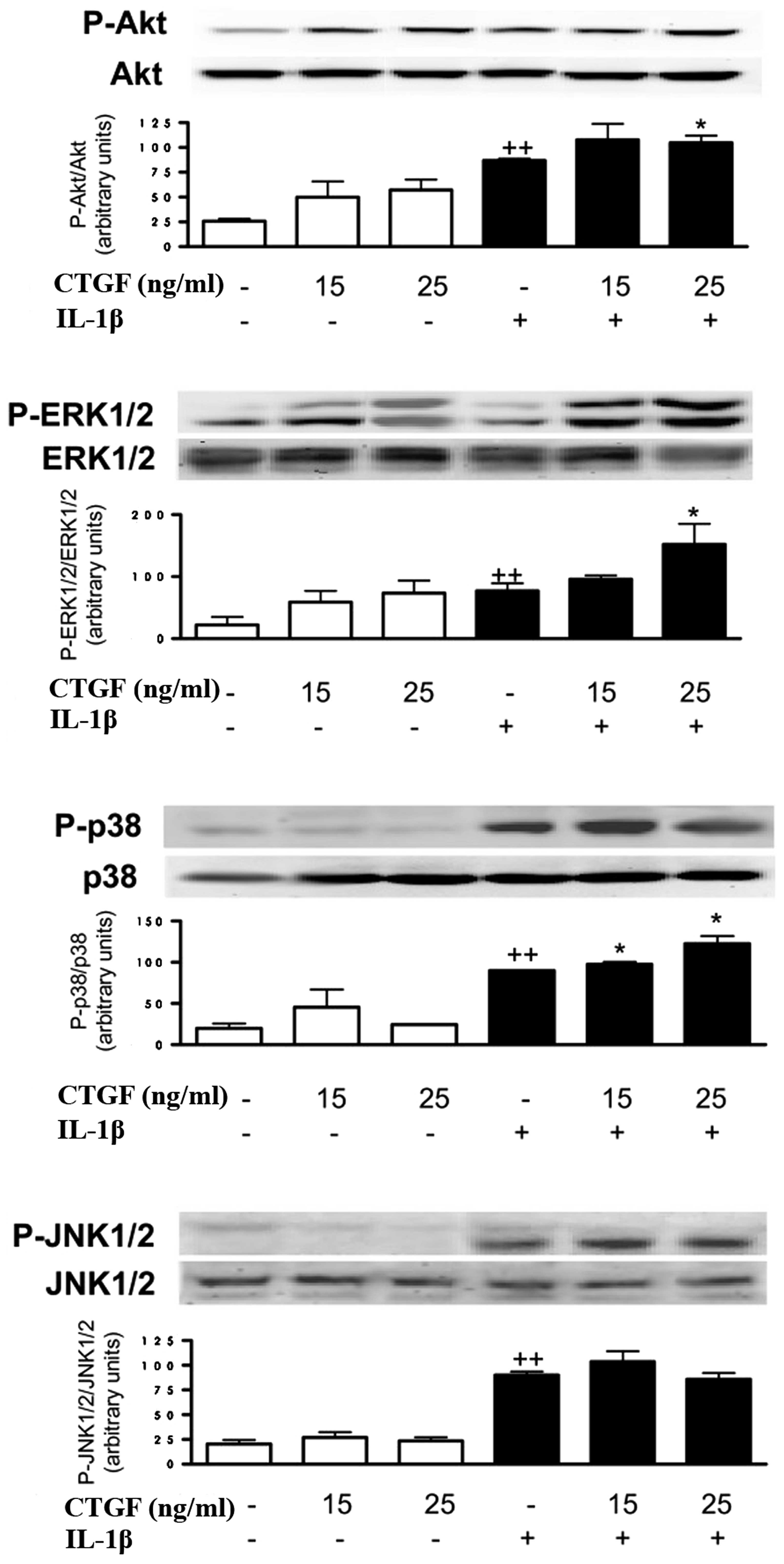
Effect of CTGF on Akt and p38MAPK
phosphorylation induced by IL-1β
To determine the possible mechanism of action of
CTGF, we determined whether this protein acted on Akt and p38MAPK
activation. As shown in Fig. 5,
CTGF, at the concentrations studied (15 and 25 ng/ml), increased
the phosphorylated Akt and ERK1/2 levels. In the presence of IL-1β,
CTGF enhanced the phosphorylated ERK1/2 and p38MAPK levels, with a
lower effect on phosphorylated Akt. In contrast, JNK1/2
phosphorylation was not affected by CTGF in the presence or absence
of IL-1β stimulation.
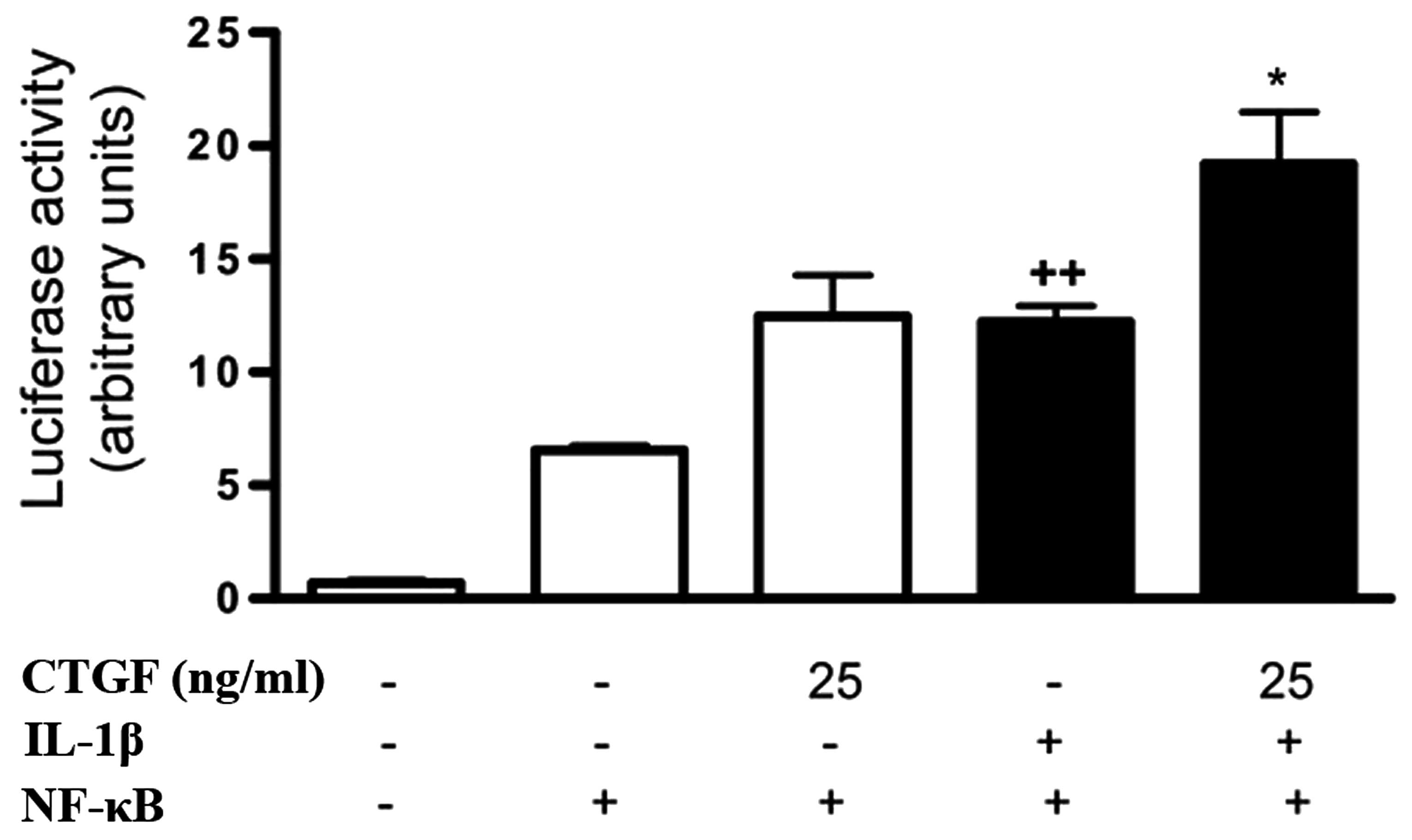
Effect of CTGF on NF-κB activation
induced by IL-1β
NF-κB is a regulator of proinflammatory and
degradative genes in the joint. The possible effect of CTGF on
NF-κB activation induced by IL-1β in FLSs was investigated. CTGF
treatment in nonstimulated cells resulted in the increased
transcriptional activity of NF-κB, although this was not
significantly different compared with that of control. Of note,
CTGF at 25 ng/ml significantly potentiated NF-κB activation in the
presence of IL-1β (Fig. 6).
Discussion
OA is a heterogeneous group of conditions associated
with the defective integrity of the articular cartilage and
associated changes in the underlying bone. The chronic inflammatory
process is mediated by a complex cytokine network. Synovial
inflammation has been demonstrated in tissue samples of OA patients
and may be associated with disease progression (15). Accumulating data support the
critical role of FLSs in OA cartilage degradation through the
production of inflammatory and catabolic mediators (16–18).
Proinflammatory cytokines, such as IL-1β, are involved in driving
synovitis during OA, and affecting the production of cytokines and
MMPs (19). CTGF is involved in
the regulation of the inflammatory response and increases IL-6
expression in human synovial fibroblasts through the
integrin-dependent signaling pathway (20). In addition, CTGF induces monocyte
chemoattractant protein-1 expression to enhance monocyte migration
in the human synovial fibroblasts (21). In the present study, the results
indicated that CTGF cooperated with IL-1β to amplify the
inflammatory response, which led to the production of a number of
cytokines, chemokines and MMPs in OA FLSs. The data demonstrated
that CTGF and IL-1β synergistically enhanced IL-6 production, which
is in accordance with a previous study regarding the stimulation of
IL-6 release by CTGF (20).
Chemokines, such as IL-8, CCL2 and CCL20, are able
to attract inflammatory cells and regulate gene transcription and
cell proliferation (22). The
upregulation of chemokines in FLSs upon stimulation with IL-1β
promotes inflammation and cartilage degradation through the
activation of MMPs and other degradative enzymes (2). CCL2 and CCL20 are chemokines
implicated in arthritis synovitis (23–24)
and are produced by the OA synovium in the presence of
proinflammatory cytokines. In particular, IL-1β has been
demonstrated to be a more potent inducer of CCL20 than TNFα or
IL-17 (25). The results of the
present study revealed that CTGF acted on OA FLSs in vitro
to enhance the production of IL-8, CCL2 and CCL20. IL-8 is produced
by fibroblasts and macrophages (26), and CCL2 has been demonstrated in
the induction of synovial macrophage and fibroblast chemotaxis
(27). Therefore, the enhanced
production of these mediators indicates that CTGF may be involved
in the amplification of the inflammatory response induced by IL-1β
in OA FLSs.
MMPs are important in articular tissue degradation
in OA. The present study demonstrated the potentiating effect of
CTGF on the IL-1β induction of MMP-1 and MMP-3 in human OA FLSs. As
MMP-1 degrades collagen in the extracellular matrix and MMP-3
activity leads to the activation of collagenases (28–29),
the results suggested that catabolic responses are amplified by
CTGF during joint inflammation.
Studies have indicated that the spontaneous or
stimulated production of numerous inflammatory and degradative
mediators by OA FLSs are correlated with NF-κB activation (30–31).
In particular, the transcription of IL-6, IL-8, CCL2, CCL20 and
MMPs is NF-κB dependent. This study demonstrated that IL-1β and
CTGF synergistically increased the transcriptional activity of
NF-κB, resulting in the enhanced production of inflammatory
mediators.
p38α-MAPK activity regulated the activation of
transcription factors relevant in inflammatory responses and OA
(32). CTGF enhanced p38
phosphorylation, which participated in IL-6 and IL-8 transcription
in human FLSs (33–34). As the production of MMP-1 and MMP-3
upon stimulation of synoviocytes with IL-1β was dependent on ERK
activation (35), the effects of
CTGF on ERK phosphorylation were investigated. The results
indicated that CTGF potentiated the effects of IL-1β on ERK
phosphorylation, which may be important in the upregulation of
MMP-1 and MMP-3 by CTGF. Notably, CTGF potentiated Akt
phosphorylation by IL-1β, a pathway involved in cell survival and
the proliferation of fibroblasts in rheumatoid arthritis synovium
(36). In addition, Akt activation
may be involved in human cartilage breakdown, as it has been
implicated in MMP-13 and aggrecanase-1 expression induced by
oncostatin M (37), as well as the
synergistic induction of MMP-1 and MMP-13 expression following
oncostatin M + IL-1β stimulation of human chondrocytes (38). Therefore, data from the present
study suggest that the potentiation of ERK, p38 and Akt activation
by CTGF may be a mechanism relevant to the increase in the
intensity and persistence of synovitis, as well as the expression
of catabolic factors, in OA.
In conclusion, the results of the present study
support the view that CTGF acts as a proinflammatory cytokine,
which enhances the OA synovial inflammatory process. CTGF was
observed to synergize with IL-1β to induce the phosphorylation of
ERK1/2, p38 and Akt, and the activation of NF-κB. These effects
resulted in the production of proinflammatory and catabolic
mediators that contribute to synovitis and articular destruction
during OA.
References
|
1
|
Li N, Rivéra-Bermúdez MA, Zhang M, et al:
LXR modulation blocks prostaglandin E2 production and matrix
degradation in cartilage and alleviates pain in a rat
osteoarthritis model. Proc Natl Acad Sci USA. 107:3734–3739. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mor A, Abramson SB and Pillinger MH: The
fibroblast-like synovial cell in rheumatoid arthritis: a key player
in inflammation and joint destruction. Clin Immunol. 115:118–128.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen PC, Wu CL, Jou IM, et al: T helper
cells promote disease progression of osteoarthritis by inducing
macrophage inflammatory protein-1γ. Osteoarthritis Cartilage.
19:728–736. 2011.PubMed/NCBI
|
|
4
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. New Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sakkas LI and Platsoucas CD: The role of T
cells in the pathogenesis of osteoarthritis. Arthritis Rheum.
56:409–424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sucosky P, Balachandran K, Elhammali A, Jo
H and Yoganathan AP: Altered shear stress stimulates upregulation
of endothelial VCAM-1 and ICAM-1 in a BMP-4- and
TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol.
29:254–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qureshi MH, Cook-Mills J, Doherty DE and
Garvy BA: TNF-alpha-dependent ICAM-1- and VCAM-1-mediated
inflammatory responses are delayed in neonatal mice infected with
Pneumocystis carinii. J Immunol. 171:4700–4707. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perbal B: CCN proteins: multifunctional
signalling regulators. Lancet. 363:62–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kular L, Pakradouni J, Kitabgi P, Laurent
M and Martinerie C: The CCN family: a new class of inflammation
modulators? Biochimie. 93:377–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Omoto S, Nishida K, Yamaai Y, et al:
Expression and localization of connective tissue growth factor
(CTGF/Hcs24/CCN2) in osteoarthritic cartilage. Osteoarthritis
Cartilage. 12:771–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blaney Davidson EN, Vitters EL, Mooren FM,
et al: Connective tissue growth factor/CCN2 overexpression in mouse
synovial lining results in transient fibrosis and cartilage damage.
Arthritis Rheum. 54:1653–1661. 2006.PubMed/NCBI
|
|
12
|
Altman R, Asch E, Bloch D, et al:
Development of criteria for the classification and reporting of
osteoarthritis. Classification of osteoarthritis of the knee
Diagnostic and Therapeutic Criteria Committee of the American
Rheumatism Association. Arthritis Rheum. 29:1039–1049. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Y, Chang H, Zou J, Jin X and Qi Z:
The effect of atorvastatin on mRNA levels of inflammatory genes
expression in human peripheral blood lymphocytes by DNA microarray.
Biomed Pharmacother. 65:118–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meier FM, Frommer KW, Peters MA, et al:
Visfatin/pre-B-cell colony-enhancing factor (PBEF), a
proinflammatory and cell motility-changing factor in rheumatoid
arthritis. J Biol Chem. 287:28378–28385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Haywood L, McWilliams DF, Pearson CI, et
al: Inflammation and angiogenesis in osteoarthritis. Arthritis
Rheum. 48:2173–2177. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nair A, Kanda V, Bush-Joseph C, et al:
Synovial fluid from patients with early osteoarthritis modulates
fibroblast-like synoviocyte responses to toll-like receptor 4 and
toll-like receptor 2 ligands via soluble CD14. Arthritis Rheum.
64:2268–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kloesch B, Liszt M, Krehan D, Broell J,
Kiener H and Steiner G: High concentrations of hydrogen sulphide
elevate the expression of a series of pro-inflammatory genes in
fibroblast-like synoviocytes derived from rheumatoid and
osteoarthritis patients. Immunol Lett. 141:197–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu Z, Liu P, Yang D, et al:
Interleukin-18-induced inflammatory responses in synoviocytes and
chondrocytes from osteoarthritic patients. Int J Mol Med.
30:805–810. 2012.PubMed/NCBI
|
|
19
|
Bondeson J, Wainwright SD, Lauder S, Amos
N and Hughes CE: The role of synovial macrophages and
macrophage-produced cytokines in driving aggrecanases, matrix
metalloproteinases, and other destructive and inflammatory
responses in osteoarthritis. Arthritis Res Ther. 8:R1872006.
View Article : Google Scholar
|
|
20
|
Liu SC, Hsu CJ, Chen HT, Tsou HK, Chuang
SM and Tang CH: CTGF increases IL-6 expression in human synovial
fibroblasts through integrin-dependent signaling pathway. PLoS One.
7:e510972012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu SC, Hsu CJ, Fong YC, Chuang SM and
Tang CH: CTGF induces monocyte chemoattractant protein-1 expression
to enhance monocyte migration in human synovial fibroblasts.
Biochim Biophys Acta. 1833:114–1124. 2013.PubMed/NCBI
|
|
22
|
Hayashida K, Nanki T, Girschick H, Yavuz
S, Ochi T and Lipsky PE: Synovial stromal cells from rheumatoid
arthritis patients attract monocytes by producing MCP-1 and IL-8.
Arthritis Res. 3:118–126. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hatano Y, Kasama T, Iwabuchi H, et al:
Macrophage inflammatory protein 1 alpha expression by synovial
fluid neutrophils in rheumatoid arthritis. Ann Rheum Dis.
58:297–302. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schutyser E, Struyf S and Van Damme J: The
CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor
Rev. 14:409–426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chabaud M, Page G and Miossec P: Enhancing
effect of IL-1, IL-17, and TNF-alpha on macrophage inflammatory
protein-3alpha production in rheumatoid arthritis: regulation by
soluble receptors and Th2 cytokines. J Immunol. 167:6015–6020.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Wu L and Qu JM: Inhibited
proliferation of human lung fibroblasts by LPS is through IL-6 and
IL-8 release. Cytokine. 54:289–295. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
García-Vicuña R, Gómez-Gaviro MV,
Domínguez-Luis MJ, et al: CC and CXC chemokine receptors mediate
migration, proliferation, and matrix metalloproteinase production
by fibroblast-like synoviocytes from rheumatoid arthritis patients.
Arthritis Rheum. 50:3866–3877. 2004.
|
|
28
|
Poole AR, Nelson F, Dahlberg L, et al:
Proteolysis of the collagen fibril in osteoarthritis. Biochem Soc
Symp. 70:115–123. 2003.PubMed/NCBI
|
|
29
|
Wu W, Billinghurst RC, Pidoux I, et al:
Sites of collagenase cleavage and denaturation of type II collagen
in aging and osteoarthritic articular cartilage and their
relationship to the distribution of matrix metalloproteinase 1 and
matrix metalloproteinase 13. Arthritis Rheum. 46:2087–2094. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amos N, Lauder S, Evans A, Feldmann M and
Bondeson J: Adenoviral gene transfer into osteoarthritis synovial
cells using the endogenous inhibitor IkappaBalpha reveals that
most, but not all, inflammatory and destructive mediators are
NFkappaB dependent. Rheumatology (Oxford). 45:1201–1209. 2006.
View Article : Google Scholar
|
|
31
|
Bondeson J, Lauder S, Wainwright S, et al:
Adenoviral gene transfer of the endogenous inhibitor IkappaBalpha
into human osteoarthritis synovial fibroblasts demonstrates that
several matrix metalloproteinases and aggrecanases are nuclear
factor-kappaB-dependent. J Rheumatol. 34:523–533. 2007.
|
|
32
|
Rasheed Z, Akhtar N and Haqqi TM:
Pomegranate extract inhibits the interleukin-1β-induced activation
of MKK-3, p38α-MAPK and transcription factor RUNX-2 in human
osteoarthritis chondrocytes. Arthritis Res Ther. 12:R1952010.
|
|
33
|
Miyazawa K, Mori A, Miyata H, Akahane M,
Ajisawa Y and Okudaira H: Regulation of interleukin-1beta-induced
interleukin-6 gene expression in human fibroblast-like synoviocytes
by p38 mitogen-activated protein kinase. J Biol Chem.
273:24832–24838. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Suzuki M, Tetsuka T, Yoshida S, et al: The
role of p38 mitogen-activated protein kinase in IL-6 and IL-8
production from the TNF-alpha- or IL-1beta-stimulated rheumatoid
synovial fibroblasts. FEBS Lett. 465:23–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pillinger MH, Rosenthal PB, Tolani SN, et
al: Cyclooxygenase-2-derived E prostaglandins down-regulate matrix
metalloproteinase-1 expression in fibroblast-like synoviocytes via
inhibition of extracellular signal-regulated kinase activation. J
Immunol. 171:6080–6089. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang HG, Wang Y, Xie JF, et al:
Regulation of tumor necrosis factor alpha-mediated apoptosis of
rheumatoid arthritis synovial fibroblasts by the protein kinase
Akt. Arthritis Rheum. 44:1555–1567. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
El Mabrouk M, Sylvester J and Zafarullah
M: Signaling pathways implicated in oncostatin M-induced
aggrecanase-1 and matrix metalloproteinase-13 expression in human
articular chondrocytes. Biochim Biophys Acta. 1773:309–320.
2007.PubMed/NCBI
|
|
38
|
Litherland GJ, Dixon C, Lakey RL, et al:
Synergistic collagenase expression and cartilage collagenolysis are
phosphatidylinositol 3-kinase/Akt signaling-dependent. J Biol Chem.
283:14221–14229. 2008. View Article : Google Scholar : PubMed/NCBI
|