Introduction
The adult human intervertebral disc (IVD) consists
of an extracellular matrix combined with a small number of cells.
The external part of the IVD is composed of an annulus fibrosus
(AF) that surrounds the nucleus pulposus (NP) in the centre. The
IVD possesses low regeneration capabilities mainly due to the
avascular system of metabolite transport. NP damage is usually
irreversible, therefore numerous attempts have been undertaken to
stimulate regeneration of the nucleus pulposus cells (NPCs)
(1).
Studies on the interaction between the IVD and
multipotent mesenchymal stromal cells (MSCs) have been performed in
human and animal culture systems (2–4).
Direct co-culture between human MSCs and human AF cells was
required to observe an increase in the glycosaminoglycan (GAG)
content (5). The increase of mRNA
of the NP marker genes COL2A1, COL6A2, ACAN
and SOX9 has been reported as the main result of direct
co-culture of human NPCs and MSCs (6). Indirect co-culturing of NPCs and MSCs
in the insert system promoted COL2A1 expression in the MSCs
and the proliferation of NPCs as well as the expression of
ACAN (7). A study of human
MSCs that were co-cultured with NP revealed higher expression of
COL2A1 and ACAN (8).
Direct co-culture has been reported as the most efficient way of
inducing the NP-like phenotype in MSCs observed as an increase in
SOX9, COL4 and ACAN expression including
growth factor production (9).
Degenerate NPCs regained a non-degenerate NP phenotype as a result
of close contact with MSCs (9).
It has recently been demonstrated that cell fusion
or gap-junction are a marginal form of intercellular communication
between NPCs and MSCs (10). It
was revealed that NPCs and MSCs exchanged CFDA and SNARF
hydrophilic dyes and after 7 days 0.75% of the cells were hybrids
of NPCs and MSCs (10). By
contrast, the lipophilic dye DiI was transported from labeled to
unlabeled cells much more efficiently and after 7 days of
co-culture 80% of the unlabeled cells were stained (10). The amount of microvesicles (MVs)
detected in the culture media collected from the DiI-stained cells
was insufficient to explain such intensive membrane transfer
(10). Tunnelling nanotubes (TnTs)
are structures connecting two cells of the same phenotype,
different phenotypes or tissues. Their diameter ranges from 50 to
200 nm and may reach several cell diameters long. TnT structures
are present in various types of cells including fibroblasts,
neurons, immune cells and cancer cells (11). An increasing number of data have
demonstrated that TnTs are highways of intercellular transport
(11). Findings of previous
studies (12) have demonstrated
TnT in various cell types, nevertheless, there are no studies
demonstrating TnT in NP-MSC co-cultures.
In the present study NPCs and MSCs were obtained
from patients with scoliosis. The cells were then co-cultured in
common medium on separate surfaces (called: non contact, indirect
or insert system) and with one-surface conditions (called direct
system). Using a cell sorter and a confocal microscope human NPCs
and MSCs were found to create protrusions similar to TnTs. In these
tubes a reciprocal exchange of lipophilic dyes DiO (green) and DiD
(red) occurred. This exchange was more efficient between cells
growing in direct co-culture in comparison with the porous membrane
(insert system).
The present study focused on the issue of
determining which type of structure is required for the direct
interaction of NPCs and MSCs. The objective of this study was to
discriminate the subpopulation of cells that underwent membrane
interchange using DiO and DiD dyes and to verify the hypothesis
that NP and MS cells are able to establish contact channels to
exchange components of membranes.
Materials and methods
Patients and tissues
Human NPCs were collected using an anterior approach
from 12 patients undergoing treatment to correct thoraco-lumbar or
lumbar scoliosis during routine preparation of the site for
anterior spodylodesis. All the patients were recruited into the
study consecutively.
The eligibility criteria for the study were: i)
adolescent idiopathic scoliosis; ii) 10–19 years of age; iii) a
Cobb angle of over 40° and iv) scoliosis correction from an
anterior approach with routine removal of an IVD in preparation of
the site for anterior spodylodesis. This process complies with
ethical considerations with regard to the collection of human NPCs.
NPCs extracted from the IVDs of adolescent subjects were presumed
to be non-degenerative.
The exclusion criteria were: i) use of analgesic,
antibiotic or steroid medication prior to hospital admittance and
ii) previous surgery in the spinal area.
Patients who fulfilled the inclusion criteria
received in-depth information on the aim of the study and were
assured of anonymity. Informed consent from the legal guardians of
each patient was obtained prior to the request to collect NPCs from
the donors was made. The mean ± SD age of the patients was 16±2.3
years (range 14–19). The study design was approved by the Ethics
Committee of Poznan University of Medical Sciences (Poznan, Poland;
approval no. 838/09) and was carried out in accordance with
universal ethical principles.
Cell culture
Nucleus pulposus
Non-degenerate IVD tissue was dissected mainly from
Th12 to L3 to separate the NP from the AF tissue. The NP was
enzymatically digested overnight at 37°C with 0.02% collagenase
type II (Sigma, St. Louis, MO, USA) in serum-free medium containing
100 units penicillin, 100 μg streptomycin and 250 ng amphotericin B
per ml (ABAM, Sigma). The digested tissue/cell suspension was
filtered through a sterile nylon fabric to remove remaining tissue
debris. The cells were centrifuged at 300 g for 5 min, seeded onto
a tissue culture flask and cultured at 37°C in 5%
CO2/95% air, in (1:1 v/v) Dulbecco’s modified Eagle’s
medium/nutrient F-12 Ham supplemented with 10% fetal bovine serum,
ABAM (all from Sigma) solution and vitamin C (5 mg/ml). For gene
expression experiments, 6-well plates were used and 150,000 cells
were placed in each well.
Bone marrow
Non-degenerative vertebrae were extracted mainly
from Th12 to L3. The vertebrae were minced mechanically and
enzymatically digested overnight in similar conditions as for the
NPCs. The digested tissue/cell suspension was filtered and
resuspended in 1 ml PBS buffer and 20 μl fetal bovine serum
(Sigma). Subsequent steps of stem cell enrichment were performed
according to the stem cells kit procedure (RosetteSep Human
Mesenchymal Stem Cell Enrichment Cocktail, StemCell Technologies,
Vancouver, BC, Canada). Briefly, to the bone marrow cells
resuspended in 1 ml PBS buffer and 20 μl fetal bovine serum 50 μl
of RosetteSep Human Mesenchymal Stem Cell Enrichment Cocktail
(StemCell Technologies) was added. Subsequent to a 20 min
incubation the cells were placed in the ficoll solution and
centrifuged. The intermediate layer of the cells was transferred
into a cell culture dish and placed in the incubator in a medium
similar to the one used for the NPCs except that in this case the
culture medium, Dulbecco’s modified Eagle’s medium/nutrient F-12
Ham, was not enriched with vitamin C. For the first 4 days of
culture the cells were washed daily to enable the selection of
adhesive cells only. The MSCs obtained were cultured in a
chondrocyte selective cell culture medium (Human Mesenchymal Stem
Cell Functional Identification Kit, R&D Systems Inc.,
Minneapolis, MN, USA). The MSC phenotype was confirmed by
quantitative reverse transcription PCR (RT-qPCR). MSCs destined for
co-culture with NPCs were stained with lipophilic dyes and
subsequently cultured in the insert system or in direct co-culture
with NPCs. The MSCs were analysed with a flow cytometer (BD
FACSAria™ III; Becton Dickinson, Franklin Lakes, NJ, USA) and
confocal laser scanning microscope (Olympus Fluoview FV10i;
Olympus, Tokyo, Japan).
Staining cells for flow cytometry and
confocal microscopy
For flow cytometry and confocal microscopy the cells
were cultured and stained with lipophilic dyes DiO (green) or DiD
(red) (Molecular Probes, Inc., Eugene, OR, USA). The cells were
removed from the culture dish using trypsin (Sigma), in accordance
with the manufacturer’s instructions, and subsequently counted. The
required number of cells were incubated for 30 min with 10 μl of
DiO or DiD dye solutions in 1 ml of cell suspension in PBS. The
cells were subsequently washed with PBS and cultured overnight in a
culture medium appropriate to the cell type. The following day the
stained cells were subcultured to the new culture dishes depending
on the experiment requirements.
NPCs and MSCs were stained with DiD, cultured in the
1-μm insert system (Cell Culture Inserts for 6-well plates, 1 μm,
translucent PET membrane, BD Falcon™, Franklin Lakes, NJ, USA) and
placed in culture dishes (6-well Cell Culture Plate, BD Falcon™).
In the 6-well culture dishes the same number of non-stained MSCs
and NPCs were cultured, respectively, under the inserts. In an
alternative version of this experiment NPCs and MSCs were stained
with DiD and cultured together with non-stained MSCs or NPCs
respectively in 6-well culture dishes at a ratio of 50:50.
Following 4 days of culture, the cells were detached from the
dishes by trypsin and then analysed using the BD FACSAria™ III
(Becton Dickinson).
The cells designated for the flow cytometry in the
double staining system were stained with DiD or DiO and cultured in
6-well culture dishes at a ratio of 50:50. The experiment was
performed in two versions, the first with NP/DiO and MSC/DiD and
the second with NP/DiD and MSC/DiO. Following 8 days of culture the
cells were detached from the dishes by trypsin, sorted on BD
FACSAria III and the subsequent double-stained population was
cultured again. For laser confocal microscopy the cells were
cultured on glass bottom dishes (3.5 cm diameter) from Mattek
(Ashland, MA, USA).
Confocal and fluorescent
microscopy
The Olympus Fluoview FV10i confocal laser scanning
microscope with the 60× water immersion objective lens (NA=1.35)
was used (Olympus). Images were captured in two fluorescence
channels: DiO, DiD and in the phase-contrast channel. The images
were prepared using FV10-ASW software (Olympus). Images from the
three channels were combined into one.
Flow cytometric analysis combined with
cell sorting
Culture cells were digested with 1X trypsin and
analyzed using a BD FACSAria™ III (Becton Dickinson) flow cytometer
(cell sorter), equipped with four lasers (375, 405, 488 and 633
nm), 11 fluorescence detectors, forward scatter (FSC) and side
scatter (SSC) detectors. Side scatter signals and fluorescent light
was collected by a gel-coupled, NA 1.2 collection lens. The
instrument setup (optical alignment), stability and performance
test was performed using the Cytometer Setup and Tracking system by
Becton Dickinson. FACSFlow solution (Becton Dickinson) was used as
a sheath fluid. The configuration of the flow cytometer included a
100 μm nozzle and 20 psi sheath fluid pressure. The cells were
characterized by the non-fluorescent parameters FSC and SSC, and
the fluorescent parameters green fluorescence from DiO reagent
collected using a 530/30 band pass filter and red fluorescence from
the DiD reagent collected using a 660/20 band pass filter. The 488
and 633 nm lasers were employed in excitation of DiO and DiD
fluorescent reagents, respectively. Flow cytometric analyses were
performed using logarithmic gains and specific detector settings.
The threshold was set at the FSC signal. Data were obtained in a
four-decade logarithmic scale as area signals and analyzed with the
FACS DIVA software (Becton Dickinson). The populations were defined
by gating in the dot plots of DiO versus DiD. Each sample was
analyzed in triplicate. Cell sorting preceded the doublet
discrimination procedure with the use of height versus width
scatter signal measurements, in order to discriminate single cells
from conglomerates. The cells were sorted in 5 ml cytometric
tubes.
Glycosoaminoglycans
NPCs were grown in appropriate culture media, then
digested with papain following the indicated time elapsed.
Subsequently, Blyscan Sulfated Glycosaminoglycan Assays (Biocolor
Ltd., Carrickfergus, UK) were performed according to the
manufacturer’s instructions.
Gene expression
The NP total RNA was extracted from the cultured
cells with the use of TRItidy G, according to the manufacturer’s
instructions (Applichem, Darmstadt, Germany). In total, 1 μg total
RNA was reverse-transcribed using a Superscript Reverse
Transcriptase Kit (Life Technologies, Carlsbad, CA, USA).
Oligo(dT)15 primed cDNAs were amplified by qPCR, using
the primers listed in Table I, and
the LightCycler FastStart DNA Master SYBR-Green I kit (Roche,
Mannheim, Germany). The crossing-point values were calculated
automatically based on the second derivative algorithm, and the
results were analyzed by the relative expression method.
HMBS and MRPL19 were used as reference genes.
 | Table IPrimers used in quantitative PCR. |
Table I
Primers used in quantitative PCR.
| Gene | Primer
sequence | Amplicon length,
bp | GenBank accession
nos. |
|---|
| ACAN |
5′-ACCAGACTGTCAGATACCCC-3′ | 156 | NM_001135 |
|
5′-CATAAAAGACCTCACCCTCC-3′ | | |
| SOX9 |
5′-GAAGAACGGGCAGGCGGA-3′ | 181 | NM_000346 |
|
5′-TTTGGGGGTGGTGGGTGG-3′ | | |
| COL2A1 |
5′-ACCAGGACCAAAGGGACA-3′ | 246 | NM_033150 |
|
5′-GCAGCAAAGTTTCCACCA-3′ | | |
| COL1A1 |
5′-GAAGGGACACAGAGGTTTCAG-3′ | 179 | NM_000088 |
|
5′-TTCCACGAGCACCAGCAG-3′ | | |
| TGFB1 |
5′-GAAACCCACAACGAAATC-3′ | 300 | NM_000660 |
|
5′-AATTTCCCCTCCACGGCT-3′ | | |
| MRPL19 |
5′-TCCAACCGCCGCCGAAAC-3′ | 197 | NM_014763.3 |
|
5′-AACACGAAGAATACTTCCAACA-3′ | | |
| HMBS
(PBGD) |
5′-CCCTGGAGAAGAATGAAGTG-3′ | 254 | NM_000190 |
|
5′-TCCCCGAATACTCCTGAA-3′ | | |
Results
Cell line identity
The NPCs obtained surgically were cultured and the
mRNA was subsequently extracted. Reverse transcription and PCR
revealed the expression of COL1A1, COL2A1,
TGFB2, SOX9 and ACAN (results not shown). In
addition, the GAG levels in cultures were measured using the
Blyscan kit (results not shown) and the presence of GAG in the
cultured cells was detected.
MSCs were selected from total bone marrow cells by
their ability of adhesion to a plastic surface. This method is
commonly used to obtain multipotent MSCs (13). The cells were cultured in a
chondrogenesis-stimulating medium to control their differentiation
potential. MSCs cultured in the medium stimulating chondrocyte
differentiation revealed increased levels of mRNA of SOX9,
TGFB1 and ACAN in correlation to MSCs cultured in the
standard medium (data not shown). TGFB1, transforming growth
factor β 1 gene, is responsible for MSC differentiation and is one
of the main components of chondrocyte differentiation media.
ACAN aggrecan gene encodes the protein component of
proteoglycans. SOX9 is a gene of transcription factor SOX-9
and is important in chondrocyte differentiation. These experiments
confirmed the differentiation potential of our primary MSC
lines.
Differences in the DiD transport between
the NPCs and MSCs in two systems of co-culture
NPCs and MSCs were stained with DiO and DiD
lipophilic dyes and cultured in the following combinations: NP/DiO
with NP/DiD and MSC/DiO with MSC/DiD. The two co-cultures revealed
exchange of dyes following 24 h of culture (data not shown).
The comparison of DiD transport in two co-culture
systems between NPCs and MSCs was measured by flow cytometry. The
first system contained a culture dish and a 1-μm pore insert. The
second culture system was a one surface co-culture. DiD prestained
NPCs were cultured in the insert and non-stained MSCs were cultured
with under-stained NPCs. Following 4 days of co-culture the two
cell lines underwent flow cytometric analysis. The stained NPCs
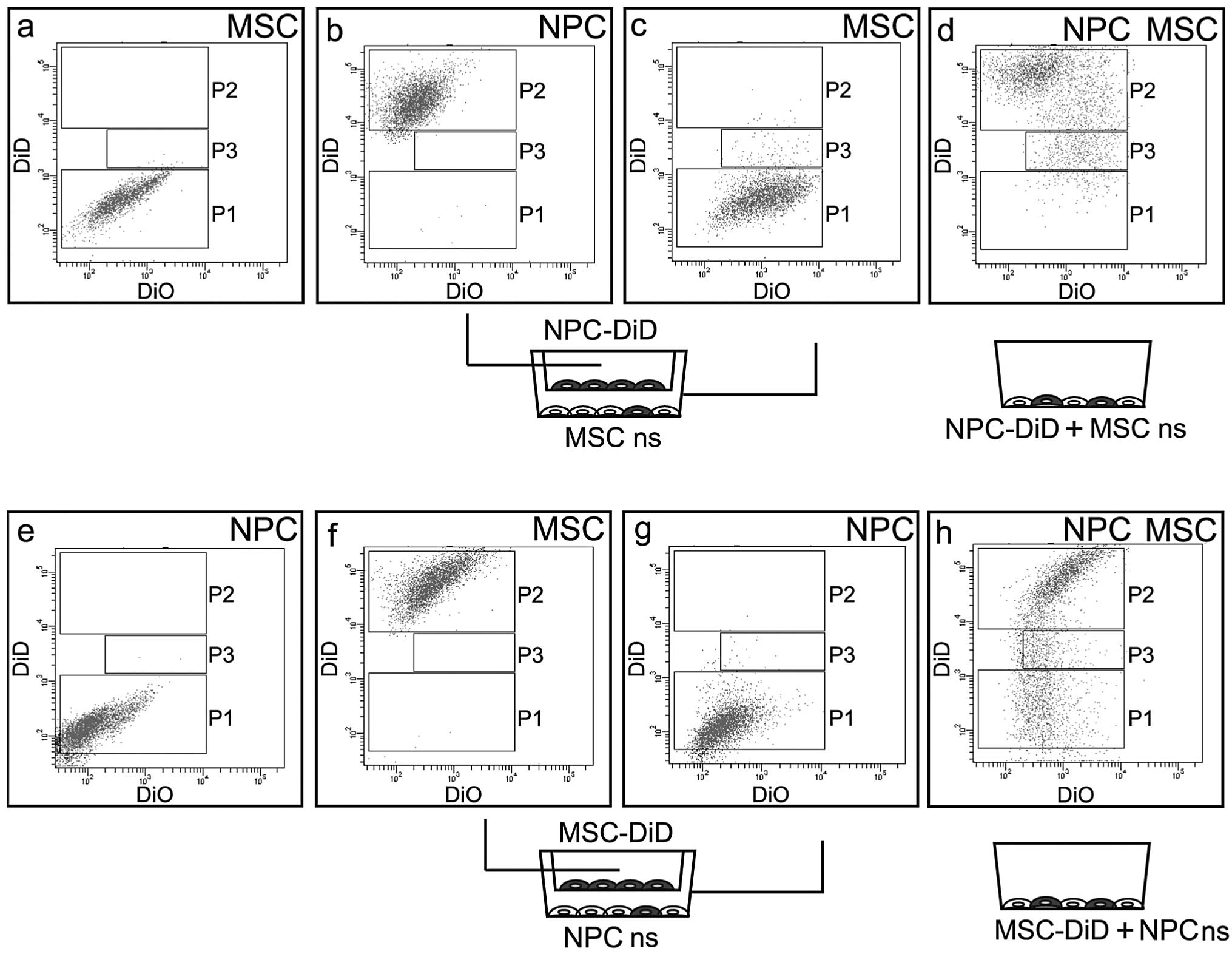
were detected and are marked as P2 in the diagram (Fig. 1b). The population of the MSCs (P1)
was grouped in the region corresponding to the population of the
non-stained control (P1 in Fig.
1a, P1=99.0%, P2=0.0% and P3=0.5% and Fig. 1c, P1=96.4%, P2=0.4% and P3=2.7%),
however, this population was slightly diffused and the
subpopulation P3 was increased. (Fig.
1c). The subpopulation P3 revealed intermediate intensity of
the DiD signal between non-stained MSCs and DiD-stained NPCs
(population P2). The co-culture of prestained NPCs with non-stained
MSCs on the direct system revealed a population corresponding to
DiD-stained NPCs (P2) (Fig. 1d,
P1=5.2%, P2=69.0% and P3=14.5%). The subpopulation of
intermediately stained cells (P3) was more numerous compared with
the corresponding populations in the indirect system (14.5 vs.
2.7%). The population P1 corresponding to non-stained MSCs was
almost absent. Thus, the subpopulation P3 is likely to be mainly an
MSC population, stained by DiD dye absorbed from the prestained
NPCs. Moreover, the dye exchange in the direct, one-surface system
is more efficient compared with the indirect co-culture (insert
system).
To compare the cell lines in their DiD exchange
efficiency, MSCs were also stained with DiD and were co-cultured
with non-stained NPCs. DiD prestained MSCs were cultured in the
insert and non-stained NPCs were cultured with under-stained MSCs.
Following 4 days of co-culture the two cell lines were analyzed by
a flow cytometer. The stained MSCs were detected and marked as P2
in the diagram (Fig. 2f, P1=0.1%,
P2=96.7% and P3=0.0%). The dominant population of NPCs (P1) was
grouped in the region corresponding to the population of the
non-stained control (Fig. 1e,
P1=84.9%, P2=0.0% and P3=0.0%), however, this population was
diffused and the subpopulation P3 was discriminated (Fig. 1g, P1=93.9%, P2=0.1% and P3=0.5%).
The subpopulation P3 revealed an intermediate intensity of DiD
signal between non-stained NPCs and DiD-stained MSCs. Co-culture of
prestained MSCs (P2) with non-stained NP (P1) in the direct system
revealed a great population corresponding to the DiD-stained MSCs
(P3) (Fig. 1h, P1=29.5%, P2=51.3%
and P3=12.6%). The subpopulation of intermediately stained cells
(P3) was greater than the corresponding population in the insert
system (12 vs. 0.5%). The population P1 corresponding to
non-stained NPCs was present (Fig.
1e). We hypothesized that the subpopulation P3 is likely to be
mainly an NP population that was stained by DiD dye absorbed from
prestained MSCs. By comparing the populations P1 (non-stained MSCs)
and P1 (non-stained NPCs) in Fig. 1d
and h, respectively, it was found that in the one-surface
system with direct contact of cells, the flow of the DiD dye was
more efficient from MSCs towards NPCs compared with that in the
opposite direction.
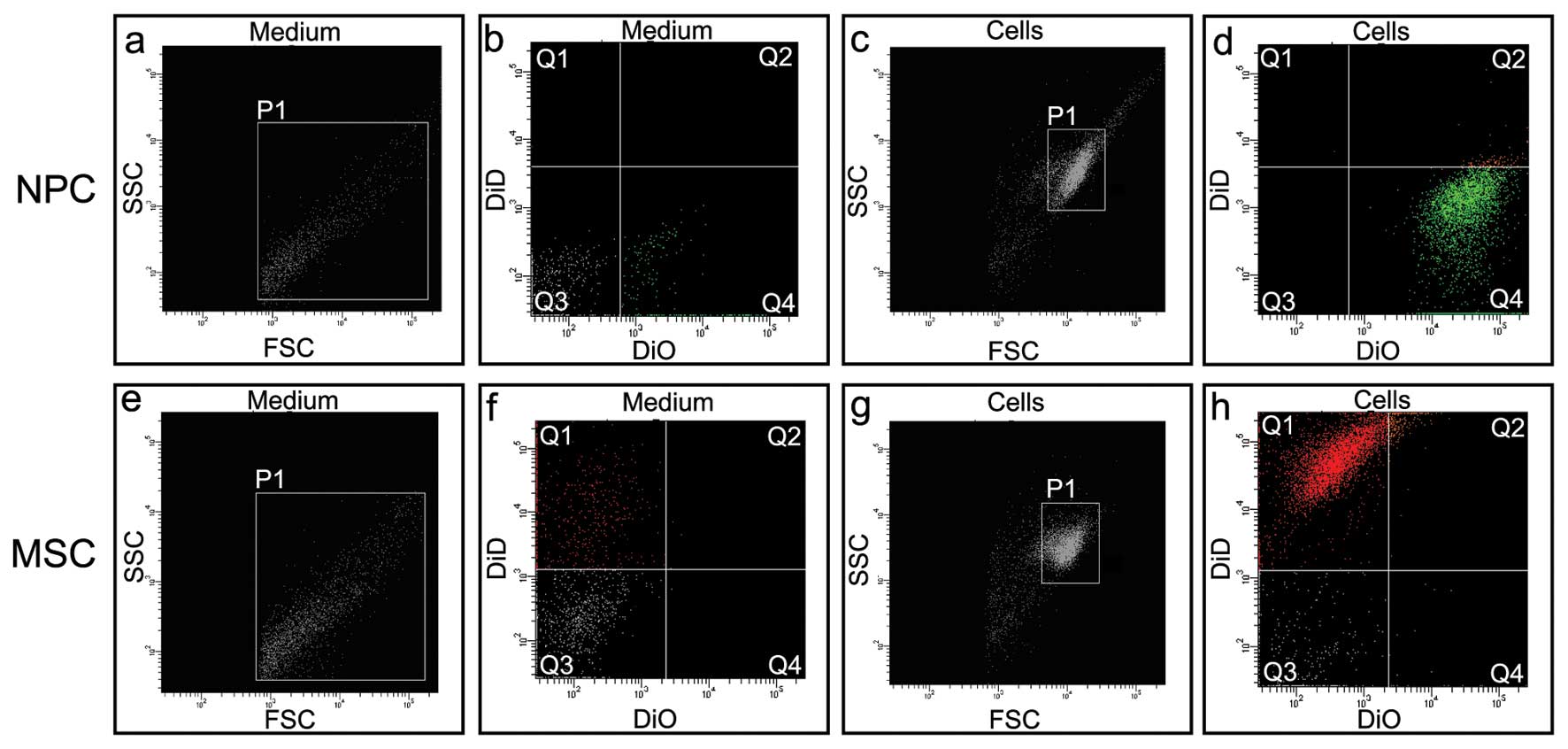
MV detection
To verify the hypothesis that a flow of particles
between the cells co-cultured in the insert system exists a
cytometric analysis of the culture media was performed. Two
possible paths of lipophilic dye transport were considered: free
dye diffusion or transport in secreted microvesicles. The culture
media from NPCs prestained with DiO or MSCs prestained with DiD
were collected subsequent to 2 days of culture. A flow cytometric
analysis of the cell culture medium from DiO prestained NP revealed
the presence of DiO-stained particles (Fig. 2b, Q4) of a diameter smaller than
the NPCs (Fig. 2a vs. c, P1). The
cytometric analysis of the cell culture medium from over
DiD-prestained MSCs revealed the presence of DiD stained particles
(Fig. 2f, Q1) of a diameter
smaller than that of an MSC (Fig. 2e
vs. g, P1).
Cell-to-cell communication in direct
co-culture of NPCs and MSCs
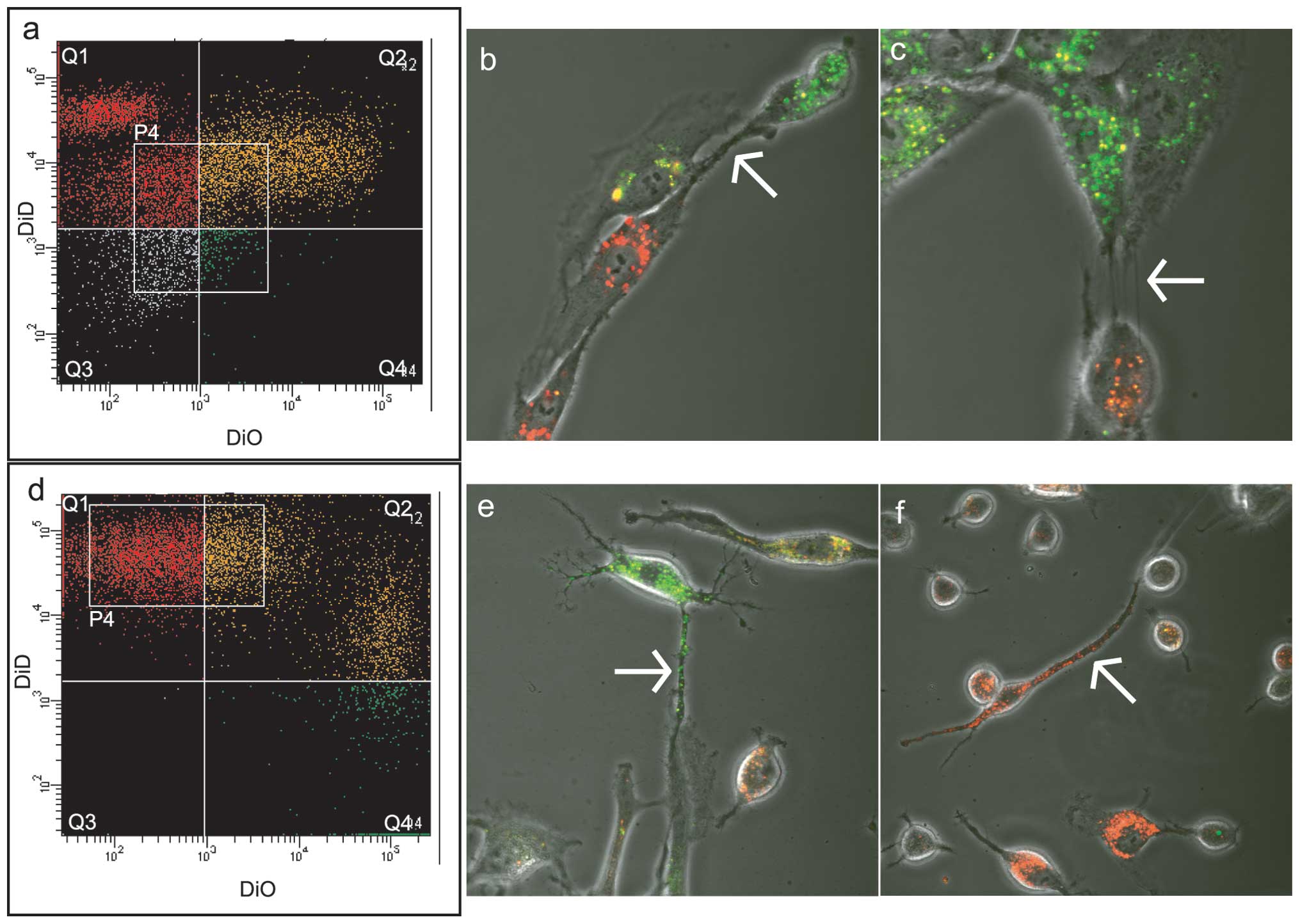
Prestained NP/DiO with MSC/DiD were cultured at a
ratio of 1:1 on 3.5 cm diameter culture dishes for 8 days. In a
parallel alternative experiment the same amount of MSC/DiO and
NP/DiD was cultured in 3.5 cm culture dishes for 8 days.
Co-cultured NP-MSC cells were then separated on the BD FACSAria
III. The distribution of dyes was asymmetric (Fig. 3a and d) and therefore it was
concluded that the NP-MSC communication is likely to be asymmetric.
The separated population P4 (Fig.
3a) from the NP/DiO and MSC/DiD experiment was selected for
subsequent culture. The population P4 (Fig. 3d) from the MSC/DiO and NP/DiD
experiment was also selected for further culture. The two cultures
were analyzed by confocal microscopy subsequent to 4 days in
culture and revealed exchange of DiO and DiD between the two cell
lines in the post-sorting culture (Fig. 3b and c, e and f). Analysis of the
cells stained by confocal microscopy by NP/DiO and MSC/DiD
including NP/DiD and MSC/DiO revealed that 4 days of culture is
sufficient to enable detection of at least two types of cell
structures that may be responsible for lipophilic dye transport.
Thin and thick linear structures that connect NPCs and MSCs were
visible (arrows in Fig. 3b, c, e and
f). It may be that the thin linear structures are pre-TnTs,
which later expand to form thicker communication arteries.
Different phases of exchange in the two dyes are also visible: from
clear green through light green to orange and yellow and from deep
red through light red to orange and yellow.
Discussion
It has previously been revealed that the exchange of
cellular membranes between NPCs and MSCs is dependent on MVs
(10). It has been indicated that
the intensity of lipophilic dye exchange requires other structures
including TnTs (10,12). In the present study it was
confirmed that when NPCs and MSCs were grown on one surface (direct
co-culture) the exchange of lipophilic dye (DiD) was more efficient
in comparison with the transport mediated by the culture medium in
the insert system. Two types of structures were observed similar to
TnTs formed between NPCs and MSCs grown on one surface. The high
rate of lipophilic dye transport between the cells in culture on
one surface in comparison with co-culture in the insert system is
the additive effect; on the one hand engaging known phenomena of
gap junctions, cell fusion and MV release, and on the other, the
transient formation of TnTs.
Therapies based on cells obtained from patients,
cultured and subsequently reintroduced into the patient’s body may
be used in the treatment of IVD degeneration (14). Studies performed in animal and
human cells have indicated the possibility of reciprocal
stimulation of differentiated IVD cells and MSCs (2,5).
Although this has been known for at least a decade, little is known
regarding the molecular mechanisms of cell-to-cell communication.
MSCs from various sources, mainly bone marrow, were used for the
development towards a chondrocyte-like phenotype (6). The International Society for Cellular
Therapy recommends applying the term ‘multipotent MSCs’ to the
fibroblast-like plastic-adherent cells (13). However, the same organization also
indicates that the term MSCs may be used. To promote
differentiation of animal or human MSCs, several methods were
developed which involved growth factors from the transforming
growth factor family (15–18). An alternative approach is the
co-culture of IVD cells with MSCs in the ‘one-surface system’ (also
known as direct co-culture or contact system), three-dimensional
systems (alginate beads) and culture of two cell lines separated by
a porous membrane (also termed insert system, indirect co-culture
or non-contact system) (19).
Injection of MSCs into the disc space was also assayed (16,20).
The superiority of direct co-culture of NPCs and
MSCs over the indirect systems has been observed in earlier studies
demonstrating the modulation of gene expression in NPCs and MSCs in
a direct co-culture system (9). In
the experiments of the present study, non-stained MSCs were
co-cultured with DiD-stained NPCs, or DiD-stained MSCs with
non-stained NPCs. This approach revealed that the culture medium is
a path for lipophilic dye transport and non-stained cells adsorbed
DiD from stained cells. In comparison with the insert system,
co-culture on one surface caused a relatively higher exchange of
the lipophilic dye between NPCs and MSCs. Similar observations have
been made in other cell lines. PKH67-labeled CHO and CD36+ cells
were cultured using a 0.4 μm insert system and the one-surface
system. In comparison with the one-surface, the transfer of
proteins and lipids was much lower in the insert system (21). The abovementioned results and those
of the present study provide strong evidence that a type of
additional means of transport occurs during a one-surface
co-culture, known as the spontaneous intercellular transfer of
cellular components (21). As a
result of membrane contact, the outer phospholipid bilayers may
provoke a transient fusion called hemifusion. Subsequently, the two
membranes might locally form fusion pores or activation of
fusogenic proteins leading to complete cell-cell fusion (21,22).
To determine whether or not the culture medium
contained particles detectable in the cytometric analysis the media
from DiD-stained cells were analyzed. Stained structures smaller
than the cells were detectable in the media and may be responsible
for indirect cell contact. This observation is similar to earlier
data from the indirect co-culture system of NPCs and MSCs stained
by DiI (10). DiI was exchanged in
a more intense way, between cells cultured in the direct system in
correlation to the insert indirect system (10). It has been indicated that in
addition to MV, alternative ways of cell contact occur in the
direct co-culture (10).
The NPCs and MSCs were analysed in a double-stained
co-culture system. In the images from the confocal microscopy of
NPCs and MSCs stained with DiO and DiD, respectively, we observed
an intense exchange of dyes. The majority of the double-stained
cells were not larger than a single NPCs and MSCs. However, there
was a small fraction of doublets that may indicate the existence of
hybrids. A small amount of double-stained cells were grown again in
a one-surface culture system revealing thin protrusions connecting
the cells. Several of these structures were stained, thus that the
exchange of dyes via this structure is possible.
Several studies (2–10)
have been undertaken to induce a reciprocal stimulation of animal
NPCs and MSCs in a co-culture system. In experiments using rabbit
cells, NPCs and MSCs were grown on opposite sites of the same 0.4
μm pore size membrane (insert system) where direct contact of cells
was possible (2). Fiber-like
structures in the electron microscope image obtained from the
membrane of the cell culture insert, may be a form of intercellular
channels known as TnTs (12).
Besides TnTs, further changes in the cell phenotype were observed
subsequent to co-culture of rabbit NPCs cultured in the porous
membrane insert system with MSCs revealing higher proliferation and
proteoglycan synthesis by NPCs (2). Formation of TnTs by human MSCs with
other cell types was observed in the co-culture of MSCs with
vascular smooth muscle cells (23). TnTs in the system promoted the
exchange of mitochondria and increased the proliferation of MSCs
initiated by contact with vascular smooth muscle cells (23). In the present study TnT-like
structures are shown, which form cytoplasmic channels observed
earlier in other cell types, including MSCs. However, the reason
for the hydrophilic dyes, used in earlier studies of NP-MSC
co-cultures, not exchanging as efficiently as the lipophilic dyes
remains to be clarified.
Previous studies demonstrated that human cell
contact and exchange of cytoplasm components with the use of
fluorescent proteins or hydrophilic dyes have been produced
(8,9). Paradoxically, this approach did not
produce a large double-stained population of cells. When various
lipophilic dyes were applied in rat and human NP and MS cell
systems the result was different. Thus, lipophilic dyes may only be
exchanged with external phospholipids of the plasma membrane. Since
experiments with cytoplasm soluble dyes or fluorescent proteins
exhibited a small fraction of double-stained cells, this
demonstrates that these dyes are not efficiently packed into MVs or
TnTs. The cellular cargo is selectively packed into the MVs of
three classes: apoptotic bodies, ectosomes and exosomes (24). The TnTs are plasma membrane
protrusions that connect the cytoplasms of communicating cells
(12). In addition to enabling the
relocation of organelles, TnTs are involved in Ca2+
fluxes between cells (12). As the
hydrophilic dyes are not efficiently transported in co-culture
systems, this means that TnTs are not as important for the
intercellular transport of cytosol components as for lipid
components. Another, although less likely hypothesis, is the
selection on entry to these structures, a type of ‘gate control’.
Further studies are required into this hypothesis. The existence of
TnTs in vivo has been documented for certain cell types,
therefore TnTs between NPCs might enable contact of these remote
cells in the structure of NP (12).
Co-culture of DiO-stained NP and DiD-stained MSCs
and invert dye DiD-stained NP and DiO-stained MSCs revealed that
the exchange of dyes is asymmetrical. Lipophilic dye exchange has
been studied in rabbit and human NPCs and MSC co-culture systems
(4,10). In rabbit culture, PKH26 (red) and
PKH67 (green) lipophilic dyes were used. Subsequent to a two-week
incubation period on alginate beads 42% of the cells were
double-stained (4). A flow
cytometric analysis revealed that the exchange of dyes between
rabbit NPCs and MSCs is balanced and the cytometric diagram was
less asymmetrical (in relation to a 45° axis) than our diagram,
nevertheless, it is probable that the MSCs secreted more PKH26 due
to fewer PHK67-stained NPCs observed (4). The co-culture resulted in the
generation of a double-stained cell population characterized by
increased Col2a1 and Acan mRNAs levels. Double
nucleolar hybrids were also detected (4).
In comparison to former results, the experiments of
this study demonstrated a more rapid flow of DiO and DiD between
NPCs and MSCs and after 8 days the majority of the cells were
double-stained. Comparing our flow cytometric results from direct
co-culture experiments: NP/DiO and MSC/DiD vs. MSC/DiO and NP/DiD
both diagrams were asymmetrical but not identical. The asymmetrical
diagrams may reflect combined effect of various properties of DiO
and DiD and reveals more efficient flow of both fluorescent
lipophilic dyes DiO and DiD from MSCs toward NPCs in the direct
systems (25). Asymmetrical
secretion of lipophylic dye DiO was already observed in co-culture
of renal tubular cells and MSCs (26). In the indirect system we did not
observe this preferred direction of the DiD flow from MSCs toward
NPCs. We concluded that direct contact and TnT formation enables
more rapid flow of dyes from MSCs toward NPCs.
Based on previous results of experiments performed
in NPCs and MSCs co-culture systems in which only <1% of cells
formed hybrids, a hypothesis of cell fusion was abandoned as a rare
event (27). A hypothesis of
transient, short-term fusion and fission cannot be excluded nor
confirmed based on the present and previous results. A hypothesis
of the presence of TnTs was confirmed by the present results where
double-staining of NPCs and MSCs with DiO and DiD was applied and
followed by the observation under a confocal microscope. A
hypothesis of hemifusion of membranes is markedly supported by the
current results but not proven. The hemifusion hypothesis was able
to explain the conflicting results. In previous studies ~1% of
co-cultured NPCs and MSCs were double-stained (fused) following
staining with hydrophilic dyes or fluorescent proteins (8,10,28).
By contrast, in similar culture systems in which lipophilic dyes
were applied, up to 42% of double-stained cells were observed. Our
cytometric measurements revealed >50% of cells were
double-stained by DiO and DiD. Hemifusion may concern both MVs in
the insert system and whole cells in the one-surface system where
only membrane phospholipids are exchanged without pore formation
(29). The transfer of membrane
lipids and lipophilic dyes was higher compared with that of the
membrane proteins (21). Membrane
lipids, gangliosides and sphinogosine-phosphate, may promote
differentiation of MSCs independently or with growth factors
(30–32). Independent cell-cell membrane
contact may be a factor initiating the potential changes which are
a functional determinant of MSC differentiation and cell function
(33).
However, issues concerning the nature of the
information which is transported via MVs and TnTs remain. The most
efficient way of chondrocyte-like NP phenotype induction from MSCs
remains under debate and requires additional studies concerning
phenotype induction.
Hybrids in the co-cultured cells account <1% and
it is likely that they do not contribute to the net phenotype of
the cell co-culture (8,10). However, no clear rules emerged as
to which phenotype may predominate in hybrids (34). The mechanisms by which one
phenotype predominates over another in such hybrids remains poorly
understood. The same consideration may be applied in co-culture
systems. The resulting phenotype of the two co-cultured cells is
unknown and unpredictable. Degenerated NPCs co-cultured with MSCs
have a higher cell division rate and gene expression. MSCs express
cartilage-specific genes. No theory predicting the phenotype of
cells which were maintained with other cells in co-culture has been
suggested. In general, it was concluded that the ‘contagious
phenotype theory’ predominates in the description of the results of
these investigations. These approaches have a default assumption
that MSCs ‘learn’ their phenotype from NPCs, and promote cell
division in NPCs. However, the manner in which the NP phenotype can
be mimicked by MSCs and whether or not this method is safe with
regard to possible chromosomal rearrangement due to hybrid
formation remain to be determined (22).
Acknowledgements
This study was supported by a National Science
Centre grant, no. N N 403600538. We would like to thank Ms Beata
Raczak and Bogumila Ratajczak for their indispensable help during
the preparation of this manuscript.
References
|
1
|
Roberts S, Evans H, Trivedi J and Menage
J: Histology and pathology of the human intervertebral disc. J Bone
Joint Surg Am. 88(Suppl 2): 10–14. 2006. View Article : Google Scholar
|
|
2
|
Yamamoto Y, Mochida J, Sakai D, et al:
Upregulation of the viability of nucleus pulposus cells by bone
marrow-derived stromal cells: significance of direct cell-to-cell
contact in coculture system. Spine (Phila Pa 1976). 29:1508–1514.
2004. View Article : Google Scholar
|
|
3
|
Wei A, Chung SA, Tao H, et al:
Differentiation of rodent bone marrow mesenchymal stem cells into
intervertebral disc-like cells following coculture with rat disc
tissue. Tissue Eng Part A. 15:2581–2595. 2009. View Article : Google Scholar
|
|
4
|
Niu CC, Yuan LJ, Lin SS, Chen LH and Chen
WJ: Mesenchymal stem cell and nucleus pulposus cell coculture
modulates cell profile. Clin Orthop Relat Res. 467:3263–3272. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Visage C, Kim SW, Tateno K, Sieber AN,
Kostuik JP and Leong KW: Interaction of human mesenchymal stem
cells with disc cells: changes in extracellular matrix
biosynthesis. Spine (Phila Pa 1976). 31:2036–2042. 2006.
|
|
6
|
Richardson SM, Walker RV, Parker S, et al:
Intervertebral disc cell-mediated mesenchymal stem cell
differentiation. Stem Cells. 24:707–716. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang SH, Wu CC, Shih TT, Sun YH and Lin
FH: In vitro study on interaction between human nucleus pulposus
cells and mesenchymal stem cells through paracrine stimulation.
Spine (Phila Pa 1976). 33:1951–1957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vadalà G, Studer RK, Sowa G, et al:
Coculture of bone marrow mesenchymal stem cells and nucleus
pulposus cells modulate gene expression profile without cell
fusion. Spine (Phila Pa 1976). 33:870–876. 2008.PubMed/NCBI
|
|
9
|
Strassburg S, Richardson SM, Freemont AJ
and Hoyland JA: Co-culture induces mesenchymal stem cell
differentiation and modulation of the degenerate human nucleus
pulposus cell phenotype. Regen Med. 5:701–711. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strassburg S, Hodson NW, Hill PI,
Richardson SM and Hoyland JA: Bi-directional exchange of membrane
components occurs during co-culture of mesenchymal stem cells and
nucleus pulposus cells. PLoS One. 7:e337392012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gerdes HH, Rustom A and Wang X: Tunneling
nanotubes, an emerging intercellular communication route in
development. Mech Dev. 131:381–387. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abounit S and Zurzolo C: Wiring through
tunneling nanotubes - from electrical signals to organelle
transfer. J Cell Sci. 125:1089–1098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horwitz EM, Le Blanc K, Dominici M, et al:
Clarification of the nomenclature for MSC: The International
Society for Cellular Therapy position statement. Cytotherapy.
7:393–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang S, Tam V, Cheung KM, et al: Stem
cell-based approaches for intervertebral disc regeneration. Curr
Stem Cell Res Ther. 6:317–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie LW, Fang H, Chen AM and Li F:
Differentiation of rat adipose tissue-derived mesenchymal stem
cells towards a nucleus pulposus-like phenotype in vitro. Chin J
Traumatol. 12:98–103. 2009.PubMed/NCBI
|
|
16
|
Feng G, Jin X, Hu J, et al: Effects of
hypoxias and scaffold architecture on rabbit mesenchymal stem cell
differentiation towards a nucleus pulposus-like phenotype.
Biomaterials. 32:8182–8189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gantenbein-Ritter B, Benneker LM, Alini M
and Grad S: Differential response of human bone marrow stromal
cells to either TGF-β(1) or rhGDF-5. Eur Spine J. 20:962–971.
2011.
|
|
18
|
Morigele M, Shao Z, Zhang Z, et al: TGF-β1
induces a nucleus pulposus-like phenotype in Notch 1 knockdown
rabbit bone marrow mesenchymal stem cells. Cell Biol Int.
37:820–825. 2013.
|
|
19
|
Yuan M, Leong KW and Chan BP:
Three-dimensional culture of rabbit nucleus pulposus cells in
collagen microspheres. Spine J. 11:947–960. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leckie SK, Sowa GA, Bechara BP, et al:
Injection of human umbilical tissue-derived cells into the nucleus
pulposus alters the course of intervertebral disc degeneration in
vivo. Spine J. 13:263–272. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Niu X, Gupta K, Yang JT, Shamblott MJ and
Levchenko A: Physical transfer of membrane and cytoplasmic
components as a general mechanism of cell-cell communication. J
Cell Sci. 122:600–610. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oren-Suissa M and Podbilewicz B: Evolution
of programmed cell fusion: common mechanisms and distinct
functions. Dev Dyn. 239:1515–1528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vallabhaneni KC, Haller H and Dumler I:
Vascular smooth muscle cells initiate proliferation of mesenchymal
stem cells by mitochondrial transfer via tunneling nanotubes. Stem
Cells Dev. 21:3104–3113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee TH, D’Asti E, Magnus N, Al-Nedawi K,
Meehan B and Rak J: Microvesicles as mediators of intercellular
communication in cancer - the emerging science of cellular
‘debris’. Semin Immunopathol. 33:455–467. 2011.PubMed/NCBI
|
|
25
|
Chazotte B: Labeling membranes with
carbocyanine dyes (Dils) as phospholipid analogs. Cold Spring Harb
Protoc. 2011:pdb prot5555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Plotnikov EY, Khryapenkova TG, Galkina SI,
Sukhikh GT and Zorov DB: Cytoplasm and organelle transfer between
mesenchymal multipotent stromal cells and renal tubular cells in
co-culture. Exp Cell Res. 316:2447–2455. 2010. View Article : Google Scholar
|
|
27
|
Chen EH and Olson EN: Unveiling the
mechanisms of cell-cell fusion. Science. 308:369–373. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ferrand J, Noël D, Lehours P, et al: Human
bone marrow-derived stem cells acquire epithelial characteristics
through fusion with gastrointestinal epithelial cells. PLoS One.
6:e195692011. View Article : Google Scholar
|
|
29
|
Chernomordik LV and Kozlov MM: Membrane
hemifusion: crossing a chasm in two leaps. Cell. 123:375–382. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nincheri P, Luciani P, Squecco R, et al:
Sphingosine 1-phosphate induces differentiation of adipose
tissue-derived mesenchymal stem cells towards smooth muscle cells.
Cell Mol Life Sci. 66:1741–1754. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang HJ, Jung KY, Kwak DH, et al:
Inhibition of ganglioside GD1a synthesis suppresses the
differentiation of human mesenchymal stem cells into osteoblasts.
Dev Growth Differ. 53:323–332. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang L, Chang N, Liu X, et al: Bone
marrow-derived mesenchymal stem cells differentiate to hepatic
myofibroblasts by transforming growth factor-β1 via sphingosine
kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J
Pathol. 181:85–97. 2012.PubMed/NCBI
|
|
33
|
Sundelacruz S, Levin M and Kaplan DL:
Membrane potential controls adipogenic and osteogenic
differentiation of mesenchymal stem cells. PLoS One. 3:e37372008.
View Article : Google Scholar
|
|
34
|
Essentials of Stem Cell Biology. Lanza R,
Gearhart J, Hogan B, Melton D, Pedersen R, Donnall Thomas E,
Thomson AJ and Wilmut I: Academic Press; pp. 105–110. 2009
|