Introduction
Temporal lobe epilepsies (TLEs) are a group of
medical disorders that result in recurrent epileptic seizures
arising from one or both temporal lobes of the brain.
The presence of gap junction proteins termed
pannexins (Panx) was first reported by Panchin et al in 2000
(1). These proteins release large
signaling molecules, including ATP and arachidonic acid
derivatives. Three subtypes of the Panx family have been identified
as Panx1, 2 and 3 (2,3). Human and mouse Panx1 mRNA is
ubiquitously expressed in normal tissues of humans and mice,
respectively; however, human Panx2 is a brain-specific gene and
Panx3 is expressed predominantly in osteoblasts and synovial
fibroblasts in in silico evaluation. Furthermore, Panx1 has
been shown to form functional hemichannels by itself or in
conjunction with Panx2 (1,4).
Panx1 channel opening has been indicated to
contribute to epileptiform seizure activity. Thompson et
al(5) identified that Panx1
hemichannel opening is triggered by N-Methyl-D-aspartate
stimulation and may be a significant target for the treatment of
epilepsy. Furthermore, it has been demonstrated that reduced levels
of extracellular glucose due to fasting or adhering to a ketogenic
diet may induce Panx1 hemichannel-mediated ATP release from CA3
neurons (6). This in turn
hyperpolarizes neuronal membrane potentials via ATP-sensitive
potassium channels. The role of Panx2 in the pathology of epilepsy
is less certain than that of Panx1. However, since Panx2 may
participate in the functional hemichannels with Panx1, this subtype
was also included in the present study in order to investigate
whether the expression pattern changed in epileptic brain
tissues.
To the best of our knowledge, the current study
compared for the first time the expression pattern of Panx1 and
Panx2 in brain tissues in patients with TLE and non-epileptic
patients using immunohistochemistry and western blotting
techniques, to aid in our further understanding of the pathogenesis
of TLE.
Patients and methods
Patient selection
Epileptic brain tissue was obtained from 37 patients
with TLE and from nine control patients with traumatic brain injury
(Tables I and II) (7).
 | Table IData for patients with temporal lobe
epilepsy. |
Table I
Data for patients with temporal lobe
epilepsy.
| Sample | Age (years) | Gender | Disease duration
(years) | Lobectomya | Types of
seizures |
|---|
| E1 | 22 | M | 14 | L | CPS, SGS |
| E2 | 27 | M | 21 | R | CPS, SGS |
| E3 | 28 | M | 12 | R | CPS, SGS |
| E4 | 20 | M | 18 | R | SPS, SGS |
| E5 | 27 | M | 4 | R | CPS |
| E6 | 39 | F | 37 | R | CPS, SGS |
| E7 | 20 | M | 8 | R | CPS, SGS |
| E8 | 30 | M | 18 | L | CPS, SGS |
| E9 | 28 | F | 12 | L | CPS, SGS |
| E10 | 27 | M | 11 | R | SPS, SGS |
| E11 | 32 | F | 4 | L | CPS |
| E12 | 26 | M | 3 | R | CPS |
| E13 | 17 | M | 7 | L | SPS, SGS |
| E14 | 22 | M | 18 | R | CPS |
| E15 | 25 | M | 8 | R | GTCS, SPS |
| E16 | 29 | M | 8 | R | GTCS, TS |
| E17 | 32 | M | 4 | L | CPS |
| E18 | 20 | M | 8 | R | CPS, SGS |
| E19 | 25 | M | 6 | R | GTCS, TS |
| E20 | 17 | M | 4 | L | CPS, SGS |
| E21 | 24 | M | 3 | L | SPS, SGS |
| E22 | 23 | M | 20 | L | CPS, SGS |
| E23 | 29 | M | 25 | R | GTCS, SPS |
| E24 | 30 | M | 2 | L | CPS |
| E25 | 18 | F | 7 | L | CPS |
| E26 | 30 | F | 12 | R | SPS, SGS |
| E27 | 22 | M | 10 | L | SPS, SGS |
| E28 | 30 | M | 5 | L | SPS, SGS |
| E29 | 24 | F | 14 | R | CPS, SGS |
| E30 | 22 | M | 17 | R | SPS, SGS |
| E31 | 32 | F | 28 | R | SPS, SGS |
| E32 | 23 | M | 12 | R | SPS, SGS |
| E33 | 21 | M | 17 | R | CPS, SGS |
| E34 | 35 | F | 20 | R | CPS, SGS |
| E35 | 31 | F | 10 | L | SPS, SGS |
| E36 | 32 | M | 20 | L | CPS, SGS |
| E37 | 34 | F | 21 | L | SPS, SGS |
 | Table IIData for patients used as
controls. |
Table II
Data for patients used as
controls.
| Sample | Age (years) | Gender | Lobectomya | Etiological
diagnosis | Adjacent tissue
pathology |
|---|
| C1 | 39 | M | L | Trauma | Normal |
| C2 | 32 | F | R | Trauma | Normal |
| C3 | 28 | M | R | Trauma | Normal |
| C4 | 40 | M | L | Trauma | Normal |
| C5 | 22 | F | R | Trauma | Normal |
| C6 | 27 | M | L | Trauma | Normal |
| C7 | 21 | M | R | Trauma | Normal |
| C8 | 10 | M | L | Trauma | Normal |
| C9 | 34 | F | R | Trauma | Normal |
The patients with TLE had typical clinical
manifestations and characteristic electroencephalography (EEG)
findings. The diagnosis of the seizure type was confirmed according
to the 1981 International Classification of Epileptic Seizures of
the International League Against Epilepsy (8). Prior to surgery, the epileptic
lesions were located in all patients by brain magnetic resonance
imaging (MRI) and 24-h or video-EEG recordings. Sphenoidal
electrode monitoring and intraoperative electrocorticography were
performed to localize the epileptic lesion prior to resection.
For the control experiments, temporal lobe tissue
from nine patients with traumatic brain injury was obtained from
the files of the Department of Neurosurgery of Xiangya Hospital
(Changsha, China). These patients had undergone surgery due to
severe brain trauma and had no history of epilepsy or other
abnormal pathology and exposure to anti-epileptic drugs. The
samples comprised temporal neocortical tissue adjacent to the
trauma-induced lesion.
Written informed consent was obtained from the
patients or their relatives with regard to the use of data and
tissues for the purpose of research studies. The study complied
with guidelines for the conduct of research involving human
subjects as established by the National Institute of Health and the
Committee on Human Research at Xiangya Hospital.
Tissue processing
The brain tissue from the epileptic patients and the
patients with traumatic brain injury was obtained during surgery.
Following dissection, the specimens were divided into two portions.
One portion was immediately frozen in liquid nitrogen, stored at
−80°C and subsequently used for western blotting. The other portion
was fixed in 4% paraformaldehyde, embedded in paraffin blocks, cut
into 5.0-μM transverse sections on a Model RM2135 microtome (Leica,
Wetzlar, Germany) and preserved for future use at room
temperature.
Immunohistochemistry
Temporal lobe tissue sections of patients with
epilepsy were processed for Panx immunohistochemistry using
avidin-biotin peroxidase methods. Following dewaxing and
rehydration, endogenous peroxidases were inactivated by adding 1%
H2O2 for 30 min. The sections were rinsed in
0.01 M phosphate-buffered saline (PBS) and incubated in 10% normal
goat serum in PBS for 2 h to reduce any non-specific binding.
The sections were then incubated overnight with a
Panx antiserum at 4°C. Panx1 (1:150, rabbit polyclonal antibody;
Abcam, Cambridge, UK) and Panx2 (1:250, rabbit polyclonal antibody;
Abcam) antisera were diluted in PBS containing 2% normal goat
serum. Following rinsing, the sections were incubated in
biotinylated secondary antiserum (rabbit anti-goat IgG, 1:200;
Vector Laboratories, Burlingame, CA, USA) at room temperature for 1
h. Following a further rinsing, the sections were incubated in
avidin-biotin peroxidase complex (1:200; Vectastain Elite ABC;
Vector Laboratories) in PBS for 1 h.
To visualize the peroxidase labeling, the sections
were processed with 0.06% diaminobenzidine tetrahydrochloride and
0.006% H2O2 diluted in 0.075 M PBS for 10
min. Following rinsing, the sections were dehydrated and placed
under a cover slip. Visual field images were obtained layer by
layer from every section using an Nikon TE2000 automatic microscope
(Nikon, Tokyo, Japan) and Nikon DS-Fi1 (Nikon) pathology system.
The full featured photo of the cortex was captured by PT Gui Pro
9.0.4 (New House Internet Services B.V., Rotterdam, The
Netherlands).
Western blotting
A western blot analysis was performed to compare
Panx immunoreactivity in the TLE and control groups. Tissue samples
were cut into small sections, homogenized in
radio-immunoprecipitation assay lysis buffer [0.05 mol/l Tris-HCl
(pH 7.4), 0.15 mol/l NaCl, 1% Triton X-100, 1% (w/v) sodium
deoxycholate and 0.1% sodium dodecyl sulfate (SDS)], including 1 mM
phenylmethylsulfonyl fluoride, and centrifuged at 12,000 × g at 4°C
for 5 min. The protein concentration of the lysates was determined
using a bicinchoninic acid protein assay kit (Pierce Biotechnology,
Rockford, IL, USA). The extracts (40 μg) were resolved by 10%
SDS-polyacrylamide gel electrophoresis and electrotransferred to a
polyvinylidene difluoride (PVDF) membrane. The PVDF membranes were
divided into two sections according to the location of molecular
weight markers to detect Panx2 (69 kDa) and β-actin (42 kDa), which
was used as a loading control. The PVDF membrane was blocked with
5% milk prepared in tris-buffered saline with 0.05% Tween-20 (TBST)
then incubated for 1 h at room temperature.
Following extensive washing with TBST, the membranes
were incubated in Panx2 (1:200, rabbit polyclonal antibody, Abcam,
UK) and β-actin (mouse monoclonal IgG, 1:1,000; Santa Cruz
Biotechnology, Santa Cruz, CA, USA) antibodies for 12 h at 4°C.
Following three 10-min washes with TBST, each blot was incubated
with a secondary antibody (goat anti-rabbit IgG for Panx2 blot and
goat anti-mouse IgG for β-actin blot; Beyotime Company, Haimen,
China) at a dilution of 1:1,000 for 1 h. Following three further
washes with TBST and one wash with TBS (15 min each), the Panx2
protein and β-actin were detected using an enhanced
chemiluminescence detection kit according to the manufacturer’s
instructions (Thermo Scientific Pierce, Waltham, MA, USA).
Panx1 was assayed by incubating the PVDF membranes
with β-actin in stripping buffer [700 μl 14.4 mmol/l
β-mercaptoethanol, SDS 1 g, 0.5 mol/l Tris-HCl (pH 6.8) and 12.5 ml
in 100 ml ultra-pure water] for 30 min. The blot was then
re-hybridized with the anti-Panx1 primary antibody (1:100, rabbit
polyclonal antibody; Abcam) for 12 h at 4°C. Following washing with
TBST three times, the blot was incubated with goat anti-rabbit IgG
(1:1000, Beyotime Company). Following washing three times with TBST
and once with TBS, the Panx1 level was detected by enhanced
chemiluminescence as described.
Statistical analysis
Statistical analysis was performed using SPSS
(version 17.0; SPSS, Inc., Chicago, IL, USA). Data were presented
as the mean ± SD and any differences between active and control
groups were determined using χ2 tests and t-tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
No significant differences were identified in the
ratios of age, gender and lobectomy among the two groups of
patients who donated tissues for the study (Table III). The TLE group included 27
male and 10 female patients with a mean age of 26.3 years (range,
17 to 39 years). The duration of epilepsy (time since onset of
symptoms) ranged from 2 to 37 years (mean, 12.6 years). In total,
26 cases were categorized as partial seizures, secondarily
generalized as tonic, clonic or tonic-clonic seizures, seven cases
as complex partial seizures and four cases as other seizure types.
The control group included six male and three female patients with
brain trauma, between 10 and 40 years of age (mean age, 28.1
years).
 | Table IIIClinical characteristics of the
subjects. |
Table III
Clinical characteristics of the
subjects.
| TLE group | Control group | P-value |
|---|
| Age | 26.29±5.33 | 28.11±9.53 | 0.443b |
| Gender |
| Male | 27 | 6 | 0.706c |
| Female | 10 | 3 | |
| Lobectomya |
| Left | 16 | 4 | 0.948c |
| Right | 21 | 5 | |
Panx expression
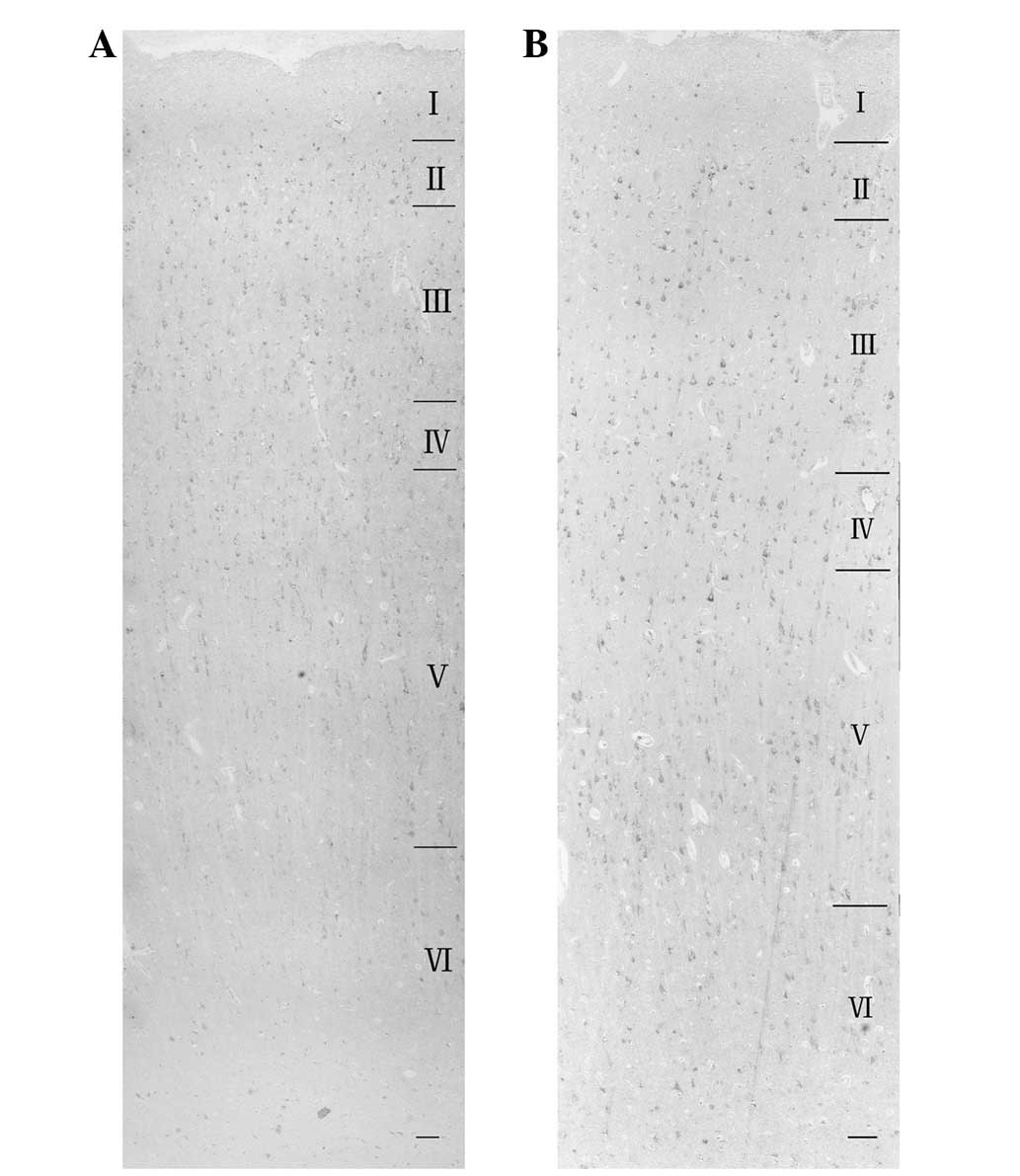
Panx1 and Panx2 expression was detected in the
temporal lobe cortex of patients with TLE and in the control
tissues. In the control group, Panx1 and Panx2 proteins were
expressed predominantly in formation layers II and III of the
cortex of the temporal lobe formation (Fig. 1). In the TLE group, Panx1 and Panx2
were expressed in all the six layers of the epileptic cortex, but
less in layer I than in the other five layers (Fig. 2).
 | Figure 1Microphotographs of
immunohistochemistry. Distinct localization of Panx1 and Panx2 in
layer III of control (A, B, E, F) and epileptic (C, D, G, H)
temporal lobe cortex. The pyramidal cell bodies highly expressed
Panx1 and Panx2 (A–D) in layer III of the temporal lobe in both
groups. Scale bar, (A, C, E and G) 50 μm; (B, D, F and H) 10 μm.
Pannexin, Panx. |
At a higher magnification, the cell bodies of
pyramidal cells were shown to be immunoreactive for Panx1 (Fig. 1B and D) and Panx2 (Fig. 1F and H) in layer III. In the TLE
group, the nuclei of several small-sized cells exhibited moderate
immunoreactivity (Fig. 1D and
F).
The expression level of Panx protein in the brain
tissue of the TLE patients was subject to semi-quantitative
analysis using western blotting (Fig.
3A). The variance between the two groups of patients was
examined by calculating the ratio of the optical density of Panx to
β-actin (Fig 3B and C). The
Panx1/β-actin ratio was 0.80±0.01 for the TLE samples and 0.39±0.08
for the control samples (P<0.05). Thus strong upregulation of
Panx1 immunoreactivity was present in all samples from TLE patients
compared with the control group.
The Panx2/β-actin ratio of the TLE samples was
0.67±0.02 and the corresponding ratio for the control samples was
0.75±0.05. This difference was not statistically significant
(P>0.05).
Discussion
In the present study, the expression patterns of
Panx1 and Panx2 in the brain tissues of epileptic patients and
controls were compared. To the best of our knowledge, this was the
first time changes in Panx expression have been studied in human
epileptic brains.
Panx1 and Panx2 are expressed in the central nervous
system (1,4,9,10).
The expression of Panx1, but not of Panx2, in Xenopus
oocytes forms functional hemichannels (1,4). In
addition, coinjection of these two Panx RNAs has been shown to
result in hemichannels with functional properties that vary from
those formed by Panx1 only. The functional characteristics of
homomeric Panx1 versus heteromeric Panx1/Panx2 channels, and the
varying expression patterns of Panx1 and Panx2 in the brain,
indicate that Panx form cell type-specific channels that have
distinct properties and that may serve different functions.
In the present study, it was demonstrated that the
Panx1 and Panx2 proteins were expressed predominantly in layers II
and III of the cortex in the control group. Previous studies have
shown that Panx1 and Panx2 mRNAs are present in all regions of the
cerebral cortex of adult rats; however, the intensity of expression
varies in the individual layers. For example, cells in layers
II/III and V have been shown to exhibit a stronger signal for Panx
mRNAs than those in layers I, IV and VI (4). This was not quite consistent with the
results of the present study in the control groups. This apparent
difference between the results was likely to be due to two reasons.
The first was that the subjects studied varied; one subject was a
rat while the other was human. The second reason may be due to
different methods being adopted; the previous study conducted in
situ hybridization in an animal, while the present study used
immunohistochemistry and western blotting in patients.
Cortical neurons are important in epilepsy. Layers
III, V and VI of the cortex are the layers in which pyramidal cells
are predominantly distributed and from which association fibers
arise, and layer IV is the main target of thalamocortical afferents
from thalamic type C neurons and from intra-hemispheric
corticocortical afferents. Increased excitability and inward
rectification in layer V cortical pyramidal neurons have been
recorded in epileptic mutant mice (11). In chronically injured epileptogenic
rats, excitatory synaptic connectivity is enhanced in layer V
pyramidal neurons. This is accompanied by increased total axonal
length and increased density of synaptic boutons (12). There is also evidence that
epileptic discharges may be initiated from layer V and VI neurons
(13). In the present study, Panx1
and Panx2 were expressed in all six layers of the epileptic cortex,
with the lowest expression observed in layer I. Ectopic expression
of Panx in the epileptic cortex indicates that Panx proteins may be
involved in the function of neurons in layers IV, V and VI of the
epileptic cortex.
The study of Panx1, as shown by western blot
analysis, confirmed previous results obtained with
Co2+-treated brain slices (14). However the Panx2 findings are at
variance. With the Co2+-treated brain slices there was a
1.5-fold increase in Panx1 and a 1.4-fold increase in Panx2 mRNA;
significant post-translational modifications of Panx1 protein were
also observed after Co2+ treatment. While the present
study identified significantly increased Panx1 levels in the TLE
group, the change in Panx2 protein levels was not significant. With
the exception of the difference between the species of the subjects
studied and the research methods, this inconsistency was most
likely due to the choice of the brain slice model vs. TLE patients
to detect changes in Panx expression, which possibly reflects
differences in the functional mechanisms of Panx in TLE and in
acute seizure activity induced by Co2+. Therefore,
further studies are required to verify the most likely
mechanism.
These experiments provide the first evidence for the
expression of Panx protein in temporal lobe tissues of patients
with TLE. The present study observed increased Panx1 expression and
the ectopic expression of Panx1 and Panx2 in temporal lobe tissue
in epilepsy. These results may be associated with the functional
changes of cells in the epileptic cortex. The results support the
conclusion that Panx channels are important in the formation of
epilepsy.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81071048, 81100967
and 81000553) and the Specialized Research Fund for the Doctoral
Program of Higher Education (grant no. 20110162120002). The authors
would like to thank the patients and their families for their
participation in this study. The authors would also like to
sincerely thank The Second Xiangya Hospital, The Second People’s
Hospital of Hunan Province for their assistance in the brain tissue
procurement, and the local ethics committee for their support.
Moreover, the authors are grateful to Jinghui Liang and Zhiguo Wu
for their technical assistance.
References
|
1
|
Panchin Y, Kelmanson I, Matz M, Lukyanov
K, Usman N and Lukyanov S: A ubiquitous family of putative gap
junction molecules. Curr Biol. 10:R473–474. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bruzzone R, Hormuzdi SG, Barbe MT, Herb A
and Monyer H: Pannexins, a family of gap junction proteins
expressed in brain. Proc Natl Acad Sci USA. 100:13644–13649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bruzzone R, White TW and Paul DL:
Connections with connexins: the molecular basis of direct
intercellular signaling. Eur J Biochem. 238:1–27. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
White TW and Paul DL: Genetic diseases and
gene knockouts reveal diverse connexin functions. Annu Rev Physiol.
61:283–310. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vogt A, Hormuzdi SG and Monyer H:
Pannexin1 and Pannexin2 expression in the developing and mature rat
brain. Brain Res Mol Brain Res. 141:113–120. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thompson RJ, Jackson MF, Olah ME, et al:
Activation of pannexin-1 hemichannels augments aberrant bursting in
the hippocampus. Science. 322:1555–1559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawamura M Jr, Ruskin DN and Masino SA:
Metabolic autocrine regulation of neurons involves cooperation
among pannexin hemichannels, adenosine receptors, and KATP
channels. J Neurosci. 30:3886–3895. 2010. View Article : Google Scholar
|
|
8
|
No authors listed. Proposal for Revised
Clinical and Electroencephalographic Classification of Epileptic
Seizures. Epilepsia. 22:489–501. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuan J, Wang LY, Li JM, et al: Altered
expression of the small guanosine triphosphatase RhoA in human
temporal lobe epilepsy. J Mol Neurosci. 42:53–58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zappalà A, Cicero D, Serapide MF, et al:
Expression of pannexin1 in the CNS of adult mouse: cellular
localization and effect of 4-aminopyridine-induced seizures.
Neuroscience. 141:167–178. 2006.PubMed/NCBI
|
|
11
|
Zappalà A, Li Volti G, Serapide MF, et al:
Expression of pannexin2 protein in healthy and ischemized brain of
adult rats. Neuroscience. 148:653–667. 2007.PubMed/NCBI
|
|
12
|
Di Pasquale E, Keegan KD and Noebels JL:
Increased excitability and inward rectification in layer V cortical
pyramidal neurons in the epileptic mutant mouse Stargazer. J
Neurophysiol. 77:621–631. 1997.PubMed/NCBI
|
|
13
|
Jin X, Prince DA and Huguenard JR:
Enhanced excitatory synaptic connectivity in layer v pyramidal
neurons of chronically injured epileptogenic neocortex in rats. J
Neurosci. 26:4891–4900. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Polack PO, Guillemain I, Hu E, Deransart
C, Depaulis A and Charpier S: Deep layer somatosensory cortical
neurons initiate spike-and-wave discharges in a genetic model of
absence seizures. J Neurosci. 27:6590–6599. 2007. View Article : Google Scholar
|
|
15
|
Mylvaganam S, Zhang L, Wu C, et al:
Hippocampal seizures alter the expression of the pannexin and
connexin transcriptome. J Neurochem. 112:92–102. 2010. View Article : Google Scholar : PubMed/NCBI
|