Introduction
Lumbar spinal stenosis (LSS) is a medical condition
in which the spinal canal narrows, compressing the spinal cord and
nerves at the level of the lumbar vertebra. This is due to the
common occurrence of lumbar spondylolisthesis, slipped disk,
ligamentous thickening and spinal degeneration that develops with
aging. With the development of imaging methods and the increased
aging of society, the incidence of LSS has shown an annual
increase. LSS is one of the main causes of leg pain in older
individuals (1–4), including intermittent claudication,
numbness of buttocks and legs, activity disorder of waist and legs,
muscle wasting and tendon dysreflexia (5). Intermittent claudication caused by
neuropathic pain (NP) is a special chief complaint of LSS, which
may be reconstructed by clinical workers; however, its mechanisms
remain unclear. It is now commonly accepted that NP is associated
with coccygeal nerve regional pathology, regional blood flow,
neuroelectrical physiological changes, and varied compression on
the nerves or spinal cord (6–10).
Dorsal root ganglia (DRG) are the primary neurons of afferent pain
perception that synthesize diverse control factors, and are a key
relay station linking the peripheral and central nervous systems.
DRG are involved in transmitting algesia and have important
significance for the study of NP (11–15).
It has been established that a number of bioactive
compounds participate in NP, such as brain-derived neurotrophic
factor (BDNF), glial cell line-derived neurotrophic factor (GDNF),
P substance, cyclooxygenase 2 (COX2), prostaglandin E2 (PGE2) and
IE-6 (16,17). Moreover, previous studies (18,19)
demonstrated that neurotrophic factors are important for neuronal
growth, maturation and the restoration of damage, including
neuronal survival, axon growth, synaptic plasticity, transmission
of the neuronal signal and restoration of nerve injury.
Simultaneously, neurotrophic factors have been studied as
neurotransmitters that are involved in the process of pain
creation, control and restoration of nerve injury (20–25).
Thereby, we suggested that an increased BDNF expression in DRG of
rat models of LSS attenuates NP and results in intermittent
claudication.
The aim of the present study was to investigate the
expression of BDNF in DRG of rat models of LSS, and to evaluate the
correlation between BDNF expression and flat plate walking
distance. In addition, this study aimed to demonstrate that BDNF is
important for the processes of intermittent claudication caused by
LSS and NP.
Materials and methods
Animals and grouping
Healthy adult male Sprague-Dawley rats (n=136;
weight, 230–280 g) were purchased from the Animal Test Center of
Xuzhou Medical University [Lianyungang, China; animal production
license no. SCXK (su) 2012011-0003 and animal usage license no.
SYXK (su) 2010-0011]. This study was performed in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The animal
use protocol was reviewed and approved by the Institutional Animal
Care and Use Committee of the Affiliated Lianyungang Hospital of
Xuzhou Medical College (Lianyungang, China). All the animals had
access to food and water ad libitum, lived in natural light
with a temperature of 24±1°C and all testing personnel were
qualified to experiment on animals. Eight rats were selected at
random prior to surgery and used as the conjunctive control, the
remaining rats were randomly divided into the LSS (n=64) and sham
operation (n=64) groups. The animals undertook flat plate sports
every day for 3 days prior to surgery. All the rats in the control
group were sacrificed prior to surgery and enzyme-linked
immunosorbent assay (ELISA) and immunohistochemistry were performed
on four of these rats to measure BDNF content and expression. If
animals died during the test process then additional animals were
added to maintain the original test number.
LSS model building
Silica gel slices were made from 18FR two cavity
pure silicone catheter for single use (C. R. Bard, Inc., Covington,
GA, USA). A suitable section of silica gel slices were selected and
cut into thin slices (length, 4 mm; depth, 1.25 mm and thickness, 1
mm). The outer tip showed a wedge shape and the border was rubbed
blunt using abrasive paper (Huifeng abrasive paper Ltd., Guangzhou,
China). The silica gel slices were then soaked in 75% alcohol for
more than 30 min.
For the operation group, rats were anesthetized with
10% chloral hydrate via intraperitoneal injection (0.5 ml/100 g,
additional anesthetic was provided as required). At the two sides
of the iliac crest, horizontally level to the L5 vertebra, we
performed shaving and skin preparation and 75% alcohol was used for
skin degerming. A median incision was made behind the Processus
spinosus, the skin and subcutaneous fascia were cut in turn,
and the paravertebral muscles were located according to joint
motion between L6-S1 and separated. In addition, the supra- and
interspinal ligaments of L4-L6 were cut, the S1 Processus
spinosus was lifted using forceps and epidural tissues under
the lamina of vertebra were separated to expose Dura mater
spinalis. Furthermore, the silica gel slice was longitudinally
embedded under the L5 lamina of vertebra. At this time, we observed
shortened feet in the lower extremities and a tail-flick, which
confirmed the accurate location and compression of the spinal cord.
Therefore, we removed L5 Processus spinosus, lifted L4
Processus spinosus and the silica gel slice was
longitudinally embedded using the previous method. The wound was
rinsed with physiological saline and 40 units of gentamicin sulfate
were intravenously administerd. The fascia and skin were sutured in
turn.
For the sham operation group, we performed the same
process as that of the operation group; but, the silica gel slice
was not embedded.
Walking distance measurement
The walking distance was measured using a flat plate
sport instrument (SportsArt Fitness Industry Corporation, Taiwan,
China). All rats undertook flat plate sports 3 days prior to and
following surgery until they were sacrificed. The walking speed
began at 0.3 m/sec and accelerated to 0.5, 0.8 and 0.9 m/sec every
5 min. Measurements were recorded twice daily at the fixation time
and the mean value was calculated. The walking distance was not
recorded when rats had continuously fallen off the flat plate three
times.
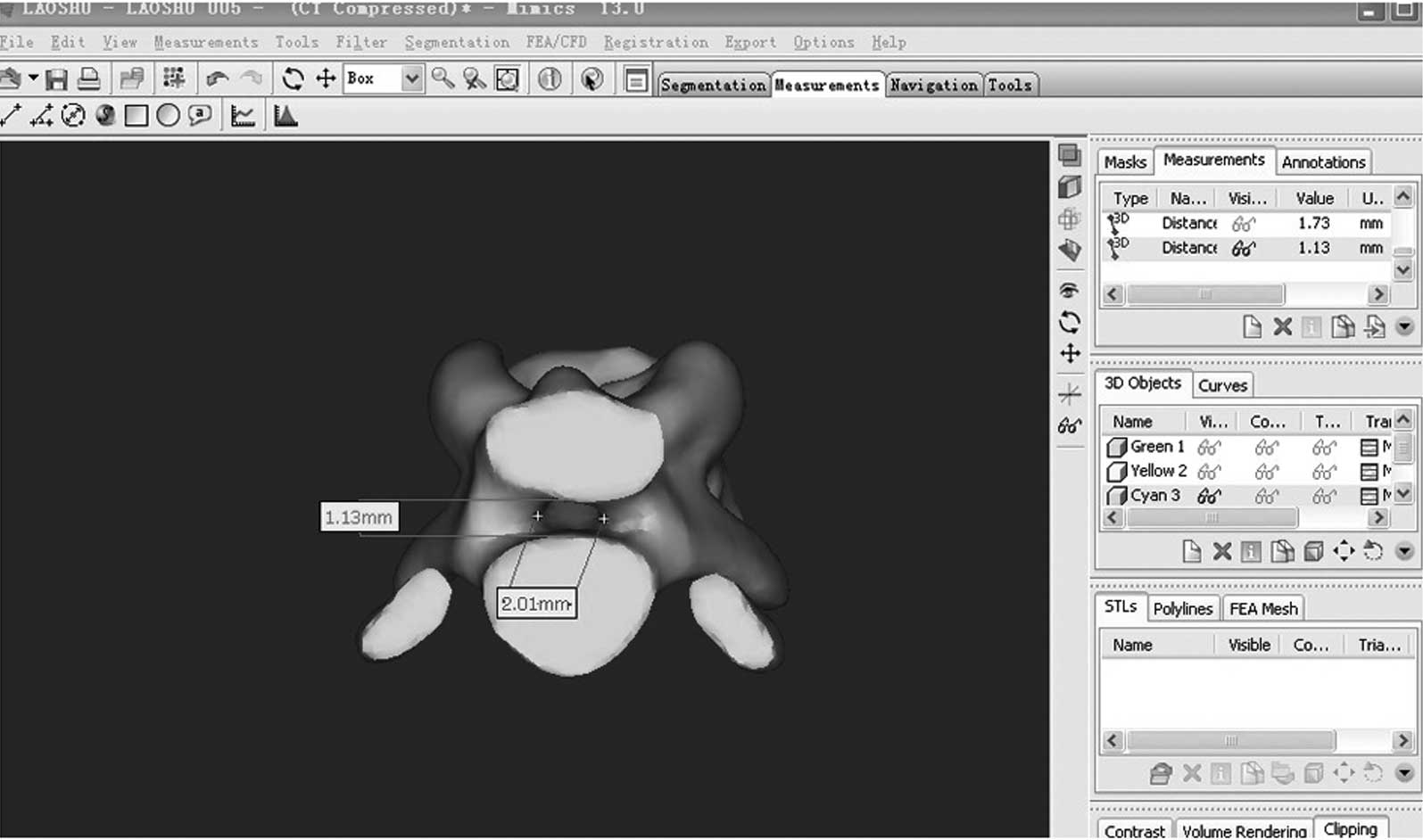
Computerized tomography (CT) scan of
rats
Four rats in the operation group were randomly
selected for the CT scan (32 row spirals, GE Company, Fairfield,
CT, USA; scan condition was 120.00 KV, 200 MA, 512×512 pixel; slice
thickness, 0.625 mm and Dicom format). The scan result was imported
into Materialise Mimics 13.0 finite element modeling software
(Materialise Company, Leuven, Belgium) to construct finite element
modeling of the lumbar in rats. Image-Pro Plus 6.0 (Media
Cybernetics Company, PA, USA) was used to measure and calculate the
sagittal and transection diameters of the spinal canal in L4 and L5
phase.
Sample clearage
PMSF crystal was added to PMSF solvents, and
following dissolution and antigrading, 10 ml 100 mM PMSF was
prepared. PMSF (1 ml 100 mM) was added to 100 ml NP-40 and the
NP-40 lysate of 1 mM PMSF was obtained. The sample was defrosted at
room temperature, and then cut into fragments and placed in a
centrifuge tube. Lysate (200 μl) was added to each centrifuge tube
and following 1 h on ice the tissue homogenate was centrifuged
(10,000 × g at 4°C for 15 min) and the supernatant was removed.
Determination of total protein
content
The reagents were purchased from Promega Corporation
(Fitchburg, WI, USA). Protein standard dispensing liquid (0.8 ml)
was added to 20 mg protein standard solution (bovine serum albumin)
following sufficient dissolution to give 25 mg/ml protein standard
solution. Moreover, 20 μl of 25 mg/ml protein standard solution was
added to 980 μl phosphate buffered-saline (PBS) and diluted to 0.5
mg/ml of the final content. BCA fluid was formed by mixing 0.8 ml
BCA reagent B and 40 ml reagent A at a ratio of 1:50. The protein
standard solution was added into the 96-well plates at 0, 1, 2, 4,
8, 12, 16 and 20 μl, 10 μl supernatant and PBS were added to
increase the concentration to 20 μl in each well. In addition, 200
μl BCA fluid was added into each well and the absorbance was
measured at 37°C for 30 min. The optical density (OD) value at 562
nm absorbance was assayed with ELISA, relative to OD 450, it was
equal to OD 450 (sample) - OD 450 (control). Protein concentration
of the samples was calculated using a standard curve, the practical
BDNF concentration was the measurement result multiplied by
two.
ELISA test
All reagents were purchased from Promega
Corporation. The rats were sacrificed by administrating an
overdosed level of anaesthetic, the L4-L5 bilateral nerve root was
quickly separated and DRG were identified (four DRG were removed
from each animal and considered as the same samples). Nerve fibers
(1 cm), including DRG were cut and removed. The blood was washed
with PBS and stored in the freezer at −80°C until the ELISA test
(Fig. 1).
BDNF protein standard liquid was prepared in the
first 24 h after the animals were sacrificed. Sample dilution (1
ml) was added to 10 ng BDNF standard preparation tube and 10 ng/ml
BDNF protein standard fluid was obtained. Moreover, 0.2 ml 10 ng/ml
was added to 0.8 ml sample dilution and 2000 pg/ml BDNF protein
standard fluid was obtained. Six centrifuge tubes were marked and
0.3 ml sample dilution was added to each centrifuge tube; 0.3 ml
2000 pg/ml fluid was added into the first tube; and 0.3 ml from the
first tube was added into the second tube, with this procedure
continuing in this manner. From the six tubes, 0.1 ml protein
standard fluid was added to a 96-well plate, 0.1 ml sample dilution
was added to the first well as the blank, and 50 μl sample
supernatant and 50 μl sample dilution were added to each empty
well. The lid was added and the well plate was incubated for 90 min
at 37°C. Furthermore, 100 μl BDNF antibody was added to 9.9 ml
antibody dilution (1:100) and 10 ml BDNF antibody fluid was
obtained. Following incubation, liquid in the well plate was
removed and the plate was dried with absorbent paper as the drying
process was slow. Antibody fluid (0.1 ml) was added to each well
and incubated for 60 min at 37°C. The plate was washed three times
with PBS (1 min per well) and liquid was removed and the plate was
dried with absorbent paper. ABC and TMB fluids (Promega
Corporation) were also incubated for 30 min at 37°C. Furthermore,
100 μl ABC was added to 9.9 ml ABC dilution and 10 ml ABC fluid was
obtained. Following the dilution, 0.1 ml ABC fluid was added into
each well and incubated for 30 min at 37°C. The plate was washed
five times with PBS (Promega Corporation; 2 min per well), liquid
was removed and the well plate was dried with absorbent paper. TMB
coloration liquid (90 μl) was added to the plate and incubated for
30 min at 37°C. Additionally, 100 μl TMB stopping buffer was added
into each well and the OD value of A450 was assessed using ELISA
for 30 min. Relative to OD 45O, it was equal to OD 450 (sample) -
OD 450 (control). Protein content of the sample was calculated
using a standard curve, practical BDNF concentration was the
measured result multiplied by two.
BDNF content equaled the BDNF concentration divided
by the BCA concentration (pg/mg total protein).
Immunohistochemistry
After the animals were sacrificed, 4%
paraformaldehyde was quickly administered into the rat heart. DRG
were removed using the previous method, soaked in 4%
paraformaldehyde liquid and fixed. Following dehydration, clearing,
routine paraffin embedding and 3-μm serial sections, we performed
immunohistochemical staining with streptavidin-peroxidase. DRG
slices were heated at 60°C overnight, dewaxed in xylol and hydrated
with gradient alcohol. To remove endogenous peroxidase, slices were
incubated at 37°C for 10 min using 3% H2O2.
Then slices were washed three times for 5 min in PBS (pH 7.4).
Slices were placed into sodium citrate liquid (pH 6.0) and heated
to boiling by microwave for 2 min. Slices were allowed to cool for
15 min, then washed three times for 5 min with PBS. In addition,
10% normal goat serum confining liquid was added for 20 min at room
temperature and any additional liquid was removed. Rabbit anti-rat
BDNF polyclonal antibody (50 μl) (1:200; Abnova Corporation, CA,
USA) was added overnight at 4°C and PBS replaced the antibody in
the blank. Furthermore, slices were washed three times for 5 min
with PBS, biotin-labeled goat anti-rabbit IgG (1:50) was added and
slices were incubated for 30 min at 37°C. Slices were then washed
three times for 5 min with PBS. Horseradish peroxidase labeling
strepto-albumin stock solution (1:300) was added, incubated for 30
min at 37°C and slices were washed three time for 5 min with PBS.
In addition, DRG slices were then stained with
3,3′-diaminobenzidine for 30 min and washed three times for 5 min
with PBS before being stained again with hematoxylin and eosin.
Slices were then dehydrated with alcohol gradient and cleared with
xylol. DRG slices were mounted with neutral gum (Promega
Corporation) and observed under a light microscope (magnification,
×200). Photographs recorded the staining of the DRG slices. We used
Image-Pro Plus software, version 6.0 to carry out the gradation
analysis for DRG slices and calculate the mean optical density
(MOD) of BDNF-positive action in DRG.
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed with SPSS software, version 16.0 (SPSS Inc., Chicago,
IL, USA). An independent sample t-test was used to compare BDNF
content difference and flat plate sports distance in each group,
between groups and before and after sports. Two- and one-way
analysis of variance and multiple comparison methods were used to
analyze BDNF expression changes between each group at all
time-points and before and after sports. Bivariate correlation was
used to analyze BDNF expression and sports distance. P<0.05 was
considered to indicate a statistically significant difference.
Results
Performance
The majority of rats in the operation and sham
operation groups were able to walk 1 day post-surgery. Eight rats
in the operation group and 1 rat in the sham operation group died
during the experiment; therefore, animals were added to maintain
the sample number. In the operation group, 7 rats were paralyzed in
the lower extremities and showed crossing. Unilateral hind legs of
five rats were paralysis and one rat was suspected to have an
infection. All the rats in the operation group demonstrated
drooping tails and were suspected to have uroclepsia or
constipation. In the sham operation group, no obvious differences
were observed in their walking abilities compared with those prior
to surgery and no animals indicated infection.
Internal diameter measurement of the
spinal canal
In the operation group, the sagittal diameter of the
spinal canal in L4 phase was 1.31±0.05 mm and the transection
diameter was 2.05±0.02 mm. In the L5 phase, the sagittal diameter
was 1.16±0.03 mm and transection diameter was 1.99±0.02 mm. Rats
were dissected and the lamina of vertebra was cut. The obvious
compression of the coccygeal nerve was exposed and compression was
~50–70%.
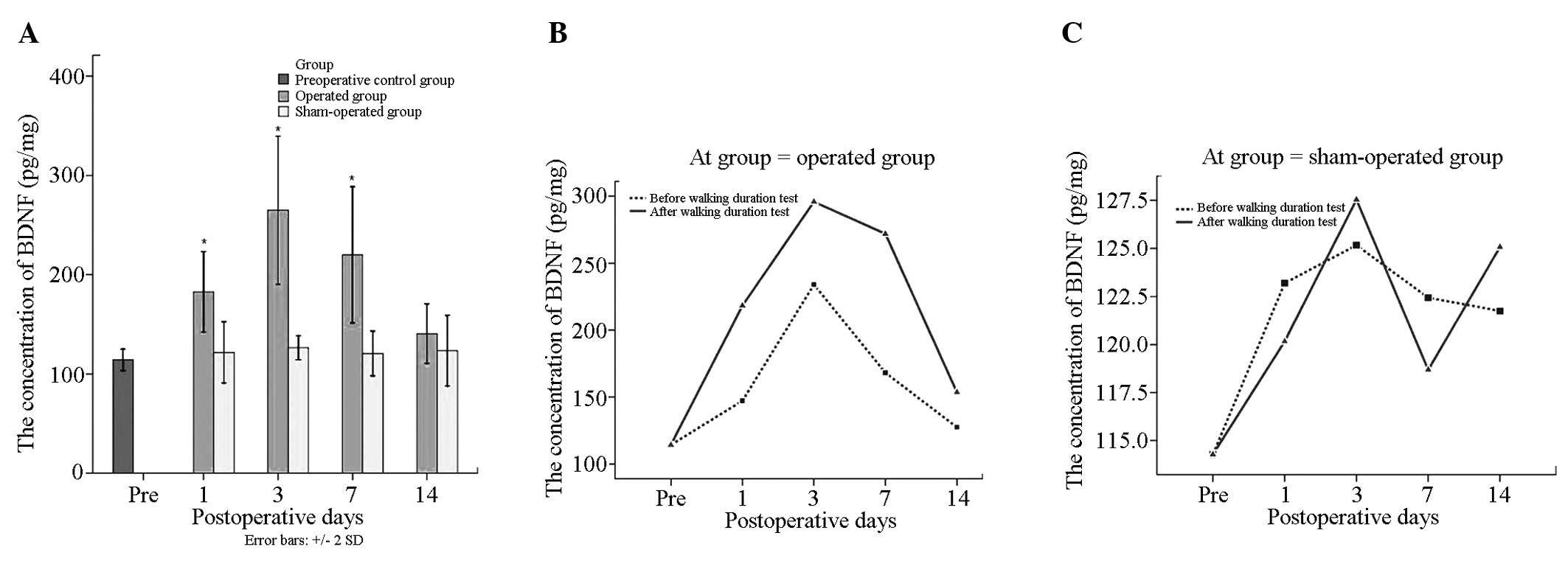
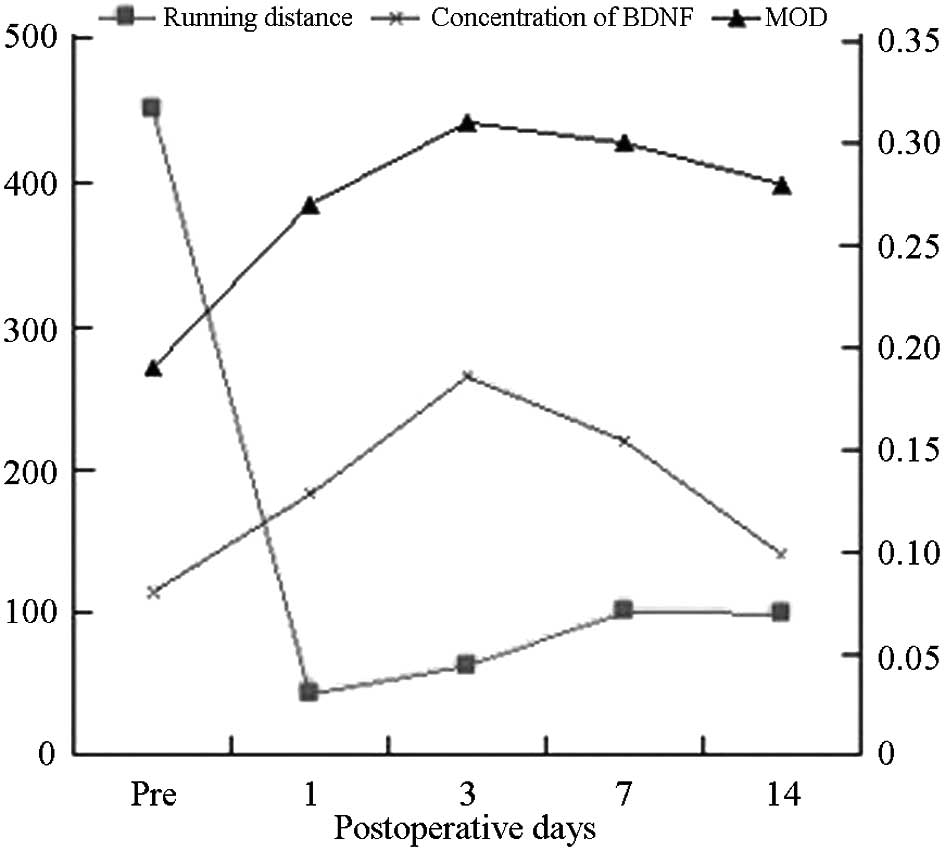
BDNF content changes
BDNF total protein levels in the operation group
were 202.06±89.25 pg/mg prior to surgery, which was significantly
higher than that of the sham operation (123.00±36.06 pg/mg) and
control groups (114.28±10.78 pg/mg) (P<0.05). There were no
differences between the sham operation and the control groups prior
to surgery (P>0.05).
In the operation group, BDNF total protein
concentration before and after sports was 169.22±70.99 pg/mg and
234.90±95.50 pg/mg, respectively (P<0.05). There were
significant differences at each time point in the operation group
(P<0.05), BDNF total protein levels were highest 3 days
following surgery and reached 264.93±105.64 pg/mg. Moreover, there
were significant differences prior to surgery compared with that 14
days following surgery (140.54±42.27) (P<0.05). There were no
significant differences at the remaining time points
(P>0.05).
BDNF content following sports in the operation group
at the same time points was higher than that prior to sports, but
there were no significant differences (P>0.05). Values before
and after sports in the sham operation group at each time point
showed no significant differences (P>0.05) (Tables I and II, Fig.
2).
 | Table IBDNF expression levels (n=4), MOD
(n=4) and walking distance (n=8) in each group. |
Table I
BDNF expression levels (n=4), MOD
(n=4) and walking distance (n=8) in each group.
| Post surgery | Group | | BDNF (pg/mg total
protein) | MOD (±s) | Sports distance
(m) |
|---|
|
|
|
|
|---|
| Control prior to
surgery | | 114.28±10.78 | 0.199±0.028 | 395.76±32.79 |
| 1 day | Operation | Before sport | 147.22±32.31 | 0.265±0.021 | 42.67±38.01 |
| | After sport | 218.27±56.90 | 0.283±0.031 | - |
| | Sum (n=8) |
182.75±57.25a | 0.273±0.026a | - |
| Sham operation | Before sport | 123.20±9.84 | 0.201±0.021 | 75.00±39.19 |
| | After sport | 120.16±65.84 | 0.198±0.022 | - |
| | Sum (n=8) | 121.68±43.61 | 0.199±0.021 | - |
| 3 days | Operation | Before sport | 234.03±116.08 | 0.298±0.019 | 62.63±31.69e |
| | After sport | 295.83±100.08 | 0.332±0.016d | - |
| | Sum (n=8) |
264.93±105.64a,b | 0.316±0.025a,c | - |
| Sham operation | Before sport | 125.17±9.80 | 0.202±0.022 | 183.66±12.13 |
| | After sport | 127.5±24.36 | 0.197±0.019 | - |
| | Sum (n=8) | 126.36±17.23 | 0.199±0.019 | - |
| 7 days | Operation | Before sport | 168.12±28.75 | 0.293±0.029 |
100.13±68.47e |
| | After sport | 271.90±118.14 | 0.314±0.031 | - |
| | Sum (n=8) |
220.01±97.02a | 0.303±0.030a | - |
| Sham operation | Before sport | 122.44±29.62 | 0.201±0.028 | 292.55±99.27 |
| | After sport | 116.68±38.47 | 0.201±0.019 | - |
| | Sum (n=8) | 120.56±31.84 | 0.201±0.022 | - |
| 14 days | Operation | Before sport | 127.51±35.91 | 0.273±0.047 | 82.69±58.93e |
| | After sport | 153.57±49.28 | 0.291±0.037 | - |
| | Sum (n=8) | 140.54±42.27 | 0.282±0.041a | - |
| Sham operation | Before sport | 121.57±51.24 | 0.197±0.034 | 279.34±106.10 |
| | After sport | 125.07±57.06 | 0.204±0.014 | - |
| | Sum (n=8) | 123.41±50.23 | 0.201±0.024 | - |
 | Table IIBDNF expression levels before and
after sports (±s) and MOD ( ±s) in each group. |
Table II
BDNF expression levels before and
after sports (±s) and MOD ( ±s) in each group.
| Group | Prior to and after
sports | BDNF content (pg/mg
total protein) | MOD |
|---|
| Operation | Before (n=16) | 169.22±70.99 | 0.281±0.031 |
| After (n = 16) |
234.90±95.50a | 0.305±0.033a |
| Sum (n=32) |
202.06±89.25b | 0.293±0.034b |
| Sham operation | Before (n=16) | 123.14±27.22 | 0.200±0.024 |
| After (n = 16) | 122.86±44.12 | 0.199±0.017 |
| Sum (n=32) | 123.00±36.06 | 0.200±0.206 |
| Control prior to
surgery (n=4) | - | 114.28±10.78 | 0.199±0.028 |
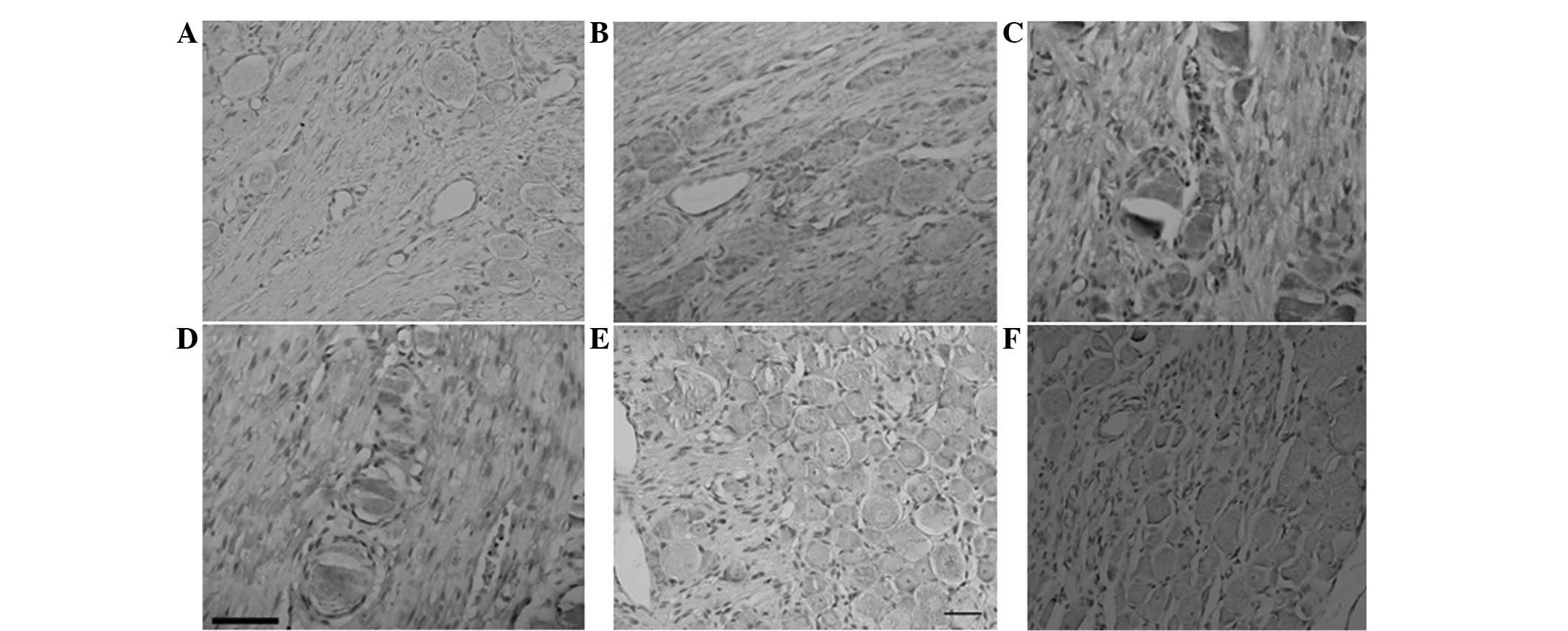
BDNF protein expression changes
In the operation group, BDNF protein expression was
upregulated 1 day following surgery. A positive reaction was
distributed in each type of neuron, but it was mainly distributed
in the intracytoplasm of neurons in the middle of DRG with a small
diameter (10–50 μm). BDNF distribution was also observed in nerve
fibers and marginal expression was identified in the nucleus
(Fig. 3).
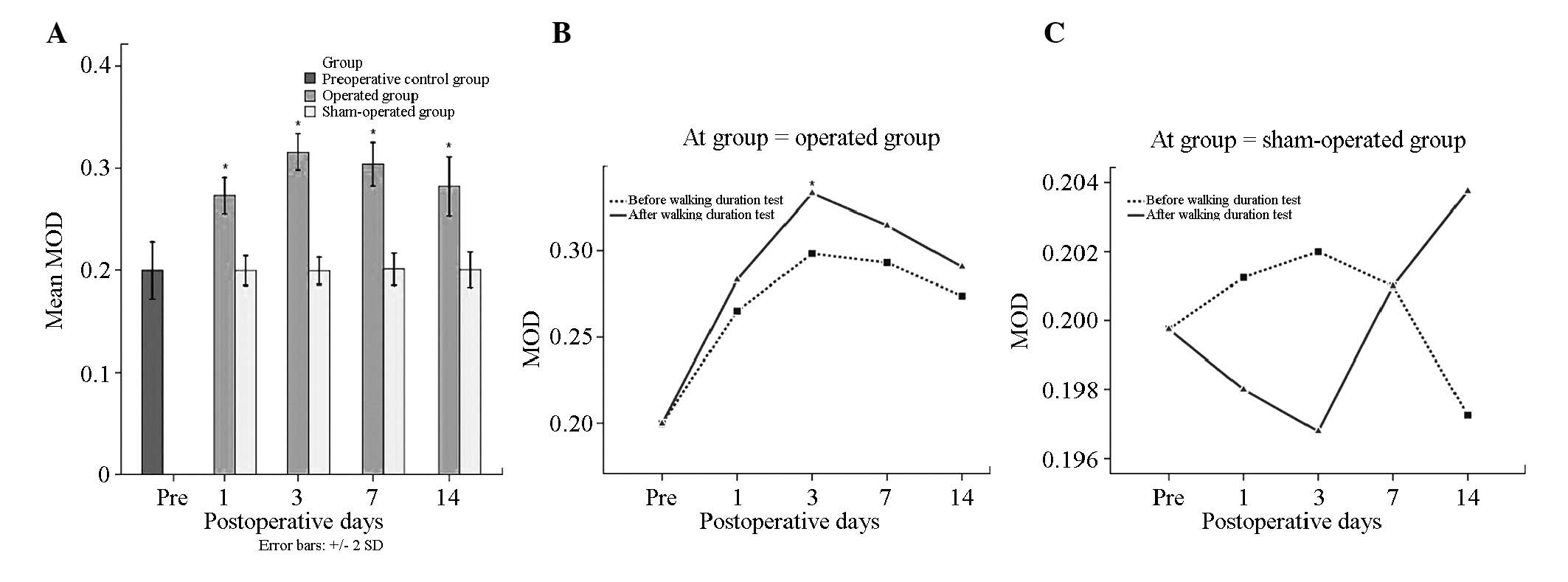
MOD in the operation group (0.293±0.034) was
significantly higher than that in the sham operation (0.200±0.206)
and control groups prior to surgery (0.199±0.028) (P<0.05).
There were no differences between the sham operation and control
groups (P>0.05). In the operation group, the MOD values before
(0.281±0.031) and after sports (0.305±0.033) were significantly
different (P<0.05). Multiple comparison with the SNK method
demonstrated significant differences at each time point in the
operation group (P<0.05). MOD was the highest 3 days post
surgery (0.316±0.025) and lowest 1 day post surgery (0.273±0.026)
(P<0.05). There were no differences at the remaining time
points.
In the operation group, the MOD following surgery
was significantly higher after sports than before sports. However,
t-test analysis compared the same time points before and after
sports and demonstrated that 3 days following surgery BDNF
expression levels after sports were significantly different than
those before sports (P<0.05). In the sham operation group, MOD
at the same time points before and after sports showed no
significant differences (P>0.05) (Tables I and II; Fig.
4).
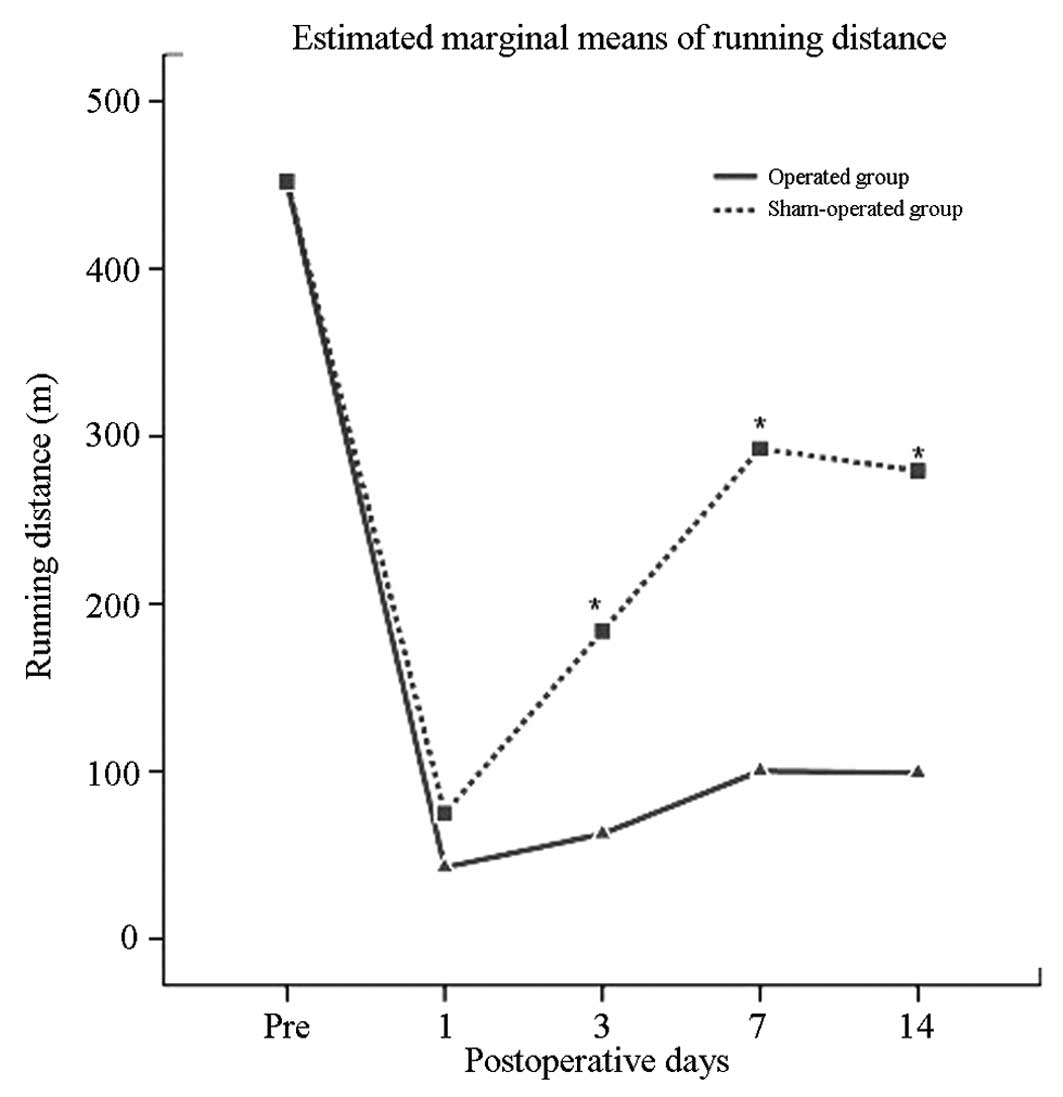
Correlation between the distance measured
following flat plate sports and BDNF expression levels
In the operation group, flat plate sports distance
following surgery was significantly less than that before surgery
(P<0.05). In the sham operation group before and after surgery,
walking distance was not significantly different. Walking distance
following surgery in the operation group was significantly less
than that in the sham operation group (P<0.05) (Table I, Fig.
5).
ELISA confirmed the results of the
immunohistochemical staining in the operation group. BDNF
expression levels showed a significant negative correlation with
walking distance (correlation coefficient r=−0.05 and −0.65,
P<0.05); however, the correlativity was not close (Fig. 6).
Discussion
There are a number of methods that can be used to
prepare a rat model of LSS, such as use of a metal device,
sacculus, composite material, lamina of vertebrae degeneration
osteophyte and tumor implantation (26–31).
Spinal stenosis caused by silica gel embedding is similar to spinal
stenosis as a result of human body degeneration. The
pressure-causing agents that compress the coccygeal nerve result in
symptoms, such as intermittent claudication. Liu et
al(32) effectively applied
silica gel embedding to establish a rat model of LSS. We found a
valid stenosis where the spinal canal was compressed by 50–70%
postoperatively. This result is inconsistent with previous results
used this specification (33). The
reason for this difference may be that spinal canal internal
diameter was not calculated effectively and directly in that study.
In the present study, when measuring the walking distance of rats
we identified that neurogenic intermittent claudication appeared in
continuous walking; however, it showed no obvious effects on the
distance measured with regard to limb sports. Yamaguchi et
al applied silica gel embedding in rats and observed flat plate
sports from 10 to 24 weeks post-operatively. The results of that
study showed that walking distance continuously decreased as
claudication increased. The pathological sections indicated
epidural edema, fibrosis and axon degeneration and regeneration
throughout the experiment (34).
The present study used silica gel to compress the
dural sac and the walking distance in rats following surgery was
observed. Finite element method with 3D software was used to
measure the internal diameter of the spinal canal and showed the
established LSS model in rats. This method is simple, feasible, may
be manipulated and repeated, and has a high achievement ratio.
DRG contain neurons, Schwann cells, fibroblasts and
satellite cells. Nerve fibers pass between all types of neurons and
are divided into large, middle and small cells according to neuron
diameter. DRG secretes and transports numerous types of
neurotransmitters and modulators, such as BDNF, GDNF and neural
cell adhesion molecule (NCAM). There are also certain receptors
that have an adjusting action, such as γ-aminobutyric acid, opium,
purine and a number of ion channels (35). Due to its unique action in signal
transduction, DRG may be a target of therapeutic medication to
decrease the generation of pain.
Stimulated DRG led to NP, which was induced by
numerous different pain factors, such as opening of BDNF channel
and TRPV channel, GDNF, NCAM, methionince encephalin (M-ENK), P
substance, COX2, PGE2 and IL-6 (36,37).
The opening of ion channels initiated a change of cell action
potential, thus algesia was generated and transmitted, and its
overexcitation resulted in a decreased pain threshold and
hyperpathia (38).
BDNF is a member of the neurotrophin family and is
important for neuronal survival and plasticity in the CNS. BDNF was
first detected and purified from a pig brain in 1982 (39). BDNF has been previously
demonstrated to promote numerous types of neuron growth
differentiation and regeneration. It adjusts neuron apoptosis,
promotes the restoration of the myelin sheath of nerve fibers, and
is important in the process of synaptic maturation and plasticity.
Furthermore, BDNF effects in injury, inflammatory pain and NP as
pain-causing factors in Cornu dorsal medullae spinalis have
been confirmed generally (40–42).
BDNF expression in different nerve cells is diverse,
which may be relevant to injury time. At an acute stress state of
nerve injury, BDNF is mainly synthesized and released from the
Schwann cell of neurons in the middle of DRG that have a large
diameter. Injury caused by chronic compression resulted in BDNF
expression in neurons from the middle of DRG with a small diameter
(43–45). In the present study, results of the
immunohistochemistry indicated that a positive reaction occurred
mainly in intracytoplasmic neurons in the middle of DRG with a
small diameter. Nerve fibers also had an obvious distribution of
BDNF, with only marginal expression in the nucleus, which is in
accordance with a previous study on nerve chronic compression
(44,46).
The present study demonstrated that flat plate
sports promoted the expression of BDNF. In injury with early stage,
the upregulation expression is more obvious, and may be the result
of the presence of various cell types, including big diameter cell,
middle diameter cell and Schwann cell. BDNF expression levels
gradually decreased with time extension, but remained higher than
that of the sham operation group. This indicated that BDNF is at a
state of high expression if compression is present. However, the
statistical results showed that BDNF expression levels after sports
were higher than those before sports. A detailed comparison at each
time point between before and after sports demonstrated that the
MOD value on day 3 following surgery was significantly different.
The reason may be that the sample quantity is less at each time
point before and after sports. Moreover, BDNF expression levels
were negatively correlated with walking distance, but this
correlation was not strong.
Inhibition of BDNF expression may effectively reduce
pain providing a novel research direction for treating intermittent
claudication clinically caused by NP. This result was confirmed in
a number of animal experiments (47,48).
When BDNF antibody, microglia inhibitor minocycline, TrkB
antagonist or p38 MAPK inhibitor were injected into the epithelium
of guard or nerve ganglion, NP was effectively relieved and
blocking DRG decreased symptoms, such as pain (46–50).
However, BDNF not only results in pain, but is also important for
the restoration and self-protection of nerves; therefore,
inhibiting BDNF expression inevitably decreased nerve protection
and restoration. Additional studies should be conducted to
determine this role of BDNF. The present study demonstrated that
BDNF expression in the LSS model was significantly upregulated
following sports. Walking distance was negatively correlated with
BDNF expression (walking distance decreased as BDNF expression
levels increased). NP development is caused by factors, such as
BDNF, as a self protection mechanism and subsequent pain restricted
walking; therefore, BDNF may heighten intermittent claudication as
a result of LSS.
BDNF expression increased, not only in the acute
stage of injury, but also in the chronic continuous compression
stage, suggesting the involvement of LSS chronic degradation
process. Silica gel slices were embedded under the L5 lamina of
vertebra resulting in immediate spinal stenosis, which is not in
accordance with LSS chronic degradation changes. BDNF changes in
the first 3 days only indicated protein expression in the acute
state, but after 3 days, BDNF expression remained at a state higher
than that before surgery in the sham operation group. BDNF may have
continued to decrease with a longer restoration time, but results
of the present have shown BDNF was at a higher state 28 days
following surgery to 2 months, demonstrating that chronic
continuous compression may cause a high expression of BDNF to a
certain extent. The change of BDNF expression before and after
sports demonstrated LSS upregulated BDNF expression during pain
attack. A shorter observation time was observed with regard to flat
plate sport ability, however, exercise tolerance for a longer
period of time following surgery remains to be clarified. The
volume of blood flow to Cauda equina may decrease during
sports inducing ischemia and anoxia of the nerve root and
generating inflammation, thereby causing pain, and the effect of
the change of blood flow was not investigated in this study. In
addition, this study did not show the sport ability change of rats
after BDNF antibody was administrated to inhibit BDNF.
In conclusion, in the present study, silica gel was
used to compress the dural sac and the sports function in rats
following surgery was observed. Flat plate sports distance in the
operation group was obviously less than that prior to surgery and
in the sham operation group. We performed CT scanning of rats, used
mimics software to set up finite element model and observed spinal
canal compression at 50–70%, which showed the model had been
successfully established. BDNF expression levels increased in DRG
in rat models of LSS and further increased following sports. The
increased BDNF levels were associated with a shortened walking
distance. We suggest that BDNF is important in NP generation and
the transmission process in rat models of LSS. The increased BDNF
expression levels indicated that BDNF may cause the pain factor of
intermittent claudication, which is caused by LSS; however,
simultaneouly BDNF may protect and repair nerve injury.
Acknowledgements
This study was supported by the Social Development
Project of Lianyungang City (Jiangsu, China) (grant no. SH1002). We
would like to thank the staff of the Experimental Animal Center,
Xuzhou Medical College (Xuzhou, China).
References
|
1
|
Rainville J, Childs LA, Peña EB, et al:
Quantification of walking ability in subjects with neurogenic
claudication from lumbar spinal stenosis - a comparative study.
Spine J. 12:101–109. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okuda T, Baba I, Fujimoto Y, et al: The
pathology of ligamentum flavum in degenerative lumbar disease.
Spine (Phila Pa 1976). 29:1689–1697. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalichman L, Cole R, Kim DH, et al: Spinal
stenosis prevalence and association with symptoms: the Framingham
Study. Spine J. 9:545–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winter CC, Brandes M, Müller C, et al:
Walking ability during daily life in patients with osteoarthritis
of the knee or the hip and lumbar spinal stenosis: a cross
sectional study. BMC Musculoskelet Disord. 11:2332010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blau JN and Logue V: Intermittent
claudication of the cauda equina. Lancet. 1:1081–1086. 1961.
View Article : Google Scholar
|
|
6
|
Molina M, Wagner P and Campos M: Spinal
lumbar stenosis: an update. Rev Med Chil. 139:1488–1495. 2011.(In
Spanish).
|
|
7
|
Lohmander LS, Gerhardsson de Verdier M,
Rollof J, Nilsson PM and Engström G: Incidence of severe knee and
hip osteoarthritis in relation to different measures of body mass:
a population-based prospective cohort study. Ann Rheum Dis.
68:490–496. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marcol W, Kotulska K, Larysz-Brysz M and
Kowalik JL: BDNF contributes to animal model neuropathic pain after
peripheral nerve transection. Neurosurg Rev. 30:235–243. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbas J, Hamoud K, May H, et al:
Degenerative lumbar spinal stenosis and lumbar spine configuration.
Eur Spine J. 19:1865–1873. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang H, Mei X, Zhang P, et al: Altered
functional properties of satellite glial cells in compressed spinal
ganglia. Glia. 57:1588–1599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Ning LP, Wang YH, et al: Nuclear
factor-kappa B mediates TRPV4-NO pathway involved in thermal
hyperalgesia following chronic compression of the dorsal root
ganglion in rats. Behav Brain Res. 221:19–24. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patte-Mensah C, Meyer L, Schaeffer V and
Mensah-Nyagan AG: Selective regulation of 3 alpha-hydroxysteroid
oxido-reductase expression in dorsal root ganglion neurons: a
possible mechanism to cope with peripheral nerve injury-induced
chronic pain. Pain. 150:522–534. 2010. View Article : Google Scholar
|
|
13
|
Van Steenwinckel J, Noghero A, Thibault K,
Brisorgueil MJ, Fischer J and Conrath M: The 5-HT2A receptor is
mainly expressed in nociceptive sensory neurons in rat lumbar
dorsal root ganglia. Neuroscience. 161:838–846. 2009.PubMed/NCBI
|
|
14
|
Vranken JH: Mechanisms and treatment of
neuropathic pain. Cent Nerv Syst Agents Med Chem. 9:71–78. 2009.
View Article : Google Scholar
|
|
15
|
Simopoulos TT, Kraemer J, Nagda JV, Aner M
and Bajwa ZH: Response to pulsed and continuous radiofrequency
lesioning of the dorsal root ganglion and segmental nerves in
patients with chronic lumbar radicular pain. Pain Physician.
11:137–144. 2008.PubMed/NCBI
|
|
16
|
Boucher TJ, Okuse K, Bennett DL, Munson
JB, Wood JN and McMahon SB: Potent analgesic effects of GDNF in
neuropathic pain states. Science. 290:124–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Y, Obata K, Yamanaka H, et al:
Activation of extracellular signal-regulated protein kinase in
dorsal horn neurons in the rat neuropathic intermittent
claudication model. Pain. 109:64–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ochs G, Penn RD, York M, et al: A phase
I/II trial of recombinant methionyl human brain derived
neurotrophic factor administered by intrathecal infusion to
patients with amyotrophic lateral sclerosis. Amyotroph Lateral
Scler Other Motor Neuron Disord. 1:201–206. 2000. View Article : Google Scholar
|
|
19
|
Dey ND, Bombard MC, Roland BP, et al:
Genetically engineered mesenchymal stem cells reduce behavioral
deficits in the YAC 128 mouse model of Huntington’s disease. Behav
Brain Res. 214:193–200. 2010.PubMed/NCBI
|
|
20
|
Martin JL, Brown AL and Balkowiec A: Glia
determine the course of brain-derived neurotrophic factor-mediated
dendritogenesis and provide a soluble inhibitory cue to dendritic
growth in the brainstem. Neuroscience. 207:333–346. 2012.
View Article : Google Scholar
|
|
21
|
Cohen-Cory S, Kidane AH, Shirkey NJ and
Marshak S: Brain-derived neurotrophic factor and the development of
structural neuronal connectivity. Dev Neurobiol. 70:271–288.
2010.PubMed/NCBI
|
|
22
|
Yoshii A and Constantine-Paton M:
Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity,
and disease. Dev Neurobiol. 70:304–322. 2010.PubMed/NCBI
|
|
23
|
Tardito D, Perez J, Tiraboschi E, Musazzi
L, Racagni G and Popoli M: Signaling pathways regulating gene
expression, neuroplasticity, and neurotrophic mechanisms in the
action of antidepressants: a critical overview. Pharmacol Rev.
58:115–134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chan JR, Jolicoeur C, Yamauchi J, et al:
The polarity protein Par-3 directly interacts with p75NTR to
regulate myelination. Science. 314:832–836. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ng BK, Chen L, Mandemakers W, Cosgaya JM
and Chan JR: Anterograde transport and secretion of brain-derived
neurotrophic factor along sensory axons promote Schwann cell
myelination. J Neurosci. 27:7597–7603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kubota M, Kobayashi S, Nonoyama T, et al:
Development of a chronic cervical cord compression model in rat:
changes in the neurological behaviors and radiological and
pathological findings. J Neurotrauma. 28:459–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sekiguchi M, Aoki Y, Konno S and Kikuchi
S: The effects of cilostazol on nerve conduction velocity and blood
flow: acute and chronic cauda equina compression in a canine model.
Spine (Phila Pa 1976). 33:2605–2611. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sekiguchi M, Kikuchi S and Myers RR:
Experimental spinal stenosis: relationship between degree of cauda
equina compression, neuropathology, and pain. Spine (Phila Pa
1976). 29:1105–1111. 2004. View Article : Google Scholar
|
|
29
|
Watanabe K, Konno S, Sekiguchi M and
Kikuchi S: Spinal stenosis: assessment of motor function, VEGF
expression and angiogenesis in an experimental model in the rat.
Eur Spine J. 16:1913–1918. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pinazo Serón MJ, Benet i Català A, Ferrer
i Santaularia J, Clotas i Sancho L, Gens i Barbera M and Cartanyà i
Benet A: Spinal cord compression caused by metastasis of soft
tissue hepatocarcinoma. An Med Interna. 16:587–589. 1999.(In
Spanish).
|
|
31
|
Takahashi N, Yabuki S, Aoki Y and Kikuchi
S: Pathomechanisms of nerve root injury caused by disc herniation:
an experimental study of mechanical compression and chemical
irritation. Spine (Phila Pa 1976). 28:435–441. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Obata K, Yamanaka H, et al:
Activation of extracellular signal-regulated protein kinase in
dorsal horn neurons in the rat neuropathic intermittent
claudication model. Pain. 109:64–72. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takenobu Y, Katsube N, Marsala M and Kondo
K: Model of neuropathic intermittent claudication in the rat:
methodology and application. J Neurosci Methods. 104:191–198. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamaguchi K, Murakami M, Takahashi K,
Moriya H, Tatsuoka H and Chiba T: Behavioral and morphologic
studies of the chronically compressed cauda equina. Experimental
model of lumbar spinal stenosis in the rat. Spine (Phila Pa 1976).
24:845–851. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Scholz J and Woolf CJ: The neuropathic
pain triad: neurons, immune cells and glia. Nat Neurosci.
10:1361–1368. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
St-Jacques B and Ma W: Role of
prostaglandin E2 in the synthesis of the pro-inflammatory cytokine
interleukin-6 in primary sensory neurons: an in vivo and in vitro
study. J Neurochem. 118:841–854. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma W: Chronic prostaglandin E2 treatment
induces the synthesis of the pain-related peptide substance P and
calcitonin gene-related peptide in cultured sensory ganglion
explants. J Neurochem. 115:363–372. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Levin JD and Alessandri-Haber N: TRP
channels: targets for the relief of pain. Biochim Biophys Acta.
1772:989–1003. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lindsay RM, Barde YA, Davies AM and Rohrer
H: Differences and similarities in the neurotrophic growth factor
requirements of sensory neurons derived from neural crest and
neural placode. J Cell Sci Suppl. 3:115–129. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pezet S, Marchand F, D'Mello R, et al:
Phosphatidylinositol 3-kinase is a key mediator of central
sensitization in painful inflammatory conditions. J Neurosci.
28:4261–4270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Merighi A, Salio C, Ghirri A, et al: BDNF
as a pain modulator. Prog Neurobiol. 85:297–317. 2008. View Article : Google Scholar
|
|
42
|
Pezet S, Cunningham J, Patel J, et al:
BDNF modulates sensory neuron synaptic activity by a facilitation
of GABA transmission in the dorsal horn. Mol Cell Neurosci.
21:51–62. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rage F, Silhol M and Tapia-Arancibia L:
IL-1beta regulation of BDNF expression in rat cultured hypothalamic
neurons depends on the presence of glial cells. Neurochem Int.
49:433–441. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ha SO, Kim JK, Hong HS, Kim DS and Cho HJ:
Expression of brain-derived neurotrophic factor in rat dorsal root
ganglia, spinal cord and gracile nuclei in experimental models of
neuropathic pain. Neuroscience. 107:301–309. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fukuoka T, Kondo E, Dai Y, Hashimoto N and
Noguchi K: Brain-derived neurotrophic factor increases in the
uninjured dorsal root ganglion neurons in selective spinal nerve
ligation model. J Neurosci. 21:4891–4900. 2001.PubMed/NCBI
|
|
46
|
Li CQ, Xu JM, Liu D, Zhang JY and Dai RP:
Brain derived neurotrophic factor (BDNF) contributes to the pain
hypersensitivity following surgical incision in the rats. Mol Pain.
4:272008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yajima Y, Narita M, Usui A, et al: Direct
evidence for the involvement of brain-derived neurotrophic factor
in the development of a neuropathic pain-like state in mice. J
Neurochem. 93:584–594. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sapunar D, Kostic S, Banozic A and Puljak
L: Dorsal root ganglion-a potential new therapeutic target for
neuropathic pain. J Pain Res. 5:31–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Quintão NL, Santos AR, Campos MM and
Calixto JB: The role of neurotrophic factors in genesis and
maintenance of mechanical hypernociception after brachial plexus
avulsion in mice. Pain. 136:125–133. 2008.PubMed/NCBI
|
|
50
|
Wang X, Ratnam J, Zou B, England PM and
Basbaum AI: TrkB signaling is required for both the induction and
maintenance of tissue and nerve injury-induced persistent pain. J
Neurosci. 29:5508–5515. 2009. View Article : Google Scholar : PubMed/NCBI
|