Introduction
The development of mouse pre-implantation embryos
involves substantial cell proliferation and differentiation, and
cell lineages are established early during embryonic development.
Various studies, over the past few years, have identified key
transcription factor networks during mouse pre-implantation
embryonic development (1–5). These transcription factors, including
octamer-binding transcription factor 4 (Oct4), Sox2, Kruppel-like
factor 4 (Klf4) and c-Myc have been demonstrated to reprogram
somatic cells in vitro leading to the generation of
pluripotent stem cells derived from mouse embryonic fibroblasts
(6). Therefore, transcription
factors are essential regulators of the transition from
germ-cell-type to embryo-type gene expression and are key in
determining cell characteristics (7). In addition, numerous studies have
shown that epigenetic regulation is a decisive factor in
pre-implantation embryo development (8,9) and
each embryonic developmental stage is characterized by a specific
epigenetic pattern (10). It has
been determined that in vitro developmental block occurs in
different species at different stages of embryonic development,
which is concurrent with embryonic genome activation (11). The developmental block in mouse,
bovine and human embryos occurs at the two-cell, four-cell and
four- to eight-cell stages (12).
In clinically assisted reproduction practices, human
embryos cultured in vitro are sensitive to developmental
arrest and the completed blastocyst formation rate is only ~50%
(13); however, the reasons for
this are not well understood. Although improvements in in
vitro culture conditions have greatly enhanced the embryo
integrity, the in vitro fertilization (IVF) success rates
are not ideal. Thus, increased knowledge of the molecular
mechanisms regulating oogenesis, fertilization, cleavage,
blastocyst formation and implantation is crucial for future
assisted reproductive technology improvements. The Serine/threonine
protein kinase 40 (Stk40) Stk40 gene has been identified
previously, by a low density array analysis, to be one of the
development-related transcription factors in mouse embryonic
pre-implantation development. This gene was initially defined as a
SINK-homologous serine/threonine kinase, designated as SHIK, which
negatively regulates NF-κB and p53-mediated transcription (14). In a previous ChIP-chip assay, the
promoter of Stk40 was demonstrated to associate with Oct4 as well
as Sox2 and Nanog (15). In
addition, it was shown that Stk40, a target gene of Oct4
transcription, was able to activate the extracellular
signal-regulated kinase (ERK)/mitogen-activated protein kinase
(MAPK) signaling pathway and induce embryonic stem cell
differentiation into extraembryonic-endoderm (ExEn) cell lineages
through reticulocalbin-2 (Rcn2) (16). However, its function and mechanism
of gene regulation in early embryonic development remains to be
further elucidated.
In the present study, the expression and
localization of the Stk40 gene was investigated during mouse
embryonic development. Downregulation of Stk40 indicated that this
gene was essential in mouse embryogenesis, particularly in
blastocyst formation. In addition, the results suggested an
association between Stk40 and the ERK/MAPK signaling pathway.
Materials and methods
Chemicals
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
(HEPES)-buffered Chatot, Ziomet and Bavister (CZB) medium was
purchased from Sigma-Aldrich (St. Louis, MO, USA), polyclonal Stk40
antibodies were purchased from Jingtiancheng Biotech Co., Ltd.
(Beijing, China).
Embryo collection and culture
A total of 200 Imprinting control region (ICR)
female mice (Shanghai Slac Labortory Animal Co., Ltd., Shanghai,
China) were maintained in a controlled environment of 20–22°C and
12/12-h light/dark cycle, with 50–70% humidity and access to food
and water ad libitum. Animal care and experimental
procedures were conducted in accordance with the Animal Research
Committee guidelines of the Nanjing Medical University (Nanjing,
China). For the collection of zygotes, female ICR mice (age, 8
weeks) were injected with human chorionic gonadotropin and mated
with ICR male mice shortly following the injection. After 15–17 h,
zygotes were collected from the oviducts of the mice and
transferred onto a HEPES-buffered CZB medium. Cumulus cells were
dispersed by treatment with 1 mg/ml hyaluronidase in HEPES-CZB.
Cumulus-free zygotes were washed with HEPES-CZB medium and cultured
in CZB medium until they grew to the later early embryo stages at
37°C in a 5% CO2 atmosphere. The study was approved by
the Ethical committee of Nanjing Normal University, Nanjing
Maternity and Child Health Care Hospital Affiliated to Nanjing
Medical University and of the Medical School of Nanjing University,
Nanjing University (Nanjing, China).
Immunoblotting analysis
Immunoblotting was performed to analyze the valence
of made-to-order Stk40 antibodies (Jingtiancheng Biotech Co.,
Ltd.). The ovary proteins of adult ICR mice were separated by
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then
electrophoresed onto polyvinylidene fluoride membranes. Following
the transfer, the membranes were blocked in Tris-buffered saline
with 0.1% Tween-20 (TBST) containing 5% skimmed milk for 2 h,
followed by overnight incubation at 4°C with rabbit polyclonal
anti-Stk40 antibodies (dilution, 1:500; NFB001; Jingtiancheng
Biotech Co., Ltd.). Following three 10 min washes with TBST, the
membranes were incubated for 1 h at 37°C with horseradish
peroxidase-conjugated goat anti-rabbit IgG (dilution, 1:1000;
Zhongshan Biotech, Beijing, China). The membranes were then
processed using an enhanced chemiluminescence detection system
(Model 920; Amersham Biosciences, Piscataway, NJ, USA).
Immunofluorescence and confocal
microscopy
For staining of Stk40, the embryos were fixed in 4%
paraformaldehyde in phosphate-buffered saline (PBS; pH 7.4) for ≥30
min at room temperature. Subsequent to permeabilization with 0.5%
Triton X-100 at room temperature for 20 min, the embryos were
incubated with 1% bovine serum albumin-supplemented PBS for 1 h and
incubated overnight at 4°C with rabbit anti-Stk40 antibodies
(dilution, 1:1000; Jingtiancheng Biotech Co., Ltd.). Following
three 5 min washes with PBS containing 0.1% Tween-20 and 0.01%
Triton X-100, the embryos were labeled with fluorescein
isothiocyanate-conjugated goat anti-rabbit IgG (dilution, 1:100;
Zhongshan Biotech) for 1 h at room temperature. Subsequent to
another three washes in PBS containing 0.1% Tween-20 and 0.01%
Triton X-100, the embryos were stained with propidium iodide (10
μg/ml in PBS). The embryos were mounted on glass slides and
examined under a confocal laser scanning microscope (Zeiss LSM 510
META, Zeiss, Jena, Germany).
Stk40 short interfering RNA (siRNA)
microinjection and in vitro culture
Stk40-siRNA (MSS232404, MSS232405 and MSS292686) was
purchased from Invitrogen Life Technologies (Carlsdbad, CA, USA).
MISSION® siRNA is a heterogeneous mixture of siRNAs that
target the same mRNA sequence. This multiple silencing approach
leads to highly specific and effective gene silencing. The
Stk40-siRNA was stored at −20°C at a concentration of 200 ng/μl.
Approximately 10 pl of individual siRNA was microinjected into the
cytoplasm of the zygotes, which were cultured in CZB medium for
further observation. The developmental status of each group was
identified and analyzed using a Nikon Eclipse TE2000-S
microscope.
Reverse-transcription polymerase chain
reaction (real-time PCR)
The primer sequences of Stk40 and related
transcription factors are shown in Table I. siRNA-injected and control
oocytes were paired to assess the differences in the mRNA quantity.
Total RNA extraction and reverse transcription were performed using
an RNeasy Micro kit (Qiagen, Valencia, CA, USA), according to the
manufacturer's instructions and oligo-dT was used as a primer.
Real-time PCR analysis was conducted using Fast start Universal
SYBR Green Master (Roche Diagnostics Co., Indiannapolis, IN, USA),
with an ABI Prism 7500 System (Applied Biosystems, Foster City, CA,
USA). We processed 15–30 embryos at a time and GAPDH was used as an
endogenous control. The 2−ΔΔCt method was used to
analyze relative changes in gene expression between the groups.
 | Table IPrimer sequences. |
Table I
Primer sequences.
| Gene name | Primer sequence |
|---|
| Stk40 | F:
5′-AGGAACTGGCATTGCTGGAAATAA-3′
R: 5′-TGCTCGGTACCGGTGAGTTG-3′ |
| GAPDH | F:
5′-AGGTTGTCTCCTGCGACTTCA-3′
R: 5′-GGGTGGTCCAGGGTTTCTTACT-3′ |
| Oct4 | F:
5′-ATGGCATACTGTGGACCTCA-3′
R: 5′-AGCAGCTTGGCAAACTGTTC-3′ |
| Nanog | F:
5′-CTCATCAATGCCTGCAGTTTTTCA-3′
R: 5′-CTCCTCAGGGCCCTTGTCAGC-' |
| Rcn2 | F:
5′-GGTCGTTCAGGCAGCTTCATC-3′
R: 5′-CTGGGTCTTCATTTGCAGTTGG-3′ |
| Cdx2 | F:
5′-CAGCAGTCCCTAGGAAGCCAA-3′
R: 5′-GTGTGGCAGCCAGCTCACTT-3′ |
Statistical analysis
All experiments were repeated three times. Prior to
significance analyses, all percentage data were subjected to
Arcsine transformation. Data were analyzed with paired Student's
t-tests and the χ2 test, using SPSS software (SPSS Inc.,
Chicago, IL, USA). Data are expressed as the mean ± standard error
of the mean. P<0.05 and P<0.01 were considered to indicate a
statistically significant difference.
Results
Expression pattern of Stk40 mRNA in early
embryonic stages
Real-time PCR was used to detect Stk40 at different
embryonic stages and compare its transcript levels between the
stages. cDNA was synthesized from mRNA isolated at the zygote,
2-cell, 4-cell, 8-cell, morula and developing blastocyst stages.
For sample analysis, the comparative 2−ΔΔCT method was
used to quantify the real-time results for the Stk40 transcripts.
All study groups were normalized with GAPDH references and the
zygote stage served as the calibrator. Therefore, the efficiencies
of target and reference gene detections were similar and the
2−ΔΔCT calculation method was used for relative
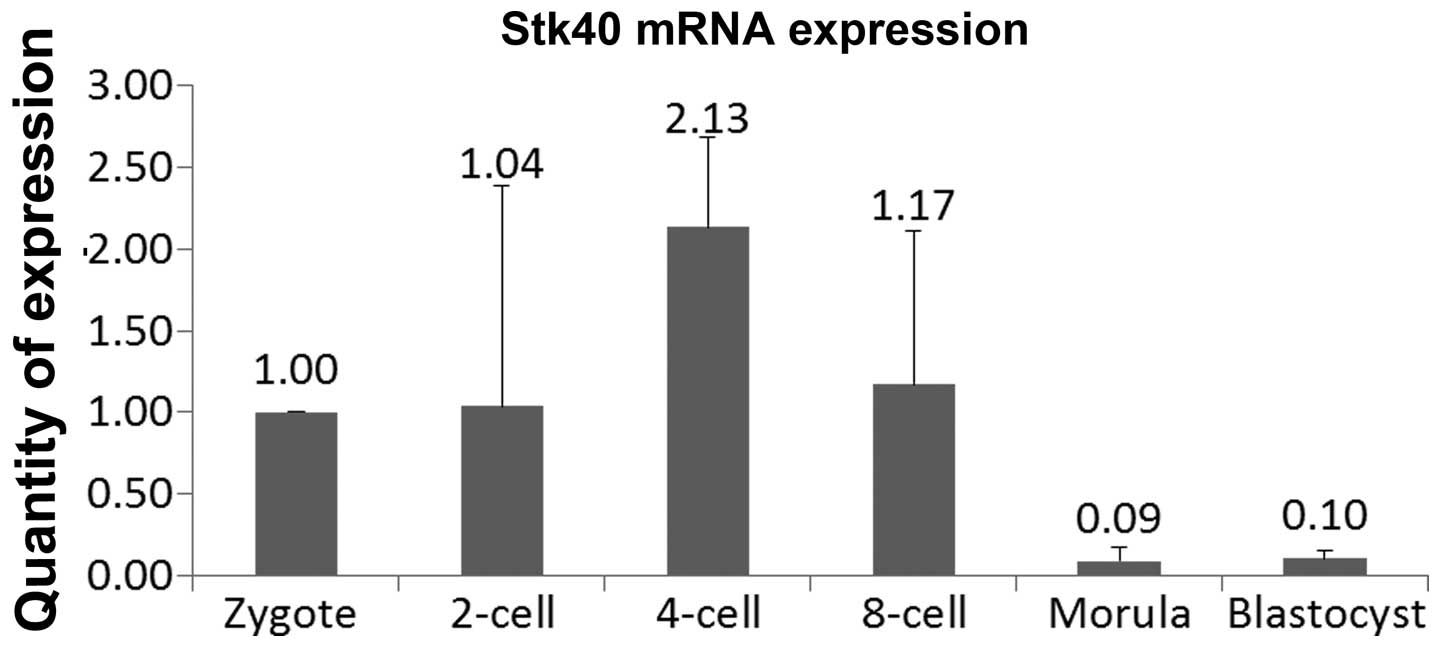
quantification. Stk40 was expressed at the highest level in the
four-cell stage, while the relative level of Stk40 mRNA in the
morula or blastocyst stage was only one-tenth that observed at the
zygote stage (Fig. 1).
Subcellular localization of Stk40 protein
in early embryos
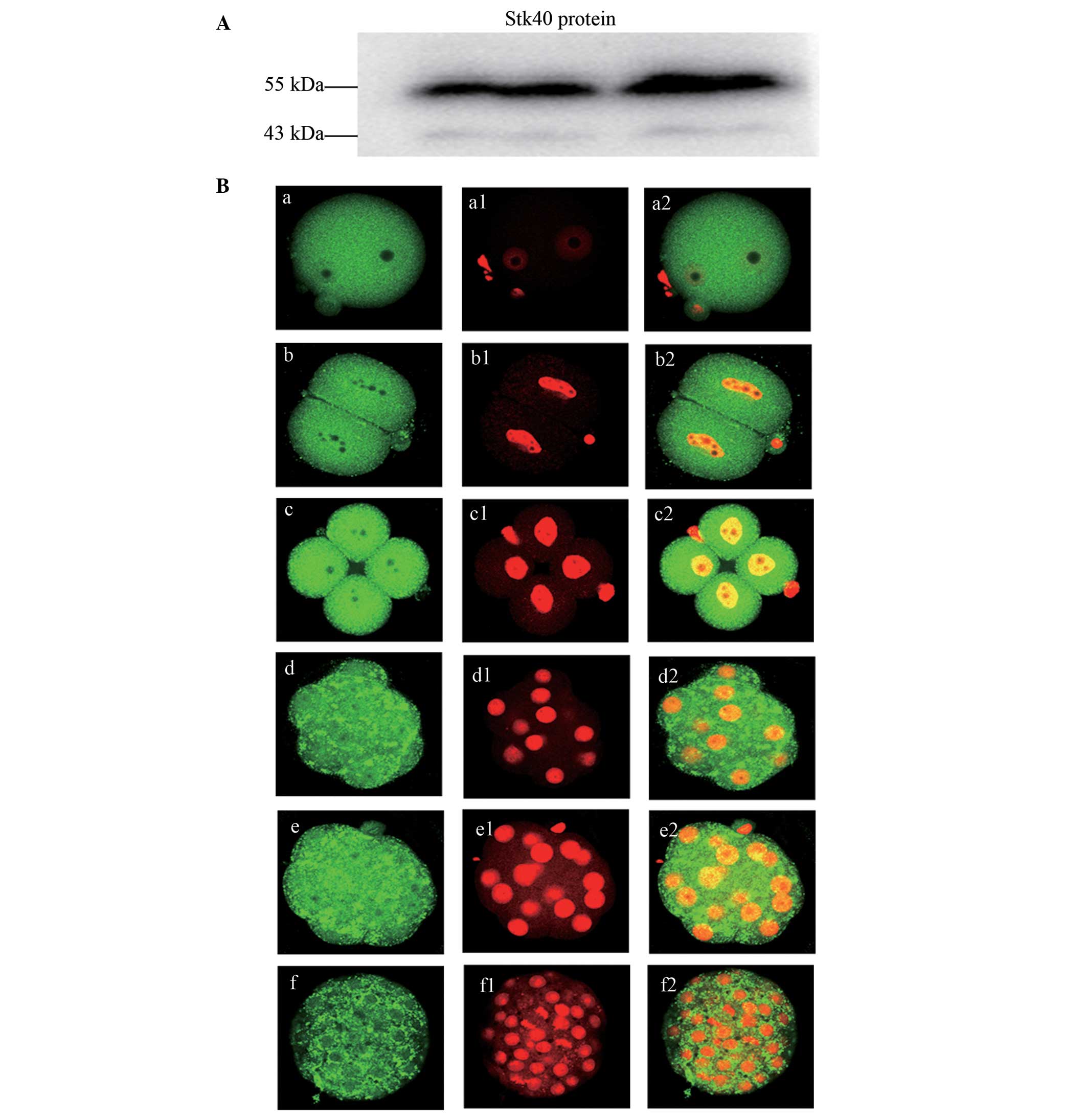
The specificity of antibody to the Stk40 protein was
investigated by western blot analysis. The molecular weight of the
anti-Stk40 antibody appeared between 43 and 55 kDa (~50 kDa) with
mouse ovary samples (Fig. 2A). The
subcellular localization of Stk40 protein in early mouse embryos
was investigated. The fluorescent staining of Stk40 was observed
equally in the cytoplasm and nuclei in all stages of the
pre-implantation mouse embryos. However, the most prominent
staining appeared in the four-cell stage (Fig. 2B).
Stk40 endoribonuclease-prepared siRNA
microinjection and in vitro culture
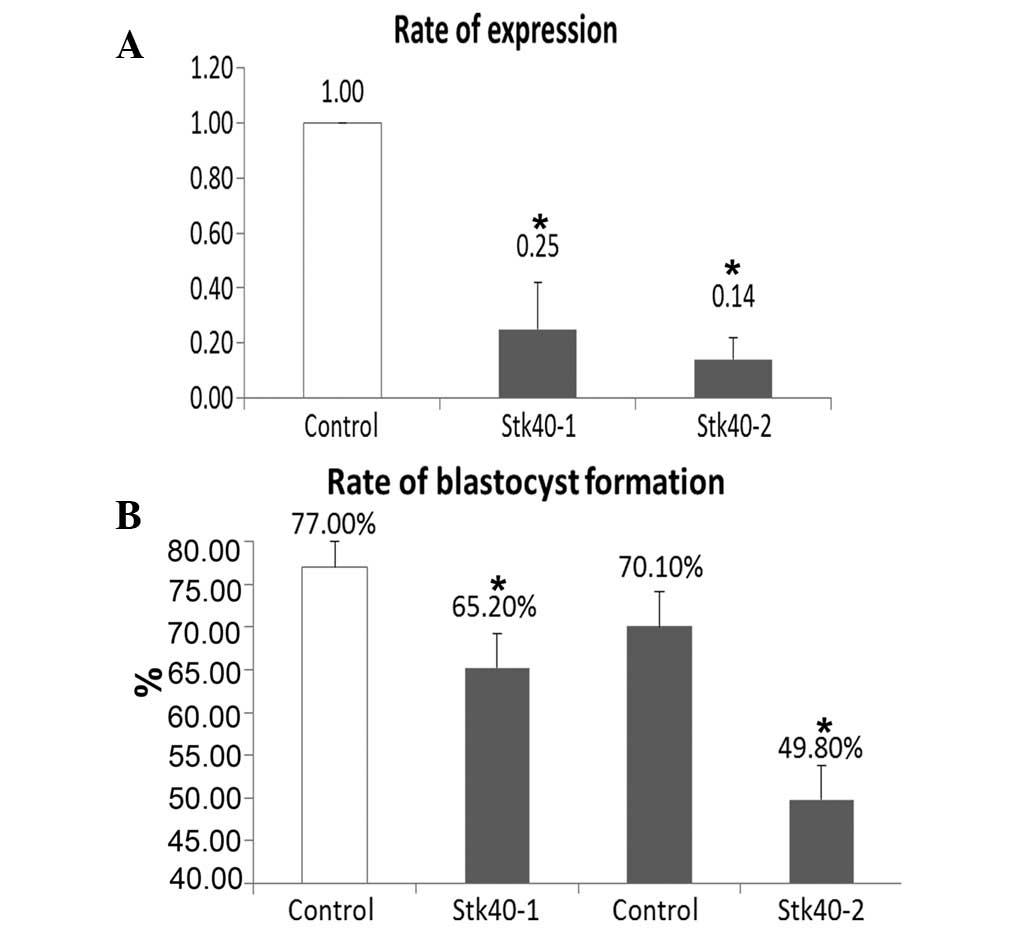
The inhibitory effect of specific Stk40-targeting
siRNA was confirmed using real-time PCR. The injection of the two
different Stk40-specific siRNAs (Stk40-1 and Stk40-2) significantly
reduced the level of Stk40 mRNA in mouse blastocysts (P<0.05;
Fig. 3A). The development of
one-cell zygotes in the Stk40 siRNA-injected groups was observably
stunted compared with the non-silencing siRNA-injected groups at
different time points including 2.5 days (4-cell stage), 3.5 days
(morula stage) and 4.5 days (blastocyst stage). In particular, the
blastocyst formation rates of the two Stk40-silenced groups showed
a significant decrease compared with that of the
non-silencing-siRNA injected control groups (Stk40-1 versus
control: 65.2% and 77.0%, P<0.05; Stk40-2 versus control: 49.8%
and 70.1%, respectively; P<0.05) (Fig. 3B).
Increase in Rcn2 expression in Stk40
silenced embryos
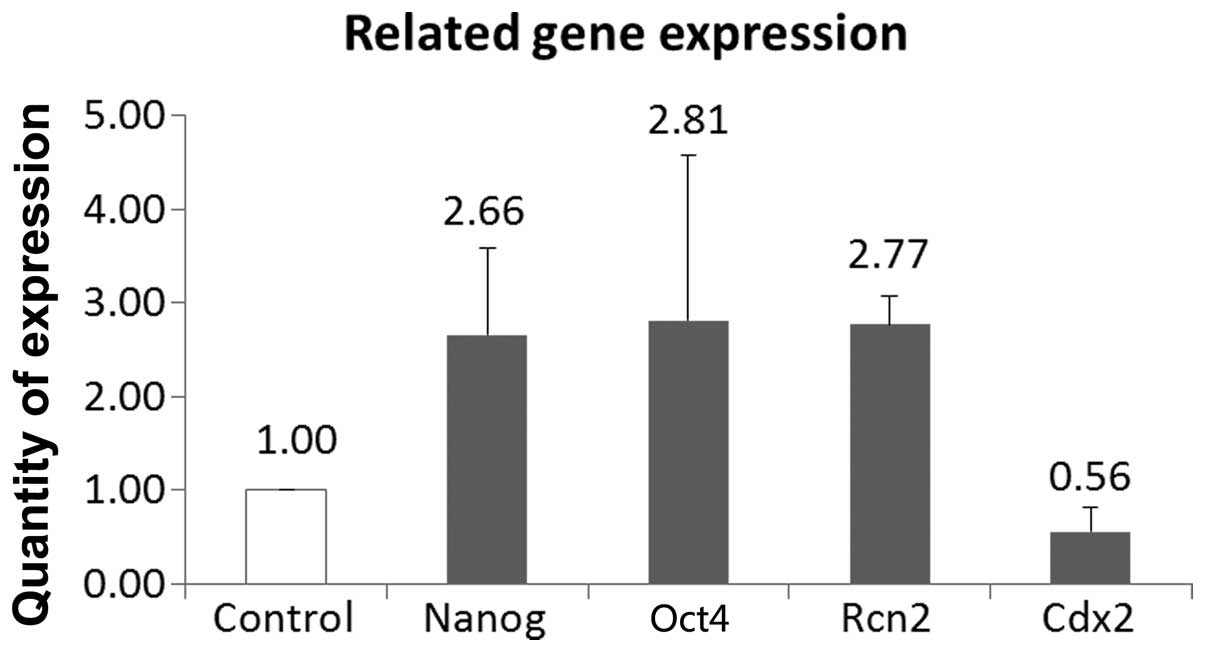
The levels of the pluripotency markers Oct4 and
Nanog, the extraembryonic endoderm marker Rcn2 and the
trophectoderm (TE)-specific gene Cdx2 were assessed using real-time
PCR in Stk40-silenced embryos and compared with those in the
non-silencing siRNA-injected embryos (negative controls). Stk40
siRNA-injected embryos showed significantly increased Rcn2 and
decreased Cdx2 levels (Fig. 4). In
every embryonic development stage of each group, at least 35
embryos were observed.
Discussion
In the present study, Stk40 levels were analyzed in
early mouse embryos at different embryonic pre-implantation stages
using real-time PCR. The results showed that Stk40 was expressed in
all investigated stages; however, the expression level began to
decrease markedly from the four-cell stage onwards. Previous
studies in mice have demonstrated that the greatest alteration in
transcription factor activities occurred between the one- and
two-cell stages, at which time zygotic genome activation (ZGA)
occurs. Moreover, basic transcription factors, which are the
general components of transcription, increased transiently at the
two-cell stage (7). These findings
are consistent with data from the present study regarding the
expression pattern of Stk40. Thus, the expression peak at the
two-cell stage and the subsequent decline suggested that Stk40 may
be important in the maternal-to-embryonic transition process. In
addition, the subcellular localization of the Stk40 protein
confirmed the results of the real-time PCR analysis and showed that
there was a translation delay with the most prominent protein
staining appearing in the four-cell stage. Furthermore, it was
demonstrated that Stk40 downregulation by siRNA markedly decreased
the rate of blastocyst formation.
It was observed that the Stk40 transcription factor
was expressed in dynamic patterns following fertilization of the
oocyte and may be important in embryonic pre-implantation
development. Identification of critical regulatory genes is a
crucial step in understanding early embryogenesis; thus, the mRNA
levels of possible related genes were measured via real-time,
following Stk40-specific siRNA microinjection. This included the
pluripotency markers Oct4, Nanog, Rcn2 and Cdx2. The results showed
that Oct4, Nanog and Rcn2 were upregulated and Cdx2 was
downregulated following Stk40-specific siRNA microinjection.
Cell proliferation is an important event in early
embryonic development and is largely affected by the regulation of
diversified signaling pathways, such as the classic ERK/MAPK
signaling pathway (2). This
pathway is a critical regulator of cellular processes in adult and
developing tissues (17), which
exerts its biological effects predominantly through the highly
selective activation of ERK1/2 (18). It has been suggested that Stk40
activated Erk1/2 through Rcn2 and participated in the ERK/MAPK
pathway through Oct4 to control the ExEn lineage differentiation
(16). According to the results of
the present study, it was suggested that the interaction between
Stk40 and the ERK/MAPK signaling pathway influenced the embryonic
mouse pre-implantation development. Oct4 and Nanog are known to be
pluripotency markers and exhibit restricted expression in
pluripotent cell types (19,20).
Previous studies indicated that Oct4 was present in all embryonic
cells until the late blastocyst stage, gradually disappearing from
the TE thereafter (21). Oct4 is
highly expressed at the 1- and 2-cell stages in embryos,
facilitating ZGA and enhancing maternal mRNA degradation (22). In the present study, the expression
pattern of Stk40 was similar to that of Oct4 and, following Stk40
downregulation, increasing Oct4 expression levels appeared in a
compensation pattern. Conversely, Nanog expression is initiated in
the late stages of pre-implantation embryos and its mRNA is first
detected at approximately the eight-cell stage (21). Thus, its expression pattern is
opposite to that of Oct4 and Stk40. Notably, Nanog is also an
activator of Oct4 through binding to the distal enhancer of Oct4
(19). The results indicated that
Nanog expression was upregulated following Stk40 downregulation.
This is consistent with a recent study, which demonstrated that
Stk40 expression was gradually upregulated when Oct4 expression was
rapidly silenced, as Nanog expression was downregulated during this
process (16). However, the
reciprocal interactions between Stk40 and Nanog remain to be fully
evaluated.
It was demonstrated that Stk40 was incapable of
promoting the extraembryonic endoderm differentiation when Rcn2 was
knocked out (16). Rcn2 is a
Ca2+-binding protein, known as an extraembryonic
endoderm differentiation marker together with Gata6 and Dab2
(23). It was shown that the Stk40
downregulation increased the Rcn2 expression level ~3-fold compared
with the control group, suggesting that Rcn2 may function
downstream of Stk40; however, the correlation between Rcn2 and
embryonic development arrest requires further investigation. Cdx2
is a known tumor repressor in intestinal epithelium (24). It was previously demonstrated that
Cdx2 is involved in trophectoderm formation at the blastocyst stage
in mice and the expression pattern of Cdx2 during pre-implantation
stages is upregulated in 8–16-cell stage embryos (25). Moreover, inactivation of Cdx2 leads
to embryonic pre-implantation lethality (26). Therefore Cdx2 was selected as a
blastocyst formation marker for analyzing the interaction with
Stk40 at the blastocyst stage. In the present study it was observed
that Cdx2 expression was significantly decreased following Stk40
gene silencing, which significantly affected blastocyst formation.
A previous study suggested that Cdx2 is essential for segregation
of the inner cell mass and TE lineages at the blastocyst stage by
ensuring repression of Oct4 and Nanog in the TE (27). Thus, it was speculated that low
Cdx2 expression following Stk40 downregulation may have been the
reason for Oct4 and Nanog upregulation. Although the correlation
between Cdx2 and Stk40 was not directly demonstrated, it was
suggested that once expression of these genes reached their
threshold levels and an imbalance between them occurred, aberrant
embryonic development is triggered.
In conclusion, it was suggested that the Stk40
transcription factor may be crucial in mouse embryonic
pre-implantation development. Further study of transcription
factors may provide a reliable molecular biological basis and
molecular targeting candidates for improving the efficiency of
in vitro embryo culture and enhancing the clinical
outcome.
Acknowledgements
This study was financially supported by the National
Natural Science Foundation of China (grant nos. 81100420 and
81270701), the Foundation of Nanjing Medical University (grant no.
2011NJMU210), the Natural Science Foundation of Jiangsu Province
(grant no. BK2012520) and the Nanjing Medical Science and Technique
Development Foundation (2010NJMU030).
References
|
1
|
Hamatani T, Carter MG, Sharov AA and Ko
MS: Dynamics of global gene expression changes during mouse
preimplantation development. Dev Cell. 6:117–131. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyanari Y and Torres-Padilla ME:
Epigenetic regulation of reprogramming factors towards pluripotency
in mouse preimplantation development. Curr Opin Endocrinol Diabetes
Obes. 17:500–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pantazis P and Bollenbach T: Transcription
factor kinetics and the emerging asymmetry in the early mammalian
embryo. Cell Cycle. 11:2055–2058. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki H: Mechanisms of trophectoderm fate
specification in preimplantation mouse development. Dev Growth
Differ. 52:263–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winger Q, Huang J, Auman HJ, Lewandoski M
and Williams T: Analysis of transcription factor AP-2 expression
and function during mouse preimplantation development. Biol Reprod.
75:324–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Carey BW, Markoulaki S, Hanna J, et al:
Reprogramming of murine and human somatic cells using a single
polycistronic vector. Proc Natl Acad Sci USA. 106:157–162. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kageyama S, Gunji W, Nakasato M, Murakami
Y, Nagata M and Aoki F: Analysis of transcription factor expression
during oogenesis and preimplantation development in mice. Zygote.
15:117–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saitou M, Kagiwada S and Kurimoto K:
Epigenetic reprogramming in mouse pre-implantation development and
primordial germ cells. Development. 139:15–31. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu FR, Liu Y, Shang MB, et al: Differences
in H3K4 trimethylation in in vivo and in vitro fertilization mouse
preimplantation embryos. Genet Mol Res. 11:1099–1108. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palini S, De Stefani S, Scala V, Dusi L
and Bulletti C: Epigenetic regulatory mechanisms during
preimplantation embryo development. Ann N Y Acad Sci. 1221:54–60.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bettegowda A, Lee KB and Smith GW:
Cytoplasmic and nuclear determinants of the maternal-to-embryonic
transition. Reprod Fertil Dev. 20:45–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Memili E and First NL: Zygotic and
embryonic gene expression in cow: a review of timing and mechanisms
of early gene expression as compared with other species. Zygote.
8:87–96. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang JQ, Li XL, Peng Y, Guo X, Heng BC
and Tong GQ: Reduction in exposure of human embryos outside the
incubator enhances embryo quality and blastulation rate. Reprod
Biomed Online. 20:510–515. 2010. View Article : Google Scholar
|
|
14
|
Huang J, Teng L, Liu T, et al:
Identification of a novel serine/threonine kinase that inhibits
TNF-induced NF-kappaB activation and p53-induced transcription.
Biochem Biophys Res Commun. 309:774–778. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim J, Chu J, Shen X, Wang J and Orkin SH:
An extended transcriptional network for pluripotency of embryonic
stem cells. Cell. 132:1049–1061. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li L, Sun L, Gao F, et al: Stk40 links the
pluripotency factor Oct4 to the Erk/MAPK pathway and controls
extraembryonic endoderm differentiation. Proc Natl Acad Sci USA.
107:1402–1407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shvartsman SY, Coppey M and Berezhkovskii
AM: MAPK signaling in equations and embryos. Fly (Austin). 3:62–67.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rajkumar R, Konishi K, Richards TJ, et al:
Genomewide RNA expression profiling in lung identifies distinct
signatures in idiopathic pulmonary arterial hypertension and
secondary pulmonary hypertension. Am J Physiol Heart Circ Physiol.
298:H1235–H1248. 2010. View Article : Google Scholar
|
|
19
|
Mitsui K, Tokuzawa Y, Itoh H, et al: The
homeoprotein Nanog is required for maintenance of pluripotency in
mouse epiblast and ES cells. Cell. 113:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nichols J, Zevnik B, Anastassiadis K, et
al: Formation of pluripotent stem cells in the mammalian embryo
depends on the POU transcription factor Oct4. Cell. 95:379–391.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dietrich JE and Hiiragi T: Stochastic
patterning in the mouse pre-implantation embryo. Development.
134:4219–4231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foygel K, Choi B, Jun S, et al: A novel
and critical role for Oct4 as a regulator of the maternal-embryonic
transition. PLoS One. 3:e41092008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gerbe F, Cox B, Rossant J and Chazaud C:
Dynamic expression of Lrp2 pathway members reveals progressive
epithelial differentiation of primitive endoderm in mouse
blastocyst. Dev Biol. 313:594–602. 2008. View Article : Google Scholar
|
|
24
|
Chawengsaksophak K, James R, Hammond VE,
Köntgen F and Beck F: Homeosis and intestinal tumours in Cdx2
mutant mice. Nature. 386:84–87. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang QT, Piotrowska K, Ciemerych MA, et
al: A genome-wide study of gene activity reveals developmental
signaling pathways in the preimplantation mouse embryo. Dev Cell.
6:133–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chawengsaksophak K, de Graaff W, Rossant
J, Deschamps J and Beck F: Cdx2 is essential for axial elongation
in mouse development. Proc Natl Acad Sci USA. 101:7641–7645. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Strumpf D, Mao CA, Yamanaka Y, et al: Cdx2
is required for correct cell fate specification and differentiation
of trophectoderm in the mouse blastocyst. Development.
132:2093–2102. 2005. View Article : Google Scholar : PubMed/NCBI
|