Introduction
Osteoarthritis (OA) is the most common degenerative
disease affecting articular cartilage and is characterized by
disrupted cartilage extracellular matrix homeostasis, ultimately
resulting in the loss of cartilage without effective replacement
(1–4). OA is caused in part by the exposure
of chondrocytes to inflammatory cytokines, including interleukin-1β
(IL-1β) and tumor necrosis factor (TNF-α), which stimulate
chondrocyte responses that promote catabolism of type II collagen
and proteoglycans, thereby compromising cartilage extracellular
matrix integrity and tissue homeostasis in OA (2). TNF-α is crucial in cartilage
degradation. It promotes the expression of cytokines and chemokines
in synovial cells and chondrocytes, thereby maintaining the renewal
of local inflammatory mediators (5). The presence of TNF-α correlates with
a general loss of cartilage matrix molecules, including type II
collagen and aggrecan (5). TNF-α
is produced by numerous cell types, including macrophages,
lymphocytes, fibroblasts and keratinocytes in response to
inflammation, infection and other environmental stresses (6). TNF-α acts by binding to its
receptors, TNFR1 (p55) and TNFR2 (p75) on the cell surface. The
majority of cells express TNFR1, which is believed to be the major
mediator of the cytotoxicity of TNF-α (6). A previous study demonstrated that p55
TNF-α receptor expression is significantly increased in OA
chondrocytes ex vivo. Enhanced expression of p55 may
contribute to OA cartilage degradation (7).
The pathogenesis of OA is poorly understood, however
a major feature is the loss of the two most important components of
cartilage extracellular matrix: Type II collagen and aggrecan
(8). Aggrecanases represent a
class of proteinases belonging to the a disintegrin and
metalloproteinase with thrombospondin motifs 4 (ADAMTS) family.
Previous studies have demonstrated that ADAMTS 1, 4, 5, 8, 9 and 15
possess aggrecanase activity (9,10).
Song et al demonstrated that the knockdown of aggrecanase-1
(ADAMTS-4), aggrecanase-2 (ADAMTS-5) or both attenuates the
degradation of aggrecan in human cartilage stimulated by TNF-α and
oncostatin M (11). It has been
stated that ADAMTS-4 is selectively overexpressed in human OA
cartilage and is positively correlated with the degree of cartilage
destruction, whereas ADAMTS-5 is similarly expressed in normal and
OA cartilages (12). The results
suggest that ADAMTS-4 is a major aggrecanase in human OA cartilage
and its induction is involved in the pathogenesis of OA.
TNF-α and ADAMTS-4 are thought to be important in OA
cartilage degradation. For the first time, to the best of our
knowledge, we explored the interaction between the two proteins by
examining the effect of TNF-α on ADAMTS-4 expression and activity
in human osteoarthritic chondrocytes.
Materials and methods
Reagents
Recombinant human TNF-α, TNFR1 inhibitor SPD304,
phosphatidylinositol-3 kinase (PI3K) inhibitor LY294002, protein
kinase C inhibitor Go6983, mitogen-activated protein kinase (MAPK)
inhibitor PD098059 and p38 MAPK inhibitor PD169316 were purchased
from Sigma (St. Louis, MO, USA). TRIzol reagent for RNA isolation
and the SYBR Green Master mix were purchased from Invitrogen
(Carlsbad, CA, USA) and Applied Biosystems (Foster City, CA, USA),
respectively. Anti-ADAMTS-4 (PA1-1749A) antibody was purchased from
Thermo Fisher Scientific (Rockford, IL, USA). Anti-phospho-p38
(Thr180/Tyr182; no. 9212) antibody, anti-p38 (no. 8690) antibody
and SignalSilence® p38 mitogen-activated protein kinase
(MAPK) siRNA (no. 6564) were purchased from Cell Signaling
Technology (Danvers, MA, USA). The SensoLyte® 520
aggrecanase-1 assay kit (no. 72114) was purchased from AnaSpec Inc.
(Fremont, CA, USA). Human ADAMTS-4 promoter-luciferase
reporter (with the ADAMTS-4 promoter sequence from 726
nucleotides upstream to 406 nucleotides downstream of the
transcription start site inserted upstream of the luciferase cDNA)
was generated as previously described (13). A dual-luciferase reporter assay
system was purchased from Promega (Madison, WI, USA). Lipofectamine
2000 transfection reagent was purchased from Invitrogen.
Cell culture and treatment
Human osteoarthritic chondrocytes (402OA-05a) and
chondrocyte growth medium (411–500) were purchased from Cell
Applications Inc. (San Diego, CA, USA). The cells were treated with
TNF-α in different concentrations (5, 15, 30, 45 and 60 ng/ml) for
different lengths of time (1, 6, 12, 18 and 24 h). All kinase
inhibitors were dissolved in dimethyl sulfoxide (DMSO; final
concentration of DMSO 0.05%). For kinase inhibitor treatment,
chondrocytes were pretreated with the kinase inhibitor for 30 min
and then incubated with the kinase inhibitor and TNF-α (60 ng/ml)
for 18 h. Chondrocytes treated with TNF-α (60 ng/ml) + DMSO (0.05%)
were used as a control in the experiments.
Real-time quantitative RT-PCR
RNA were prepared using TRIzol reagent followed by
purification with TURBO DNA-free System (Ambion, Austin, TX, USA).
The cDNAs were synthesized using SuperScript II reverse
transcriptase (Invitrogen). Real-time quantitative PCR was
performed on an Abi-Prism 7700 Sequence Detection System using the
fluorescent dye SYBR Green Master mix (Applied Biosystems) as
described by the manufacturer. The results were normalized against
that of the housekeeping gene glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) in the same sample. The primers used
were as follows: for human ADAMTS-4,
5′-GCAACGTCAAGGCTCCTCTT-3′ (forward) and
5′-CTCCACAAATCTACTCAGTGAAGCA-3′ (reverse); for human GAPDH,
5′-GACTCATGACCACAGTCCATGC-3′ (forward) and
5′-AGAGGCAGGGATGATGTTCTG-3′ (reverse). The mRNA level of treated
cells was shown as fold changes to that of untreated control cells
(designated as 1). Each experiment was repeated three times in
triplicates. Results are expressed as the mean ± SD.
Luciferase reporter assay
Human osteoarthritic chondrocytes were transfected
with human ADAMTS-4 promoter-luciferase reporter plasmids
using Lipofectamine 2000 transfection reagent (Invitrogen) and then
treated with TNF-α (30 or 60 ng/ml) for 18 h. Plasmid PRL-CMV
encoding Renilla reniformis luciferase (at one-fifth molar
ratio to test plasmids) was co-transfected with test plasmids in
each transfection as an internal control for data normalization.
Luciferase assays were performed with a dual-luciferase reporter
assay system (Promega) according to the manufacturer's
instructions. Each experiment was repeated three times in
duplicates. Untreated human osteoarthritic chondrocytes were used
as a control.
ADAMTS-4 activity assay and western blot
analysis
ADAMTS-4 activities in cell culture supernatants
were determined using a SensoLyte® 520 aggrecanase-1
assay kit (AnaSpec Inc.) according to the manufacturer's
instructions. In western blot analyses, human osteoarthritic
chondrocytes were lysed in 250 μl of 2X SDS loading buffer (62.5 mm
TrisHCl, pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, 5%
2-mercaptoethanol) and incubated at 95°C for 10 min. An equal
amount of proteins (100 g) for each sample were separated by 8–15%
SDS-polyacrylamide gel and blotted onto a polyvinylidene difluoride
microporous membrane (Millipore, Billerica, MA, USA). Membranes
were incubated for 1 h with a 1:1,000 dilution of primary antibody
and then washed and revealed using secondary antibodies with
horseradish peroxidase conjugate (1:5,000, 1 h). Peroxidase was
revealed with an ECL kit (GE Healthcare, Pittsburgh, PA, USA).
Proteins were quantified prior to being loaded onto the gel.
Statistical analysis
Statistical analyses were performed with SPSS for
Windows 10.0 (IBM, Chicago, IL, USA). Data values were expressed as
the means ± SD. Comparisons of means among multiple groups were
performed with one-way ANOVA followed by post hoc pairwise
comparisons using Tukey's tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
TNF-a increases ADAMTS-4 mRNA
expression
Cultured human osteoarthritic chondrocytes were
treated with TNF-α in different concentrations (5, 15, 30, 45, 60
ng/ml) for different lengths of time (1, 6, 12, 18 and 24 h).
ADAMTS-4 mRNA levels were examined using real-time
quantitative RT-PCR. The ADAMTS-4 mRNA level of treated
cells was shown as fold changes to that of untreated control cells
(designated as 1). As displayed in Table I, TNF-α in the concentration range
of 5 to 45 ng/ml increased the ADAMTS-4 mRNA level in a
statistically significant dose- and time-dependent manner within 18
h of treatment, which was completely eradicated by TNFR1 inhibitor
SPD304. By contrast, TNF-α demonstrated no significant effect on
ADAMTS-4 expression in normal human chondrocytes and its effect on
ADAMTS-5 expression was inconsistent in normal and osteoarthritic
human chondrocytes (data not shown).
 | Table IRelative ADAMTS-4 mRNA levels in human
osteoarthritic chondrocytes in the presence of TNF-α with or
without TNF receptor inhibitor. |
Table I
Relative ADAMTS-4 mRNA levels in human
osteoarthritic chondrocytes in the presence of TNF-α with or
without TNF receptor inhibitor.
| TNF-α (ng/ml) | 1 h | 6 h | 12 h | 18 h | 24 h |
|---|
| 5 | 1.01±0.04 | 1.03±0.03 | 1.04±0.05 | 1.04±0.05 | 1.06±0.07 |
| 15 | 1.04±0.05 | 1.27±0.07a,d | 1.83±0.10a,d,e | 2.69±0.17a,d,e,f | 2.82±0.18a,d,e,f |
| 30 | 1.07±0.05 | 1.86±0.12a,b,d | 2.71±0.14a,b,d,e | 3.15±0.20a,b,d,e,f | 3.28±0.19a,b,d,e,f |
| 45 | 1.07±0.06 | 2.76±0.19a,b,c,d | 3.05±0.13a,b,c,d,e | 3.66±0.21a,b,c,d,e,f | 3.83±0.25a,b,c,d,e,f |
| 60 | 1.08±0.09 | 2.88±0.22a,b,c,d | 3.24±0.23a,b,c,d,e | 3.79±0.26a,b,c,d,e,f | 3.92±0.25a,b,c,d,e,f |
| 60 + SPD304 (50
μm) | 0.94±0.11 | 0.99±0.05 | 0.97±0.07 | 1.03±0.07 | 1.05±0.06 |
Signaling pathways involved in
TNF-a-induced ADAMTS-4 expression and promoter activity
To determine the signaling pathways involved in the
promoting effect of TNF-α on ADAMTS-4 expression, we examined the
ADAMTS-4 mRNA levels in human osteoarthritic chondrocytes
treated with TNF-α (60 ng/ml) with or without different kinase
inhibitors or siRNA for 18 h. As displayed in Table II, inhibition of protein kinase C
(Go6983, 250 nm), MAPK (PD098059, 25 μm) and PI3K (LY294002, 50 μm)
had no significant effect on the ADAMTS-4 mRNA level. By
contrast, inhibition of p38 MAPK by the selective inhibitor
PD169316 (25 μm) or p38 MAPK-specific siRNA completely blocked the
promoting effect of TNF-α on ADAMTS-4 expression. Luciferase
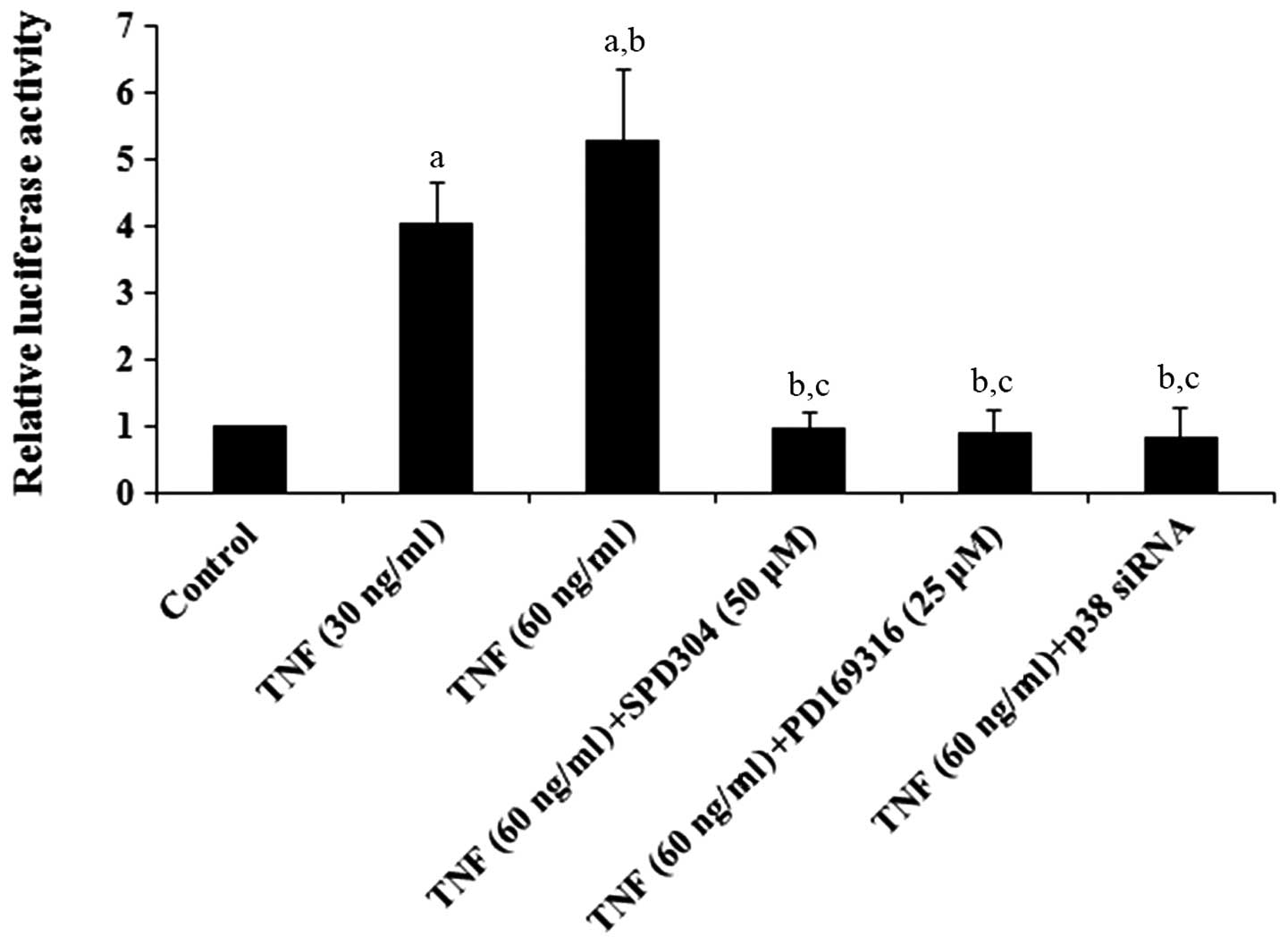
reporter assays demonstrated that TNF-α dose-dependently increased
the ADAMTS-4 promoter activity, which was eliminated by
SPD304, PD169316 or p38 MAPK siRNA (Fig. 1). Western blot analyses
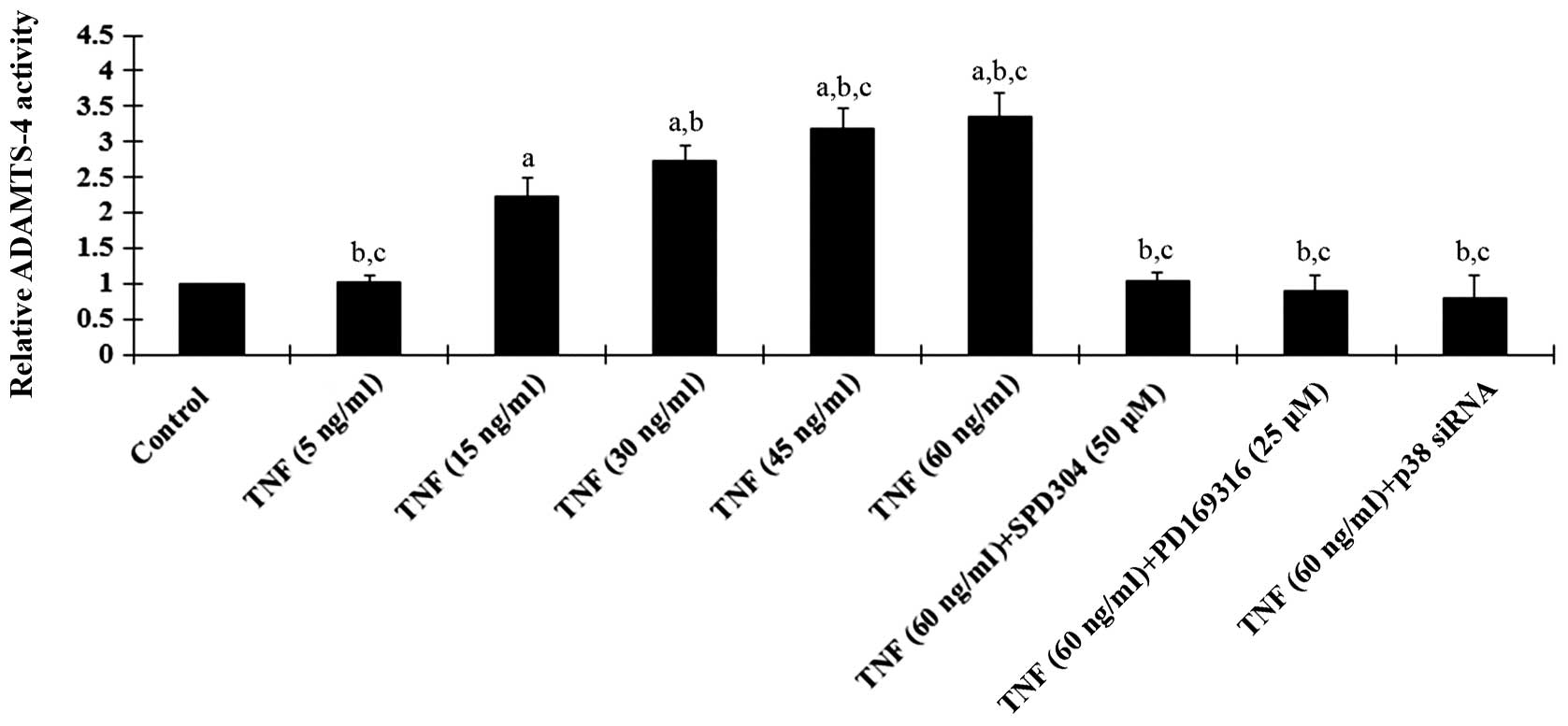
demonstrated that TNF-α treatment for 18 h dose-dependently
increased ADAMTS-4 expression in human osteoarthritic chondrocytes,
which was blocked by SPD304, PD169316 and p38 MAPK siRNA (Fig. 2). A similar data trend was observed
with ADAMTS-4 activities in the cell culture media (Fig. 3).
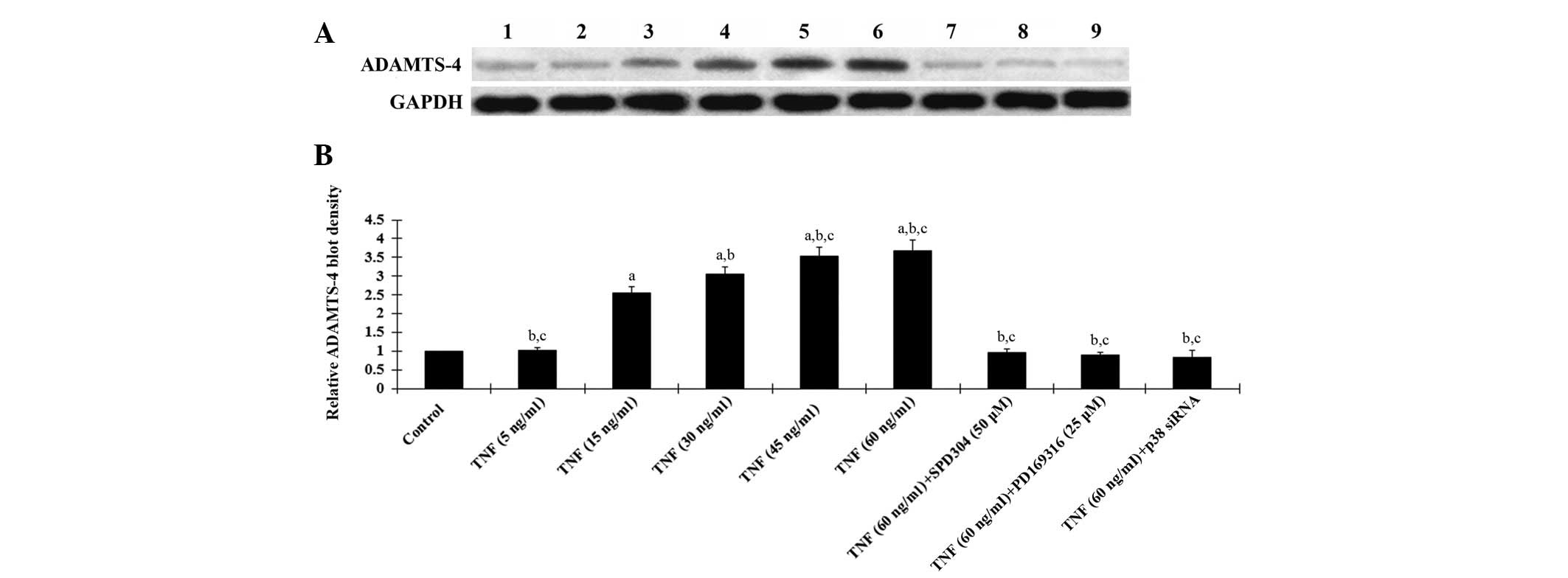 | Figure 2Western blot analysis of ADAMTS-4
expression in human osteoarthritic chondrocytes. (A) Human
osteoarthritic chondrocytes were treated with TNF-α (5, 15, 30, 45
and 60 ng/ml) with or without SPD304 (50 μm), PD169316 (25 μm) or
p38 (MAPK) siRNA for 18 h. Cell lysates were subject to western
blot analyses for ADAMTS-4 expression. Lane 1, lysates from
untreated human osteoarthritic chondrocytes were used as a control;
lane 2, TNF-α (5 ng/ml); lane 3, TNF-α (15 ng/ml); lane 4, TNF-α
(30 ng/ml); lane 5, TNF-α (45 ng/ml); lane 6, TNF-α (60 ng/ml);
lane 7, TNF-α (60 ng/ml) + SPD304 (50 μm); lane 8, TNF-α (60 ng/ml)
+ PD169316 (25 μm); lane 9, TNF-α (60 ng/ml) + p38 (MAPK) siRNA.
GAPDH blotting was used as a loading control. (B) ADAMTS-4 and
GAPDH blots were measured by densitometry. The density of the
ADAMTS-4 blot was normalized against that of GAPDH to obtain a
relative density, which was expressed as fold changes to that of
untreated control cells (designated as 1). aP<0.05
compared with untreated control cells; bP<0.05
compared with TNF-α treatment at 15 ng/ml; cP<0.05
compared with TNF-α treatment at 30 ng/ml. TNF-α, tumor necrosis
factor α; ADAMTS-4, a disintegrin and metalloproteinase with
thrombospondin motifs 4; SPD304, TNFR1 inhibitor; PD169316, p38
MAPK inhibitor; MAPK, mitogen activated protein kinase; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
 | Table IIRelative ADAMTS-4 mRNA levels in
human osteoarthritic chondrocytes in the presence of TNF-α with or
without kinase inhibitors or siRNA. |
Table II
Relative ADAMTS-4 mRNA levels in
human osteoarthritic chondrocytes in the presence of TNF-α with or
without kinase inhibitors or siRNA.
| Treatment | Relative
ADAMTS-4 mRNA level |
|---|
| Control | 3.77±0.24a |
| +PD169316 (25
μm) | 1.05±0.09 |
| +Go6983 (250
nm) | 3.68±0.21a |
| +PD098059 (25
μm) | 3.72±0.26a |
| +LY294002 (50
μm) | 3.65±0.29a |
| +p38 MAPK
siRNA | 0.97±0.15 |
Activation of p38 MAPK in TNF-a-induced
ADAMTS-4 expression
Phosphorylation and activation of p38 MAPK is
involved in TNF-α-induced biological effects (14,15).
As displayed in Fig. 4, western
blot analyses demonstrated that TNF-α treatment for 18 h
dose-dependently increased phosphorylated p38 MAPK (Thr180/Tyr182)
levels in human osteoarthritic chondrocytes, which was blocked by
SPD304, PD169316 or p38 (MAPK) siRNA. Fig. 4 also demonstrates that the p38
siRNA knocked down >70% of endogenous p38 MAPK expression. Taken
together, the results indicate that TNF-α induces ADAMTS-4
expression and activity in human osteoarthritic chondrocytes at the
transcriptional level via TNFR1 by a p38 MAPK-dependent
mechanism.
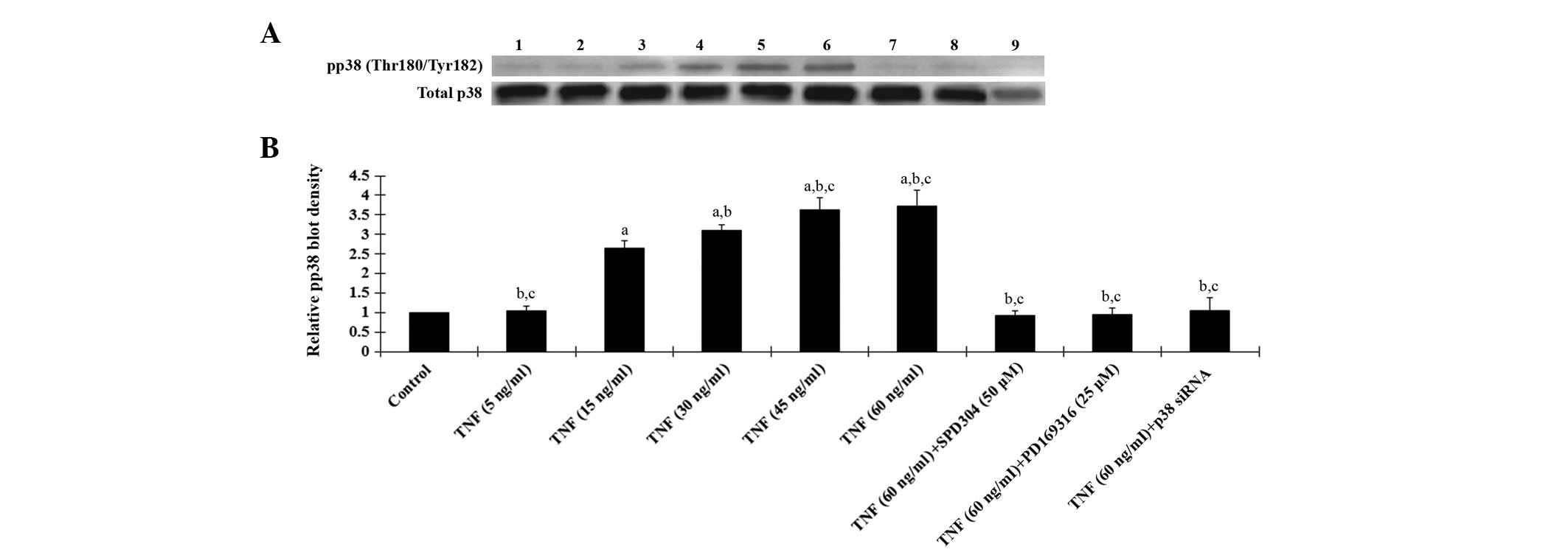 | Figure 4Western blot analysis of
phosphorylated p38 MAPK levels in human osteoarthritic
chondrocytes. (A) The levels of phosphorylated p38 MAPK (pp38) and
total p38 MAPK in human osteoarthritic chondrocytes treated with
TNF-α (5, 15, 30, 45 and 60 ng/ml) with or without SPD304 (50 μm),
PD169316 (25 μm) or p38 (MAPK) siRNA for 18 h were analyzed with
western blot analysis. Lane 1, lysates from untreated human
osteoarthritic chondrocytes were used as a control; lane 2, TNF-α
(5 ng/ml); lane 3, TNF-α (15 ng/ml); lane 4, TNF-α (30 ng/ml); lane
5, TNF-α (45 ng/ml); lane 6, TNF-α (60 ng/ml); lane 7, TNF-α (60
ng/ml)+SPD304 (50 μm); lane 8, TNF-α (60 ng/ml) + PD169316 (25 μm);
lane 9, TNF-α (60 ng/ml) + p38 (MAPK) siRNA. (B) pp38
(Thr180/Tyr182) and total p38 MAPK levels were measured by
densitometry. The density of pp38 blot was normalized against that
of total p38 MAPK levels to obtain a relative density, which was
expressed as fold changes to that of untreated control cells
(designated as 1). aP<0.05 compared with untreated
control cells; bP<0.05 compared with TNF-α treatment
at 15 ng/ml; cP<0.05 compared with TNF-α treatment at
30 ng/ml. TNF-α, tumor necrosis factor-α; SPD304, TNFR1 inhibitor;
PD169316, p38 MAPK inhibitor; MAPK, mitogen activated protein
kinase. |
Discussion
Cartilage degradation in OA constitutes a major
structural change in the joint, which may severely impair its
function and cause pain and disability (16). Among the inflammatory mediators
associated with OA, TNF-α is an established key mediator for
cartilage (5). ADAMTS-4 is
believed to be important in the degradation of aggrecan during the
progression of joint diseases (17). In the present study, we provided,
to the best of our knowledge, the first evidence for a regulatory
effect of TNF-α on ADAMTS-4 expression and activity in human
osteoarthritic chondrocytes.
TNF-α is produced in a variety of cell types,
including macrophages, lymphocytes, fibroblasts and keratinocytes
in response to inflammation, infection and other environmental
stresses (6). TNF-α in the
concentration range of 1–100 ng/ml has been used in chondrocytes
in vitro(18). In the
present study, we used 5–60 ng/ml of TNF-α to determine whether
TNF-α is able to regulate ADAMTS-4 expression in osteoarthritic
chondrocytes. Within this testing concentration range, while TNF-α
at 5 ng/ml had no significant effect on ADAMTS-4 expression, a
statistically significant dose-dependent effect of TNF-α on
ADAMTS-4 expression was observed in the concentration range of 5–45
ng/ml and the effect reached a plateau in the range of 45–60 ng/ml.
TNF-α at a concentration as low as 15 ng/ml increased ADAMTS-4
expression by >2.5 fold within 18 h, suggesting that TNF-α is a
strong positive regulator of ADAMTS-4 expression.
The depletion of aggrecan in articular cartilage is
an early event in the pathogenesis of OA. ADAMTS-4 and ADAMTS-5 are
believed to be important in the degradation of aggrecan (17). Recent studies suggest that ADAMTS-4
may be the principal aggrecanase of aggrecan degradation in human
OA as it is selectively overexpressed in human OA cartilage and is
positively correlated with the degree of cartilage destruction,
whereas ADAMTS-5 is similarly expressed in normal and OA cartilages
(12,19). Indeed, we demonstrated that TNF-α
had no induction effect on ADAMTS-4 expression in normal human
chondrocytes. In addition, the effect of TNF-α on ADAMTS-5
expression was inconsistent in normal and osteoarthritic human
chondrocytes. Thus, we only selected ADAMTS-4 as the target of the
present study.
The main extracellular matrix macromolecules of the
articular cartilage are type II collagen and aggrecan and the
pathogenesis of OA involves the degradation of aggrecan and type II
collagen (20,21). The presence of TNF-α reportedly
correlates with the loss of type II collagen and aggrecan in OA
cartilage due to increased production of matrix metalloproteinases
(5). Aggrecan has a protective
effect against collagen degradation. Mechanistically, type II
collagen is exposed due to the degradation of aggrecan and the
exposed collagen becomes an easy target for enzymatic degradation
by collagenase (20,21). Since our findings demonstrate that
ADAMTS-4 is a downstream target of TNF-α signaling in human
osteoarthritic chondrocytes, the loss of aggrecan and type II
collagen caused by TNF-α in OA cartilage is at least partially
mediated by ADAMTS-4, besides matrix metalloproteinases. Further
studies are needed to explore this issue in vivo. Our
results demonstrated that TNF-α enhanced the ADAMTS-4 promoter
activity and increased the ADAMTS-4 mRNA level, suggesting
that TNF-α induced ADAMTS-4 expression at the transcriptional
level. The underlying transcriptional regulatory mechanisms aim to
be elaborated in our future studies.
In conclusion, we demonstrated that TNF-α induces
ADAMTS-4 expression and activity in human osteoarthritic
chondrocytes at the transcriptional level via TNFR1 by a p38
MAPK-dependent mechanism. To the best of our knowledge, this is the
first evidence of crosstalk between TNF-α and ADAMTS-4 in relation
to OA cartilage degradation, which adds novel insight into the
pathophysiology of OA and cartilage degradation.
References
|
1
|
Iannone F and Lapadula G: The
pathophysiology of osteoarthritis. Aging Clin Exp Res. 15:364–372.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldring MB and Goldring SR:
Osteoarthritis. J Cell Physiol. 213:626–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davidson RK, Waters JG, Kevorkian L, et
al: Expression profiling of metalloproteinases and their inhibitors
in synovium and cartilage. Arthritis Res Ther. 8:R1242006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burrage PS, Mix KS and Brinckerhoff CE:
Matrix metalloproteinases: role in arthritis. Front Biosci.
11:529–543. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SW, Song YS, Lee SY, et al:
Downregulation of protein kinase CK2 activity facilitates tumor
necrosis factor-α-mediated chondrocyte death through apoptosis and
autophagy. PLoS One. 6:e191632011.PubMed/NCBI
|
|
6
|
Englaro W, Bahadoran P, Bertolotto C, et
al: Tumor necrosis factor alpha-mediated inhibition of
melanogenesis is dependent on nuclear factor kappa B activation.
Oncogene. 18:1553–1559. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Westacott CI, Atkins RM, Dieppe PA and
Elson CJ: Tumor necrosis factor-alpha receptor expression on
chondrocytes isolated from human articular cartilage. J Rheumatol.
21:1710–1715. 1994.PubMed/NCBI
|
|
8
|
Wieland HA, Michaelis M, Kirschbaum BJ and
Rudolphi KA: Osteoarthritis - an untreatable disease? Nat Rev Drug
Discov. 4:331–344. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Porter S, Clark IM, Kevorkian L and
Edwards DR: The ADAMTS metalloproteinases. Biochem J. 386:15–27.
2005. View Article : Google Scholar
|
|
10
|
Sandy JD: A contentious issue finds some
clarity: on the independent and complementary roles of aggrecanase
activity and MMP activity in human joint aggrecanolysis.
Osteoarthritis Cartilage. 14:95–100. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song RH, Tortorella MD, Malfait AM, et al:
Aggrecan degradation in human articular cartilage explants is
mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum.
56:575–585. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naito S, Shiomi T, Okada A, et al:
Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic
cartilage. Pathol Int. 57:703–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Salter RC, Arnaoutakis K, Michael DR, et
al: The expression of a disintegrin and metalloproteinase with
thrombospondin motifs 4 in human macrophages is inhibited by the
anti-atherogenic cytokine transforming growth factor-β and requires
Smads, p38 mitogen-activated protein kinase and c-Jun. Int J
Biochem Cell Biol. 43:805–811. 2011.PubMed/NCBI
|
|
14
|
Li YP, Chen Y, John J, et al: TNF-alpha
acts via p38 MAPK to stimulate expression of the ubiquitin ligase
atrogin1/MAFbx in skeletal muscle. FASEB J. 19:362–370. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SE, Jin B and Li YP: TNF-alpha
regulates myogenesis and muscle regeneration by activating p38
MAPK. Am J Physiol Cell Physiol. 292:C1660–C1671. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manacu CA, Martel-Pelletier J, Roy-Beaudry
M, et al: Endothelin-1 in osteoarthritic chondrocytes triggers
nitric oxide production and upregulates collagenase production.
Arthritis Res Ther. 7:R324–R332. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
He Y, Zheng Q, Simonsen O, et al: The
development and characterization of a competitive ELISA for
measuring active ADAMTS-4 in a bovine cartilage ex vivo model.
Matrix Biol. 32:143–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim AH and Song WY: TNF-alpha-mediated
apoptosis in chondrocytes sensitized by MG132 or actinomycin D.
Biochem Biophys Res Commun. 295:937–944. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang E, Yan X, Zhang M, et al:
Aggrecanases in the human synovial fluid at different stages of
osteoarthritis. Clin Rheumatol. 32:797–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eyre D: Collagen of articular cartilage.
Arthritis Res. 4:30–35. 2002. View
Article : Google Scholar
|
|
21
|
Roughley PJ: Articular cartilage and
changes in arthritis: noncollagenous proteins and proteoglycans in
the extracellular matrix of cartilage. Arthritis Res. 3:342–347.
2001. View Article : Google Scholar : PubMed/NCBI
|