Introduction
Hyperoxia exposure is a common therapeutic strategy
for patients with severe pulmonary diseases. However, prolonged
exposure to high concentrations of oxygen often causes acute and
chronic lung injury due to oxygen toxicity. Clinical studies
(1) have shown that hyperoxia
exposure is a significant risk factor for acute lung injury (ALI)
and bronchopulmonary dysplasia (BPD). Oxygen toxicity is
hypothesized to be mediated by the production and accumulation of
excessive reactive oxygen species (ROS), at levels exceeding the
capacity of the lung antioxidant defense mechanisms (2), leading to cell damage and death.
Therefore, inhibiting the oxygen toxicity or improving the survival
of epithelial cells may be a method of preventing hyperoxia lung
injury.
Hyperoxia exposure also stimulates C fibers and C
fiber activation induces the release of neuropeptide substance P
(SP) (3). SP binds primarily to
NK-1 receptors (NK-1R) (4) and
plays a role in regulating airway blood flow, airway smooth muscle
responses, airway inflammation and epithelial migration and cell
proliferation, including tracheal epithelial cells, following
injury (5,6). A previous in vitro study
showed that SP may attenuate hyperoxia-induced oxidative stress
injury, promote type II alveolar epithelial cell (AECII)
proliferation and inhibit apoptosis (7). However, whether SP is involved in or
has a positive effect in hyperoxia lung injury in vivo has
not been reported. The signaling mechanism underlying the
protective effect of SP against hyperoxia is poorly understood.
Previous studies demonstrated that sonic hedgehog
(SHH) plays a critical role in lung morphogenesis and lung
organogenesis (8,9). SHH regulates cell proliferation,
differentiation and migration in vitro and in vivo
(10–12). Therefore, the SHH signal
transduction pathways are hypothesized to play a role in the
regulatory mechanism of SP in hyperoxia-induced neonatal lung
injury. The aim of the current study was to investigate the effect
of SP on neonatal rat exposure to hyperoxia and to demonstrate the
related regulatory mechanism of SP.
Materials and methods
Experimental animals
Timed-pregnant specific-pathogen-free Sprague-Dawley
rats were obtained from the Experimental Animal Center of Chongqing
Medical University (Chongqing, China). The experiments were
conducted in accordance with the National Guidelines for the Care
and Use of Laboratory Animals. The experiment was approved by the
Ethics Committee of Chongqing Medical University.
Materials
SP was provided by Abcam (Cambridge, MA, USA).
Malondialdehyde (MDA), superoxide dismutase (SOD) assay and DAB
kits were provided by Kaiji Biological Company (Nanjing, China).
SHH polyclonal antibody was purchased from Abbiotec (San Diego, CA,
USA). RT and PCR kits and SYBR-Green I were provided by Shanghai
ShineGene Molecular Biotechnology Co., Ltd. (Shanghai, China).
Establishment of animal oxidative
model
The rats were housed in individual cages with free
access to water and laboratory chow and the rat pups were delivered
spontaneously. Neonatal rats were nested on softwood shavings and
distributed to litters of ten of equal body weight in Plexiglas
chambers. The chambers were equipped with a flow through system for
controlling the delivery of medical oxygen or room air.
Twelve-hour-old neonatal rats were randomly divided
into one of four groups: air, hyperoxia, air + SP and hyperoxia +
SP. Rats from the air and air + SP groups were exposed to air and
maintained O2 levels >21%, while the rats in
hyperoxia and hyperoxia + SP groups were placed in a sealed
Plexiglas chamber with a minimal in-and-outflow, providing 4–5
exchanges/h of chamber volume and maintaining O2 levels
>95% simultaneously. Exposure to hyperoxia was continuous, with
brief interruptions only for animal care (30 min/day). The
concentration of oxygen was maintained by the use of an oxygen
controller. The rats in the air + SP group and hyperoxia + SP group
received intraperitoneal injections of rat SP (5 μg/kg, qod). The
rats in the air and air + SP groups received intraperitoneal
injections of saline vehicle alone at the same time point.
Tissue preparation
The rat pups were sacrificed at days 3, 7 and 14 of
hyperoxia exposure, and ~5 animals at different times in each group
were used in the study. Under deep pentobarbital anesthesia (50
mg/kg, intraperitoneal injection), a midline incision was made
through the sternum and abdomen, then the whole-lung tissue was
obtained.
Histopathology
Following sacrifice, the left lungs were excised and
fixed by overnight immersion in 4% paraformaldehyde in
phosphate-buffered saline at 4°C. The specimens were dehydrated in
a graded ethanol series. Tissues were embedded in paraffin,
sectioned and stained with hematoxylin and eosin (H&E).
Oxidation and antioxidation assay
To determine the damage caused by oxygen, the
activities of MDA and endogenous antioxidant enzymes SOD were
measured according to the manufacturer’s instructions.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed using the SYBR-Green real-time
PCR method. Total RNA was extracted from lung tissue. qRT-PCR was
performed on an FTC2000 PCR instrument (Funglyn Biotech Inc.,
Toronto, ON, Canada) using the two-stage program parameters
provided by the manufacturer, as follows: 4 min at 94°C, 35 cycles
of 20 sec at 94°C and 30 sec at 60°C and 30 sec at 70°C.
Specificity of the produced amplification product was confirmed by
the examination of dissociation reaction plots. A distinct single
peak indicated that a single DNA sequence was amplified during PCR.
PCR products were run on 2% agarose gels to confirm that the
correct molecular sizes were presented. Each sample was tested in
triplicate and samples obtained from three independent experiments
were used for the analysis of relative gene expression using the
2−ΔΔCt method. The primers used for qPCR were:
glyceraldehyde-3-phosphate dehydrogenase (GAPDH): Amp forward,
5′-CCCATCTATGA GGGTTACGC-3′ and reverse, 5′-TTTAATGTCACGCACGA
TTTC-3′; and for SHH: Amp forward, 5′-TCGTGCTACGC AGTCATCG-3′ and
reverse, 5′-CGCTTCCGCTACAGAT TGC-3′.
Western blot analysis
Total tissue proteins were calculated using the BCA
protein assay and proteins (50 μg) from each sample were loaded
onto 10% SDS-polyacrylamide gels and electrophoretically
transferred to polyvinylidene fluoride membranes. The membranes
were blocked in 0.05% Tween-20/5% non-fat dried milk in TBST for 1
h, rinsed and incubated with the appropriate primary antibodies
overnight at 4°C. After washing in TBST, the membranes were
incubated with secondary antibody for 1.5 h at room temperature,
followed by three washes in TBS. The immunoreactive proteins were
visualized with peroxidase and an enhanced chemiluminescence system
(ECL kit; Pierce Biotechnology, Rockford, IL, USA).
Statistical analysis
The data are expressed as means ± SEM. The
statistical significance of the differences between the means of
the groups was determined by one-way ANOVA or two-tailed Student’s
t-tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
Lung histopathology
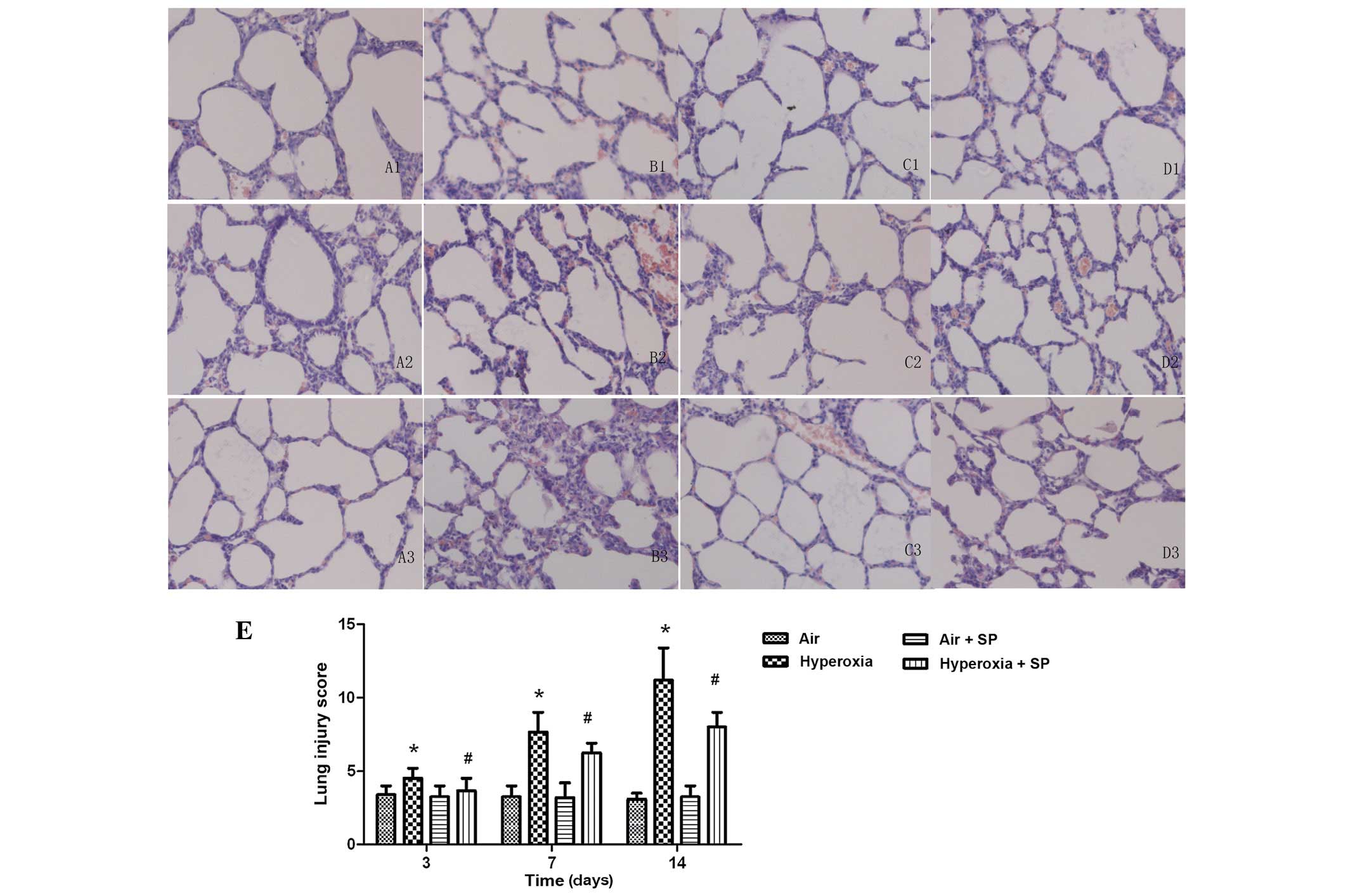
Results of H&E staining showed that, compared
with the air group, the epithelial injury, inflammatory cell
infiltrate, increased alveolar thickness and entrapped red blood
cells, characteristic of hyperoxic injury, were present in the
hyperoxia group at day 3 of hyperoxia exposure. The degree of these
histopathological changes in the lung became more serious at 7 and
14 days. The lung pathological images in the hyperoxia + SP groups
were improved significantly relative to the simple hyperoxia
exposure. Improvement in pathological images had no significant
difference between the air + SP and air groups. Representative
photomicrographs showing differences in each experimental group are
shown in Fig. 1.
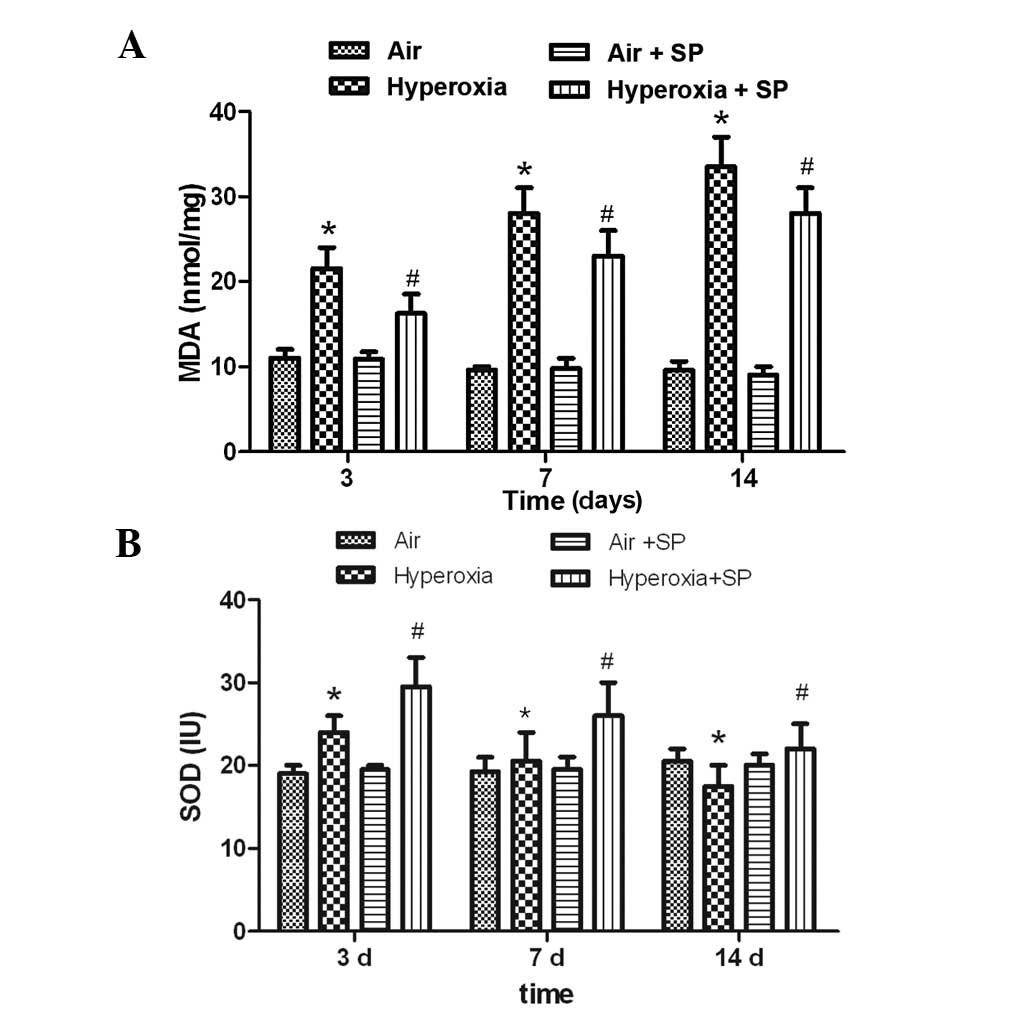
MAD and SOD activity
To test the hypothesis that treatment of SP reduced
systemic oxygen toxicity, the levels of MDA and SOD, indicators of
oxidative stress damage and potent antioxidant properties,
respectively, were measured. The results showed that the activities
of MDA were significantly increased at 3, 7 and 14 days following
hyperoxia exposure. SP may markedly inhibit the increase of MDA
(Fig. 2A). The level of SOD were
significantly increased at 3 days following hyperoxia exposure, but
decreased at 14 days compared with the air group. Moreover, the
levels of SOD were increased markedly at 3, 7 and 14 days following
administration of rat SP (Fig.
2B).
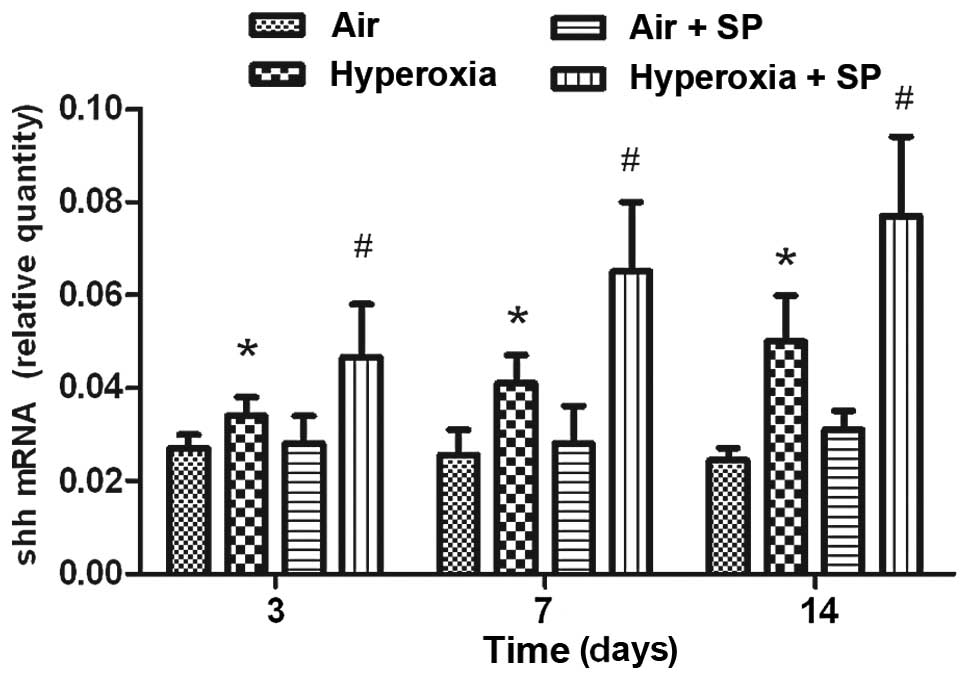
mRNA expression of SHH
Hyperoxia stimulation rapidly induced the mRNA
expression of SHH in lung tissue. Compared with air exposure, the
mRNA expression level of SHH was increased at 3, 7 and 14 days
following hyperoxia exposure (Fig.
3). SHH mRNA was expressed best at 14 days following hyperoxia
exposure. Moreover, the mRNA expression level of SHH was further
increased following additional SP treatment.
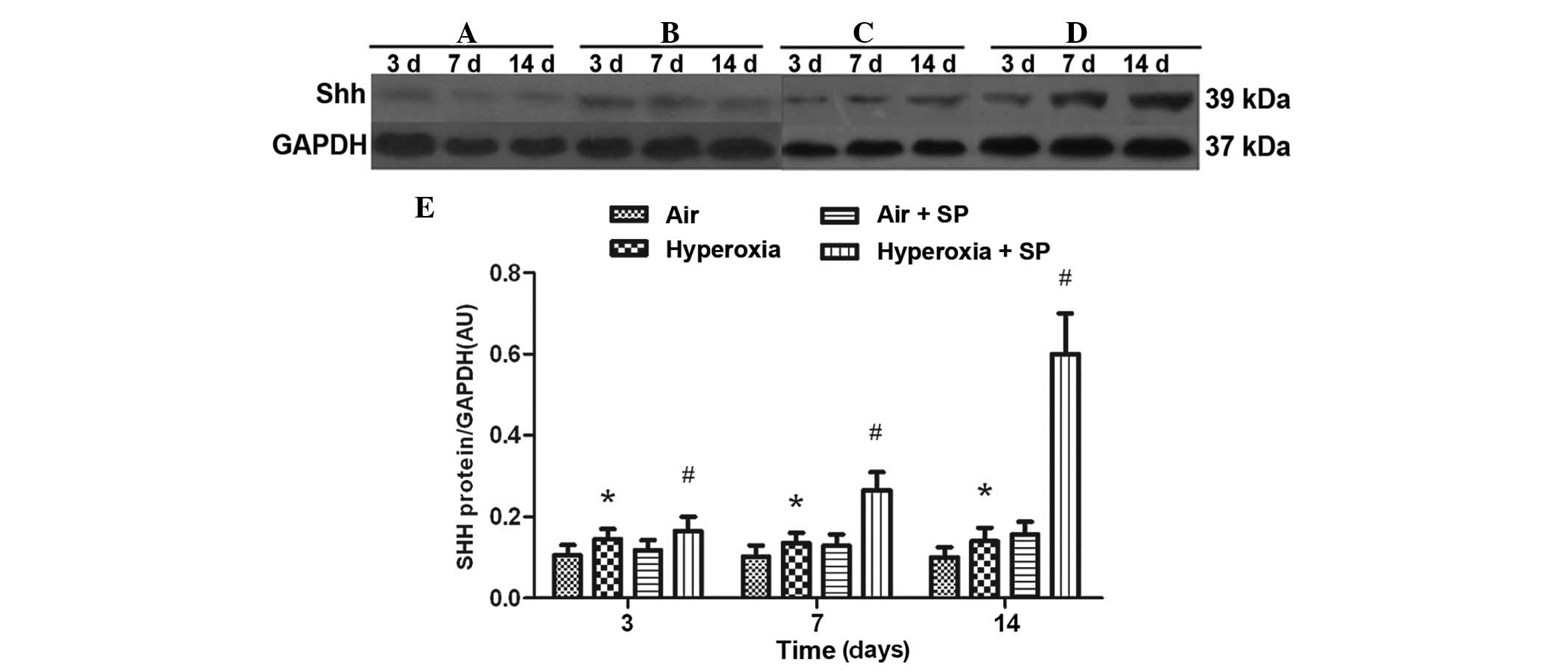
Protein expression of SHH
Compared with air exposure, hyperoxia stimulation
rapidly induced the expression of SHH in lung tissue at 3, 7 and 14
days following the hyperoxia exposure. Furthermore, the protein
expression of SHH was significantly increased following injection
of SP, in a time-dependent manner. These data, in conjunction with
the mRNA results, indicate that SP was involved in upregulating the
SHH pathway in hyperoxia-induced lung injury (Fig. 4).
Discussion
Prolonged hyperoxia exposure is likely to cause
direct oxidative damage through increased production of ROS. ROS
may cause lipid peroxidation, oxidation of proteins and DNA damage,
which induces cellular dysfunction and even cell death (13), resulting in hyperoxia lung injury
(14). Hyperoxia lung injury is
characterized by airway epithelial damage resulting from exposure
to reactive oxygen products that overwhelm the lung’s endogenous
supply of antioxidants.
A previous study suggested that hyperoxia may induce
oxidative stress injury in a time-dependent manner in vitro
(7). The present study has
demonstrated that neonatal rats exposed to 95% oxygen may cause
epithelial injury and inflammatory cell infiltration,
characteristic of hyperoxia injury. Oxidative stress was shown to
be elevated following hyperoxic exposure. Treatment with SP
decreased MDA activities and increased SOD activities. In addition,
in the present study, the pathological changes in lung tissue in
hyperoxia + SP groups were improved significantly relative to the
simple hyperoxia exposure, indicating that SP exerts antioxidant
and protective effects in hyperoxia-induced lung injury.
SP is a low-molecular weight (~1 kDa) peptide,
distributed widely in the airway endothelial cell layer, pulmonary
vessels, the trachea, bronchus smooth muscle, bronchus ganglion and
surrounding glands. Following release, SP binds primarily to NK-1R
(4) and it was observed that SP
triggers an exuberant neuroinflammatory response, regulates
proliferation, migration and differentiation of the impaired cells
(15,16). Dib et al (17) observed that sensory
neurotransmitters exhibited significant protection for
NK-1R-mediated functions in acute hyperoxic lung injury and the
study provided positive evidence for NK-1R activation in acute
hyperoxia. Oslund et al (18) observed that SP is an important
mediator in airway epithelial cell death and subsequent
proliferation following ozone exposure. The data suggested that the
SP interference may be a protective strategy for hyperoxia-induced
lung injury.
In addition to causing oxygen toxicity, previous
studies have demonstrated that hyperoxia played a role in
regulating SHH signal transduction pathways, including SHH, Patched
1 (PTCH1), smoothened (Smo) and GLI (19,20).
SHH signaling protein is important in a number of processes,
including embryogenesis, tissue repair, wound healing and lung
morphogenesis (21–23). In the past few years, a large
amount of information has emerged with regard to the mechanism and
significance of SHH-PTCH-GLI signaling in lung morphogenesis
(24,25).
SHH is known to be expressed at low levels in the
normal lung and enhanced during the repair of damaged airway
epithelium (26), but has not been
studied under the normal and hyperoxia conditions in neonatal rats.
In the current study, compared with air exposure, the expression
level of SHH was markedly increased following hyperoxia exposure at
different time points. This pathway was hypothesized to be
activated in hyperoxia and may be a compensatory reflection to
reduce hyperoxia-induced lung injury.
Whether or not activation of the SHH pathway is
required for the protective effect of SP is yet to be determined.
To highlight the effect of SP on SHH signal pathways, the mRNA and
protein expression levels of SHH were determined. The current study
also demonstrates that SHH pathway was activated by exogenous SP.
Histopathology and an oxidation assay also supported the hypothesis
that supplementary SP effectively improved lung pathological
changes accompanied by upregulating the signaling pathway of SHH.
This finding indicated that activation of SHH may be involved in
the mechanism of SP protection of hyperoxia-induced injury.
It is hypothesized that SP interference, a
protective management, produces a protective effect on neonatal
rats against hyperoxia, which may be associated with attenuation of
oxidative stress, elevation antioxidant activities and upregulation
of the signaling pathway of SHH.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 30973218).
References
|
1
|
Kallet RH and Matthay MA: Hyperoxic acute
lung injury. Respir Care. 58:123–141. 2013. View Article : Google Scholar
|
|
2
|
Buccellato LJ, Tso M, Akinci OI, Chandel
NS and Budinger GR: Reactive oxygen species are required for
hyperoxia-induced Bax activation and cell death in alveolar
epithelial cells. J Biol Chem. 279:6753–6760. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maggi CA, Giachetti A, Dey RD and Said SI:
Neuropeptides as regulators of airway function: vasoactive
intestinal peptide and the tachykinins. Physiol Rev. 75:277–322.
1995.PubMed/NCBI
|
|
4
|
Hökfelt T, Pernow B and Wahren J:
Substance P: a pioneer amongst neuropeptides. J Intern Med.
249:27–40. 2001.PubMed/NCBI
|
|
5
|
Kim JS, Rabe KF, Magnussen H, Green JM and
White SR: Migration and proliferation of guinea pig and human
airway epithelial cells in response to tachykinins. Am J Physiol.
269:L119–L126. 1995.PubMed/NCBI
|
|
6
|
Kim JS, McKinnis VS, Adams K and White SR:
Proliferation and repair of guinea pig tracheal epithelium after
neuropeptide depletion and injury in vivo. Am J Physiol.
273:1235–1241. 1997.PubMed/NCBI
|
|
7
|
Huang B, Fu H, Yang M, Fang F, Kuang F and
Xu F: Neuropeptide substance P attenuates hyperoxia-induced
oxidative stress injury in type II alveolar epithelial cells via
suppressing the activation of JNK pathway. Lung. 187:421–426. 2009.
View Article : Google Scholar
|
|
8
|
Zhang M, Wang H, Teng H, Shi J and Zhang
Y: Expression of SHH signaling pathway components in the developing
human lung. Histochem Cell Biol. 134:327–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Zhang H, Choi SC, Litingtung Y and
Chiang C: Sonic hedgehog signaling regulates Gli3 processing,
mesenchymal proliferation, and differentiation during mouse lung
organogenesis. Dev Biol. 270:214–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Watkins DN, Berman DM, Burkholder SG, Wang
B, Beachy PA and Baylin SB: Hedgehog signalling within airway
epithelial progenitors and in small-cell lung cancer. Nature.
422:313–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai K, Kaspar BK, Gage FH and Schaffer DV:
Sonic hedgehog regulates adult neural progenitor proliferation in
vitro and in vivo. Nat Neurosci. 6:21–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu M, Lui VC, Sham MH, Pachnis V and Tam
PK: Sonic hedgehog regulates the proliferation, differentiation,
and migration of enteric neural crest cells in gut. J Cell Biol.
166:673–684. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kinnula VL and Crapo JD: Superoxide
dismutases in the lung and human lung diseases. Am J Respir Crit
Care Med. 167:1600–1619. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jackson RM: Pulmonary oxygen toxicity.
Chest. 88:900–905. 1985. View Article : Google Scholar
|
|
15
|
Scott JR, Muangman P and Gibran NS: Making
sense of hypertrophic scar: a role for nerves. Wound Repair Regen.
15(Suppl 1): S27–S31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Scott JR, Muangman PR, Tamura RN, et al:
Substance P levels and neutral endopeptidase activity in acute burn
wounds and hypertrophic scar. Plast Reconstr Surg. 115:1095–1102.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dib M, Zsengeller Z, Mitsialis A, Lu B,
Craig S, Gerard C and Gerard NP: A paradoxical protective role for
the proinflammatory peptide substance P receptor (NK1R) in acute
hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol.
297:L687–L697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oslund KL, Hyde DM, Putney LF, et al:
Activation of neurokinin-1 receptors during ozone inhalation
contributes to epithelial injury and repair. Am J Respir Cell Mol
Biol. 39:279–288. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Y and Struhl G: Dual roles for
patched in sequestering and transducing Hedgehog. Cell. 87:553–563.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motoyama J, Liu J, Mo R, Ding Q, Post M
and Hui CC: Essential function of Gli2 and Gli3 in the formation of
lung, trachea and oesophagus. Nat Genet. 20:54–57. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le H, Kleinerman R, Lerman OZ, et al:
Hedgehog signaling is essential for normal wound healing. Wound
Repair Regen. 16:768–773. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katoh Y and Katoh M: Hedgehog signaling
pathway and gastrointestinal stem cell signaling network (Review).
Int J Mol Med. 18:1019–1023. 2006.PubMed/NCBI
|
|
23
|
Kusano KF, Pola R, Murayama T, et al:
Sonic hedgehog myocardial gene therapy: tissue repair through
transient reconstitution of embryonic signaling. Nat Med.
11:1197–1204. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Warburton D, Zhao J, Berberich MA and
Bernfield M: Molecular embryology of the lung: then, now, and in
the future. Am J Physiol. 276:L697–L704. 1999.PubMed/NCBI
|
|
25
|
Litingtung Y, Lei L, Westphal H and Chiang
C: Sonic hedgehog is essential to foregut development. Nat Genet.
20:58–61. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stewart GA, Hoyne GF, Ahmad SA, et al:
Expression of the developmental Sonic hedgehog (Shh) signalling
pathway is up-regulated in chronic lung fibrosis and the Shh
receptor patched 1 is present in circulating T lymphocytes. J
Pathol. 199:488–495. 2003. View Article : Google Scholar : PubMed/NCBI
|