Introduction
White adipose tissue (WAT) was, until recently, only
regarded as a predominant site of energy storage with important
roles in the control of energy homeostasis. However, WAT is now
considered to be a predominant endocrine organ in humans, secreting
a wide range of biologically active molecules, including numerous
adipokines (1). A number of these
adipokines, which are predominantly produced by WAT, exhibit the
ability to modulate metabolic and inflammatory processes and are
hypothesized to contribute to the pathophysiology of obesity-linked
diseases (2). In particular,
articular adipose tissue (AAT) is highly reactive, secreting
considerable quantities of pro-inflammatory and anti-inflammatory
cytokines and classical adipokines when stimulated (3). Thus, all the predominant
adipose-derived products, termed adipokines, participate in
inflammation and immunity. These include leptin, adiponectin,
visfatin and resistin.
Adipokine levels are elevated in the sera and
synovial fluids of patients with rheumatoid arthritis (RA). This
suggests that adipokines are involved in the pathogenesis of RA by
exerting potent modulatory effects on target tissues and cells
involved in rheumatic disease, including cartilage, synovium, bone,
and various immune cells (4,5).
Adiponectin also induces the in vitro production of
pro-inflammatory cytokines, including interleukin (IL)-6, matrix
metalloproteinase (MMP)-1 and IL-8 from RA synovial fibroblasts
(6,7). Thus, adiponectin is hypothesized to
exert significant pro-inflammatory and matrix-degrading effects.
Leptin stimulates T cell-mediated immunity, cytokine release from
monocytes/macrophages and the differentiation of hematopoietic
cells (8). Visfatin is a novel
mediator of innate immunity. It is key in the persistence of
inflammation through its capacity to inhibit neutrophil apoptosis
(9). Furthermore, visfatin
activates transcription factors, including nuclear factor (NF)-κB
and activator protein (AP)-1 and induces the production of IL-6,
IL-8, MMP-1 and MMP-3 in RA synovial fibroblasts and IL-6 and tumor
necrosis factor (TNF) in monocytes (10). Human resistin also has
pro-inflammatory functions, including the activation of
NF-κB-dependent pathways to produce TNF-α, IL-6 and IL-1β in human
peripheral blood mononuclear cells (11). It is widely accepted, that
adipokines are important in the pathogenesis of RA, yet the in
vivo role of adipokines and their association with disease
activity is poorly understood. Additional insight into the role of
adipokines may aid in the development of novel therapeutic agents.
In this study, the modulation of serum adipokines (adiponectin,
leptin, resistin and visfatin) was evaluated depending on RA
disease activity or the type of therapy, including
disease-modifying antirheumatic drugs (DMARDs) and TNF-α
blockers.
Patients and methods
Patients
A total of 40 RA patients (32 females and 8 males;
mean age ± SD, 45.2±13.6 years), who fulfilled the 1987 revised
criteria of the American College of Rheumatology (ACR) for RA
(12), were evaluated prior to and
six months following RA therapy (Table
I). All the patients were consecutively admitted to the
outpatient clinic of the Rheumatology Division of the Yonsei
University Hospital (Seoul, Korea) and were treated with DMARDs:
Monotherapy or combination therapy with methotrexate, sulfasalazine
and hydroxychloroquine. In addition, 16 patients were also treated
with TNF-α blockers [such as eternacept (Enbrel®;
Pfizer, New York, NY, USA), infliximab (Remicade®;
Janssen Biotech, Horsham, PA, USA), adalimumab (Humira®;
Abbott Laboratories, Chicago, IL, USA)]. Rheumatoid factor (RF) and
anti-cyclic citrullinated peptide antibodies (anti-CCP) were
measured at the baseline. Disease activity was assessed according
to the disease activity score in 28 joints (DAS28) based on
C-reactive protein level (CRP) at baseline and six months following
treatment (13). Health-related
quality of life was evaluated using the patient-reported Health
Assessment Questionnaire disability index (HAQ DI) (14). The therapeutic response was
evaluated six months following treatment, according to the ACR
response criteria (15). Patients
were categorized as responders or non-responders, on the basis of
fulfilling the ACR 20% improvement criteria (achieving an ACR20
response) six months following treatment. Serum samples were
collected prior to (baseline) and six months following therapy. The
present study was conducted in accordance with the recommendations
of the Declaration of Helsinki and was approved by the
Institutional Review Board of the Yonsei University Hospital. All
the patients provided written informed consent.
 | Table IClinical and demographic
characteristics of the study population. |
Table I
Clinical and demographic
characteristics of the study population.
| Characteristics | All RA patients | Responders | Non-responders |
|---|
| Patients, n (%) | 40 | 30 (75) | 10 (25) |
| Mean age ± SD
(years) | 45.2±13.6 | 45.4±14.2 | 44.5±12.2 |
| Gender
(female/male) | 32/8 | 24/6 | 8/2 |
| Disease duration,
months (range) | 5.2 (1–12) | 5.5 (1–12) | 4.6 (2–8) |
| RF-positive, n
(%) | 38 (95) | 28 (93) | 10 (100) |
| Anti-CCP positive, n
(%) | 36 (90) | 27 (90) | 9 (90) |
| Baseline DAS28
score | 5.38±1.0 | 5.65±0.9a | 4.54±0.7 |
| HAQ-DI score | 0.96±0.5 | 1.03±0.5 | 0.75±0.6 |
| Anti-TNF-α blocker
use, n (%) | 16 (40) | 15 (50) | 1 (10) |
Measurement of serum adipokine levels by
ELISA
For the assessment of adiponectin, leptin, resistin
and visfatin levels in serum, the serum was diluted with diluent
buffer (BD OptEIA; BD Bioscience, San Diego, CA, US) for the proper
detection range with ELISA, according to the manufacture’s
protocol. The adipokine levels in serum were measured using a
commercial ELISA kit. Adiponectin and leptin ELISA kits were
purchased from R&D Systems (Minneapolis, MN, USA). Resistin and
visfatin ELISA kits were purchased from Millipore (Billerica, MA,
USA) and BioVision (Mountain View, CA, USA), respectively.
Statistical analysis
Serum adipokine levels at baseline DAS28 were
compared using the Mann-Whitney U test (two-tailed). Pre- and
post-treatment levels were compared using a Wilcoxon signed rank
test (two-tailed). Data are expressed as the mean ± SD. The
differences between the groups were compared using the Mann-Whitney
U test (two-tailed). Prism software 4 (Graphpad Software, San
Diego, CA, USA) was used for statistical analysis and graphing.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline demographics and clinical
characteristics
As shown in Table
I, the majority of patients were female and had active disease
(DAS28 mean ± SD, 5.38±1.0) prior to therapy. Patients were
recently diagnosed with RA, having a mean disease duration of 5.2
months. Following DMARD and/or TNF-α blocker therapy, 30 (75%)
patients were responders and 10 (25%) were non-responders. Of the
responders, 15 were treated with DMARDs only, whereas another 15
were treated with DMARDs followed by a TNF-α blocker. Baseline
demographics and clinical characteristics were similar between
responders and non-responders, with the exception of the baseline
DAS28 score, which was significantly higher in the 30 responders
than in the 10 non-responders (5.65±0.9 vs. 4.54±0.7; P<0.05).
Due to a relatively higher disease activity, a greater number of
patients in the responder group received TNF-α blocker therapy
compared with the non-responder group.
Effect of disease activity on serum
adipokine levels
To determine how adipokine levels are regulated in
patients with RA depending on disease activity, serum was collected
prior to (at baseline) and six months following therapy. Adipokine
levels in the sera of patients with moderate disease activity on
the basis of DAS28 were not significantly different from that of
patients with high disease activity (Fig. 1). Thus, the change in serum
adipokine levels prior to and following the therapeutic treatment
of patients, who responded well to DMARD and/or TNF-α blocker
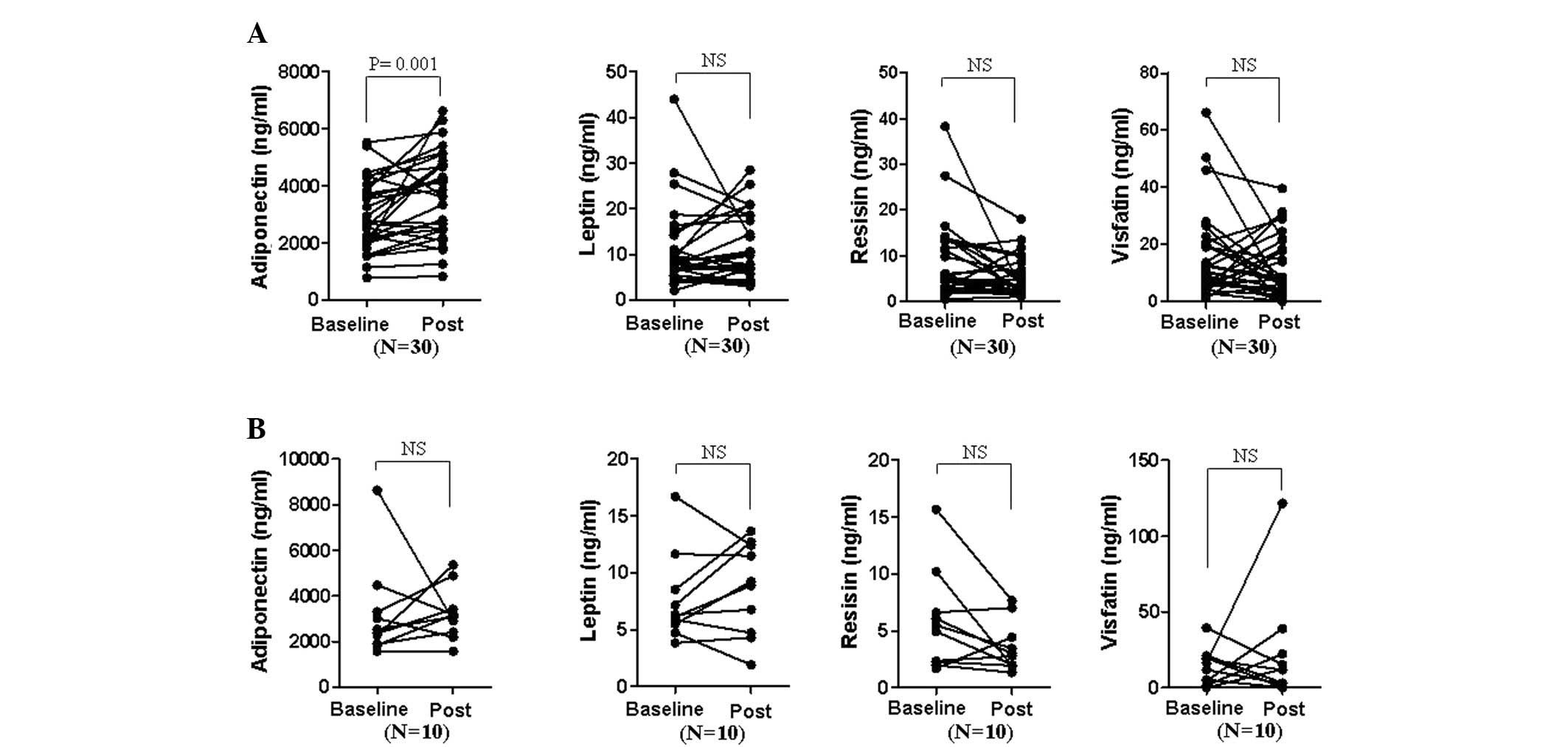
treatment in terms of ACR20 was examined (Fig. 2). Adiponectin levels were
significantly elevated in responders subsequent to treatment (mean
± SD; from 2,964±1,237 to 3,683±1,511 ng/ml, P=0.001), while
adiponectin levels in non-responders revealed no statistically
significant difference (3,192±2,090 to 3,222±1,150). By contrast,
leptin, resistin and visfatin levels were not significantly changed
in either responders or non-responders.
Effect of the type of therapeutic agent
on serum adipokine levels
To determine whether the type of therapeutic agent
differentially affects adipokine levels, the responder group was
subdivided into patients receiving only DMARDs and patients treated
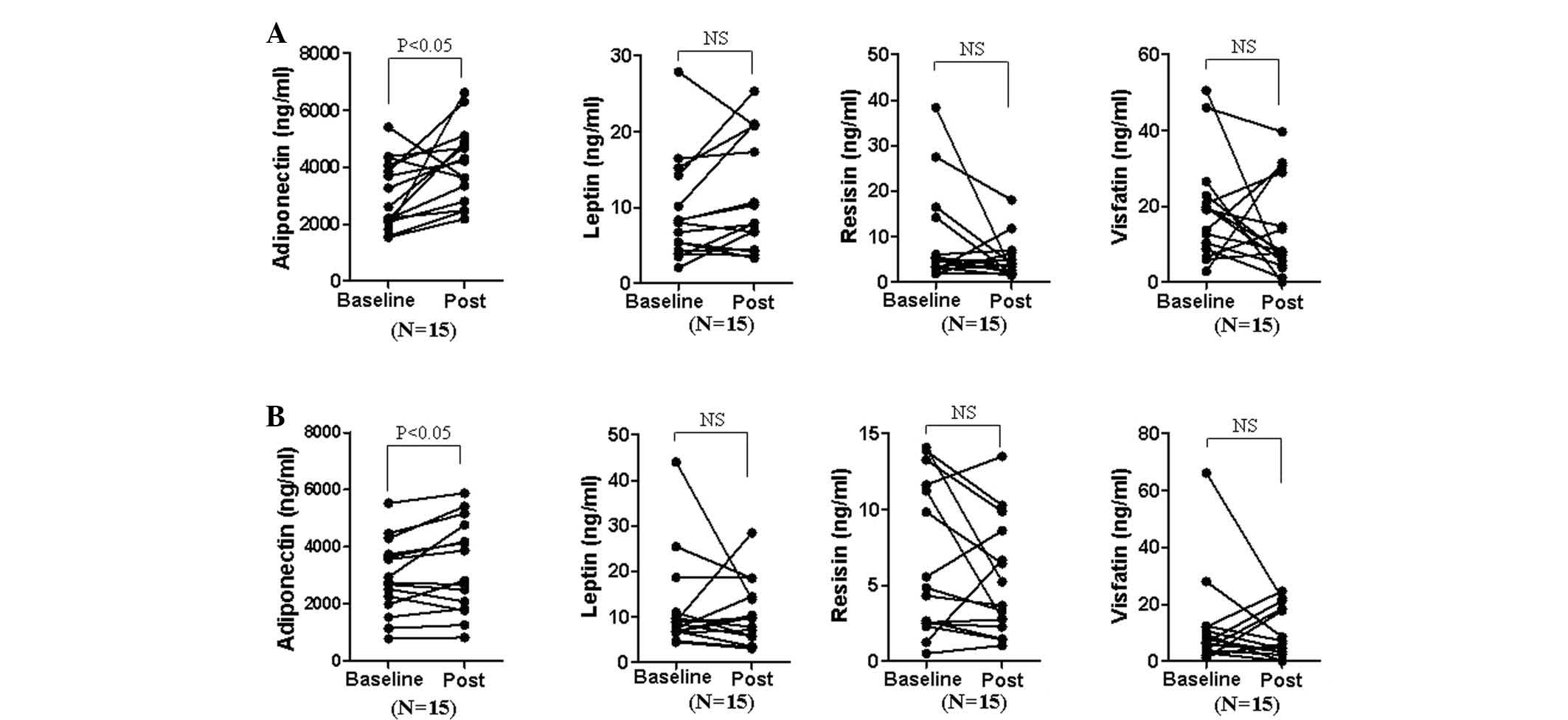
with DMARDs followed by TNF-α blockers (Fig. 3). Adiponectin levels in the DMARD
only group (3,008±1,209 to 4,089±1,345 ng/ml) showed a greater
increase compared with those of the DMARD+TNF-α blocker group
(2,921±1,305 to 3,278±1,603 ng/ml) following treatment, while other
adipokine levels were not significantly affected.
Effect of pre-treatment adipokine levels
on resistance to treatment
The effect of pre-treatment adipokine levels on
resistance to treatment was also investigated. No statistically
significant difference was identified in adipokine levels at
baseline between responders and non-responders, suggesting that the
responsiveness to therapeutic treatments was not affected by
adipokine levels (data not shown).
Discussion
In this study, the effects of RA disease activity
and type of therapeutic agent on serum adipokine levels was
evaluated, as well as the correlation between pre-treatment
adipokine levels and resistance to treatment. It was observed that
only the level of adiponectin was significantly increased following
therapeutic treatment, while other adipokine levels were not
identified to be significantly different. Increased adiponectin was
observed in patients treated with DMARDs alone or with a
combination of DMARDs and TNF-α blockers. However, the level of
adipokines at baseline was not significantly different between
patients with moderate versus high disease activity. This suggests
that moderate versus high RA disease activity does not
significantly affect adipokine expression, as the two patient
groups exhibited significant in vivo inflammation at
baseline.
Previous studies have demonstrated that adipokine
levels are affected by disease activity. DMARD treatment increased
serum adiponectin levels in RA patients (16). TNF-α blocker therapy also increased
adiponectin levels in a female RA patient three months following
treatment (17). Other studies
have shown that anti-TNF-α therapy significantly increases serum
adiponectin levels two and six weeks following therapy in RA
patients (18). Contradictory to
these results, another study reported that a short- or long-term
TNF-α blockade alone had no affect on circulating leptin and
adiponectin concentrations in RA patients, while patients treated
with anti-TNF-α and concomitant corticosteroids on a regular basis
demonstrated a significant decrease in adiponectin levels six
months after therapy (19).
Furthermore, visfatin was not associated with inflammation in
patients with severe RA undergoing anti-TNF-α therapy (20). Another study reported that
anti-TNF-α therapy resulted in a rapid reduction in serum resistin
levels, but did not modulate leptin levels in RA patients (21). It was also demonstrated that serum
leptin concentrations are inversely correlated with inflammation in
RA (22). The increased adipokine
levels post-treatment in these studies is concurrent with the
results of the present study; however, in order to clarify data
concerning additional adipokines, further studies are required.
Thus, it is currently difficult to delineate the behavior of these
adipokines during the course of the disease or the way in which
they are affected by treatment. In line with this, the resistin
level in the serum and joint fluid of patients with RA was
significantly higher compared with that in osteoarthritis (OA)
patients (23). Serum adiponectin
levels in RA patients were higher than those observed in the
healthy controls, although not significantly different from those
of OA patients (24). Serum
adiponectin levels in RA patients were significantly lower compared
with the synovial fluid levels, regardless of increased local
inflammation in the joint. A previous study has demonstrated that
serum adiponectin levels in RA patients are higher compared with OA
patients, which is contradictory to the findings mentioned
previously, stating that decreased disease activity or inflammation
increases serum adiponectin levels. In addition, leptin levels in
the joint fluid of patients with OA were significantly higher than
the serum levels (23).
Certain criticisms may be raised regarding the
parameters used for disease activity. In this study, the
correlation analysis focused on DAS28 and additional parameters
were not compared systematically. DAS28 may not be an ideal
parameter to analyze disease activity over time. It is usually
accepted that DAS28 is not an ideal parameter as it only focus on
28 joints excluding foot joints. Also, it focus on disease activity
but not radiographic damage. Thus, doctors still require more
developed parameters to evaluate disease degree. Thus, DAS28 in
combination with analysis of radiographic damage and other
parameters may be a useful application of adipokine levels with
regard to disease progression. Furthermore, additional parameters
to consider include: Age, body mass index (BMI) (with/without
normalization), disease duration, CRP, association with
RF/anti-CCP, proinflammatory cytokines, radiographic damage and
gender. Leptin levels demonstrate statistically significant
gender-specific differences (23),
while adiponectin is unrelated to age, disease duration, BMI or
disease activity in RA patients (24). In addition, systemic values of
metabolically relevant factors, such as adipokines, may not reflect
the situation in a specific organ or tissue under
pathophysiological conditions, including the joint.
Adiponectin with low molecular weight has
anti-inflammatory effects, whereas its globular form with high
molecular weight exhibits pro-inflammatory effects (25). The various adiponectin molecular
species are differentially generated depending on in vivo
physiological conditions, although no previous study has assessed
the ratios between these three forms in patients with RA. These
results indirectly indicate that inflammation may not be crucial
for the modulation of the in vivo adipokine expression
pattern of RA patients, and various unknown factors may be more
involved in the modulation of in vivo adipokine expression.
In order to evaluate the correlation between adipokine levels and
resistance to treatment, pre-therapeutic serum adipokine levels
were analyzed. As there was no statistically significant difference
between non-responders and responders, it was concluded that
pre-therapeutic adipokine levels do not contribute to resistance to
treatment.
In conclusion, serum adipokine levels in RA patients
were not significantly altered following therapy, with the
exception of adiponectin. The results suggest that serum adipokine
levels do not affect the degree of resistance to treatment and that
serum adipokine levels in RA patients may be affected by various
cell types and other unknown factors. Thus, it was hypothesized
that in RA, serum adipokine levels do not reflect and have a
limited effect on intraarticular inflammation.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science, and Technology
(grant nos. 2011-0026939 and 2011-0009061) and Korea Healthcare
Technology R&D Project, Ministry of Health and Welfare,
Republic of Korea (grant no. A102065).
References
|
1
|
Trayhurn P and Wood IS: Adipokines:
inflammation and the pleiotropic role of white adipose tissue. Br J
Nutr. 92:347–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wozniak SE, Gee LL, Wachtel MS and Frezza
EE: Adipose tissue: the new endocrine organ? A review article. Dig
Dis Sci. 54:1847–1856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kontny E, Plebanczyk M, Lisowska B, et al:
Comparison of rheumatoid articular adipose and synovial tissue
reactivity to proinflammatory stimuli: contribution to
adipocytokine network. Ann Rheum Dis. 71:262–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gomez R, Conde J, Scotece M, et al: What’s
new in our understanding of the role of adipokines in rheumatic
diseases? Nat Rev Rheumatol. 7:528–536. 2011.
|
|
5
|
Neumann E, Frommer KW, Vasile M and
Müller-Ladner U: Adipocytokines as driving forces in rheumatoid
arthritis and related inflammatory diseases? Arthritis Rheum.
63:1159–1169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ehling A, Schaffler A, Herfarth H, et al:
The potential of adiponectin in driving arthritis. J Immunol.
176:4468–4478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kitahara K, Kusunoki N, Kakiuchi T, Suguro
T and Kawai S: Adiponectin stimulates IL-8 production by rheumatoid
synovial fibroblasts. Biochem Biophys Res Commun. 378:218–223.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stofkova A: Leptin and adiponectin: from
energy and metabolic dysbalance to inflammation and autoimmunity.
Endocr Regul. 43:157–168. 2009.PubMed/NCBI
|
|
9
|
Luk T, Malam Z and Marshall JC: Pre-B cell
colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate
immunity. J Leukoc Biol. 83:804–816. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brentano F, Schorr O, Ospelt C, et al:
Pre-B cell colony-enhancing factor/visfatin, a new marker of
inflammation in rheumatoid arthritis with proinflammatory and
matrix-degrading activities. Arthritis Rheum. 56:2829–2839. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bokarewa M, Nagaev I, Dahlberg L, Smith U
and Tarkowski A: Resistin, an adipokine with potent proinflammatory
properties. J Immunol. 174:5789–5795. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arnett FC, Edworthy SM, Bloch DA, et al:
The American Rheumatism Association 1987 revised criteria for the
classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inoue E, Yamanaka H, Hara M, Tomatsu T and
Kamatani N: Comparison of Disease Activity Score (DAS)28-
erythrocyte sedimentation rate and DAS28- C-reactive protein
threshold values. Ann Rheum Dis. 66:407–409. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fries JF, Spitz P, Kraines RG and Holman
HR: Measurement of patient outcome in arthritis. Arthritis Rheum.
23:137–145. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Felson DT, Anderson JJ, Boers M, et al;
American College of Rheumatology. Preliminary definition of
improvement in rheumatoid arthritis. Arthritis Rheum. 38:727–735.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cansu B, Cansu DU, Kaşifoģlu T, Gülbas Z
and Korkmaz C: Disease-modifying antirheumatic drugs increase serum
adiponectin levels in patients with rheumatoid arthritis. J Clin
Rheumatol. 17:14–17. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewicki M, Kotyla P and Kucharz E:
Etanercept increases adiponectin level in woman with rheumatoid
arthritis. Clin Rheumatol. 27:1337–1338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komai N, Morita Y, Sakuta T, Kuwabara A
and Kashihara N: Anti-tumor necrosis factor therapy increases serum
adiponectin levels with the improvement of endothelial dysfunction
in patients with rheumatoid arthritis. Mod Rheumatol. 17:385–390.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Popa C, Netea MG, de Graaf J, et al:
Circulating leptin and adiponectin concentrations during tumor
necrosis factor blockade in patients with active rheumatoid
arthritis. J Rheumatol. 36:724–730. 2009. View Article : Google Scholar
|
|
20
|
Gonzalez-Gay MA, Vazquez-Rodriguez TR,
Garcia-Unzueta MT, et al: Visfatin is not associated with
inflammation or metabolic syndrome in patients with severe
rheumatoid arthritis undergoing anti-TNF-alpha therapy. Clin Exp
Rheumatol. 28:56–62. 2010.PubMed/NCBI
|
|
21
|
Gonzalez-Gay MA, Garcia-Unzueta MT,
Gonzalez-Juanatey C, et al: Anti-TNF-alpha therapy modulates
resistin in patients with rheumatoid arthritis. Clin Exp Rheumatol.
26:311–316. 2008.PubMed/NCBI
|
|
22
|
Popa C, Netea MG, Radstake TR, et al:
Markers of inflammation are negatively correlated with serum leptin
in rheumatoid arthritis. Ann Rheum Dis. 64:1195–1198. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Presle N, Pottie P, Dumond H, et al:
Differential distribution of adipokines between serum and synovial
fluid in patients with osteoarthritis. Contribution of joint
tissues to their articular production. Osteoarthritis Cartilage.
14:690–695. 2006. View Article : Google Scholar
|
|
24
|
Senolt L, Pavelka K, Housa D and Haluzik
M: Increased adiponectin is negatively linked to the local
inflammatory process in patients with rheumatoid arthritis.
Cytokine. 35:247–252. 2006. View Article : Google Scholar
|
|
25
|
Neumeier M, Weigert J, Schäffler A, et al:
Different effects of adiponectin isoforms in human monocytic cells.
J Leukoc Biol. 79:803–808. 2006. View Article : Google Scholar : PubMed/NCBI
|