Introduction
Acute lung injury (ALI) and the more severe form,
acute respiratory distress syndrome (ARDS), are life-threatening
respiratory failures that are caused by numerous factors (1). Despite the advances in treatment,
ALI/ARDS remains a serious problem due to its high mortality
(<40%) and morbidity rates (2).
ARDS is characterized pathologically by diffuse alveolar damage,
alveolar capillary leakage and protein-rich pulmonary edema,
leading to the clinical manifestations of poor lung compliance,
severe hypoxemia and bilateral infiltrates being observed on chest
radiographs (1). A study of
critical care units in the USA in 2005 estimated the incidence of
ARDS to be 58/100,000 individuals with 141,500 novel cases and an
annual death rate of 59,000 per year (3). Several drugs have been investigated
in the treatment of ALI/ARDS; however, to date, none of these have
been successful (4).
Circulating endothelial progenitor cells (EPCs) were
first discovered by Asahara et al in 1997 (5), and they have since been found to have
a role in neovascularization and vasculogenesis (6). In ALI, an increase in the number of
circulating EPCs is associated with an improved survival rate
(7). Lam et al (8) demonstrated that autologous
transplantation of EPCs preserves pulmonary endothelial function
and maintains the integrity of the pulmonary alveolar-capillary
barrier. Therefore, transplantation of EPCs may be a novel,
cell-based, endothelium-targeted therapeutic strategy for the
treatment and prevention of ALI/ARDS (8). Studies in rats have found that
administering EPCs results in an increase in the number of EPCs
targeted to injured lung tissue (9–11),
which significantly mitigates lung injury and improves survival of
ALI rats (10,11).
Lipopolysaccharide (LPS), a major component of the
cell membrane of Gram-negative bacteria, has a critical role in ALI
and ARDS. LPS induces ALI and this has been widely used as a model
for pathophysiological and pharmacological research. Inflammatory
stimuli have been shown to induce a rapid release of EPCs into the
circulation in humans (12). In
this study LPS was hypothesized to directly injure EPCs, as well as
influence the number and function of EPCs, thereby influencing the
endothelium repair process and disturbing the balance between the
injury and repair in ALI. To evaluate this hypothesis, the number
and activity of EPCs exposed to LPS in vitro and in
vivo were measured.
Materials and methods
Animals
Male CD-1 mice (4–8 weeks old, 20–24 g) were
obtained from the Laboratory Animal Center at the Nanjing Medical
University (Nanjing, China). CD-1 mice were used as they are the
most susceptible strains to LPS-induced injury (13). All animals in the study were
inbred, thus, of the same genetic background. Animals were raised
and used in accordance with the National Institutes of Health
Guidelines on the Use of Laboratory Animals. All experimental
procedures performed were approved by the Jinling General Hospital
Committee on Animal Care (Nanjing, China).
Isolation and culture of EPCs
EPCs were isolated from mouse bone marrow. Briefly,
mononuclear cells were separated from the tibia and femur of male
CD-1 mice (age, 4–8 weeks) using density gradient centrifugation
(Histopaque 1083, Sigma, St. Louis, MO, USA) and cultured on human
fibronectin-coated plates (ProSpec, East Brunswick, NJ, USA) in
endothelial cell growth media (EGM-2) supplemented with EGM™-2 MV
SingleQuots™ (Lonza, Basel, Switzerland), mouse recombinant
vascular epidermal growth factor (VEGF; 20 ng/ml), insulin-like
growth factor (IGF; 4 ng/ml; ProSpec), epidermal growth factor
(EGF; 20 ng/ml; ProSpec) and fibroblast growth factor (FGF; 4
ng/ml; ProSpec). The EGM-2 MV SingleQuots contained:
hydrocortisone, R3-IGF-1, human endothelial growth
factor, VEGF, human FGF-B, ascorbic acid, gentamicin amphotericin-B
(GA-1000) and 5% fetal bovine serum. Following four days in
culture, non-adherent cells were removed by washing with
phosphate-buffered saline (PBS) and adherent cells were incubated
in fresh media for a further three days for the following
experiments.
Identification of EPCs
Identification of EPCs was performed using direct
fluorescent staining to detect dual binding of fluorescein
isothiocyanate-labeled Ulex europaeus agglutinin (FITC-UEA-1;
Sigma) and 1,1-dioctadecyl-3,3,3,3-tetramethylindocarbocyanine
labeled acetylated low-density-lipoprotein (Dil-ac-LDL; Molecular
Probes®, Grand Island, NY, USA). EPCs were cultured for
seven days and then incubated with Dil-ac-LDL (10 ug/ml) at 37°C
for 4 h and fixed with 2% paraformaldehyde for 15 min. Cells were
washed with PBS for 30 min, prior to being treated with UEA-1 (10
ug/ml) for 1 h. Cells were then washed with PBS and viewed using an
inverted fluorescent microscope (IX-71, Olympus, Tokyo, Japan).
Cells demonstrating double-positive fluorescence were identified as
EPCs. EPCs were further identified by investigating the expression
of CD34, CD133 and VEGF receptor-2 (VEGFR-2) using flow cytometry
(BD Biosciences, Franklin Lakes, NJ, USA).
EPC colony-forming unit (CFU) assay
ALI was induced in mice by administering 2.5 mg/ml
LPS into the trachea (0.5 ml/kg body weight). LPS was obtained from
Escherichia coli (O55:B5; Sigma). Following treatment with
LPS, EPCs were harvested after 4, 8, 12, and 24 h, and cultured in
a fibronectin-coated 24-well plate for seven days in EGM-2, as
mentioned above. The control group was treated with PBS.
Endothelial CFUs, characterized by a central cluster surrounded by
spindle-shape cells, were counted by three independent
individuals.
Proliferation of EPCs
Monocytes from mice were seeded in a 96-well plate
and cultured for seven days, the early EPCs were harvested and the
EPCs were treated with various concentrations of LPS (10 pg/ml, 100
pg/ml, 1 ng/ml, 10 ng/ml and 100ng/ml) for 4, 8, 12, and 24 h. The
control cells were treated with PBS. The EPCs were then analyzed
using the cell counting kit-8 (CCK-8; Dojindo Laboratories,
Kunamoto, Japan) in order to assess cell proliferation. CCK-8
solution was added to each well and the cells were incubated for
1.5 h. The optical density (OD) values were read using a microplate
reader (Bio-Rad, Hercules, CA, USA). In the in vivo study,
CD-1 mice were induced by the administration of 2.5 mg/ml LPS into
the trachea and, after 4, 8, 12, and 24 h, the monocytes were
isolated and cultured for seven days. The control group were
treated with PBS. The proliferation assay was then performed in
accordance with the aforementioned method.
Senescence rate of EPCs
Cellular aging was determined using a Senescence
Cell Staining kit (Sigma). In the in vitro study, EPCs were
seeded in a 24-well plate and cultured for seven days, prior to
being treated with various concentrations of LPS (control group was
treated with PBS) for 4, 8, 12, and 24 h. EPCs were washed twice
with PBS, prior to being fixed in fixation solution for 7 min,
washed a further two times with PBS and then incubated for 12 h
with fresh staining solution at 37°C without CO2.
Following staining for 12 h, green-stained cells and total cells
were counted and the percentage of β-galactosidase-positive cells
was calculated. In the in vivo study, ALI was induced by the
administration of 2.5 mg/ml LPS into the trachea. After 4, 8, 12
and 24 h EPCs were isolated and cultured for seven days. The
control group was treated with PBS. The senescence assay was then
conducted as mentioned above.
Adhesion of EPCs
The adhesion assay was performed as previously
described (14). Briefly, the
early EPCs were cultured in three wells of a 24-well plate and were
treated with various concentration of LPS (control group was PBS)
for 4, 8, 12, and 24 h. Cells were then washed twice with PBS and
detached with 0.5 Mm EDTA (Gibco-BRL, Grand Island, NY, USA). The
EPCs were then placed in a 15-ml centrifuge tube. Cells were
centrifuged at 1,000 × g (Dingguo, Beijing, China) for 5 min prior
to the supernatant being removed and the EPCs being resuspended in
PBS. EPCs (~2×104 cells) were placed on two
fibronectin-coated wells in a 24-well plate in EGM-2 and incubated
for 30 min at 37°C with 5% CO2. Following a 30-min
incubation, EPCs were washed three times with PBS, prior to being
stained using Dil-ac-LDL and UEA-1 direct fluorescent staining in
accordance with the aforementioned method. The adherent cells were
counted by independent blinded investigators. In the in vivo
study, ALI was induced by the intratracheal administration of LPS
(2.5 mg/ml). After 4, 8, 12, and 24 h EPCs were isolated and
cultured for seven days. The control group was administered PBS.
The adhesion assay was performed as mentioned above.
Statistical analysis
All experiments were performed at least six times,
and the average result was calculated. Results are presented as the
mean ± standard error of the mean. Student’s t-test was utilized
for the comparison between two groups. Data were analyzed using
Sigma Plot software (Systat Software Inc., San Jose, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
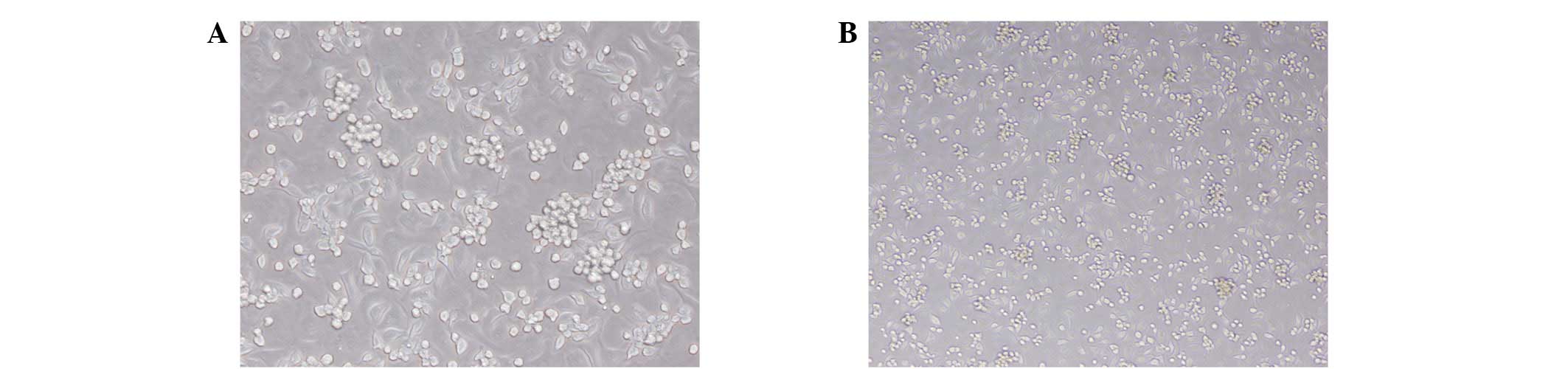
Cell morphology
Total monocytes were isolated from the bone marrow
of male CD-1 mice and cultured for seven days. The resulting cells
exhibited spindle-shaped, endothelium-like morphology, and were
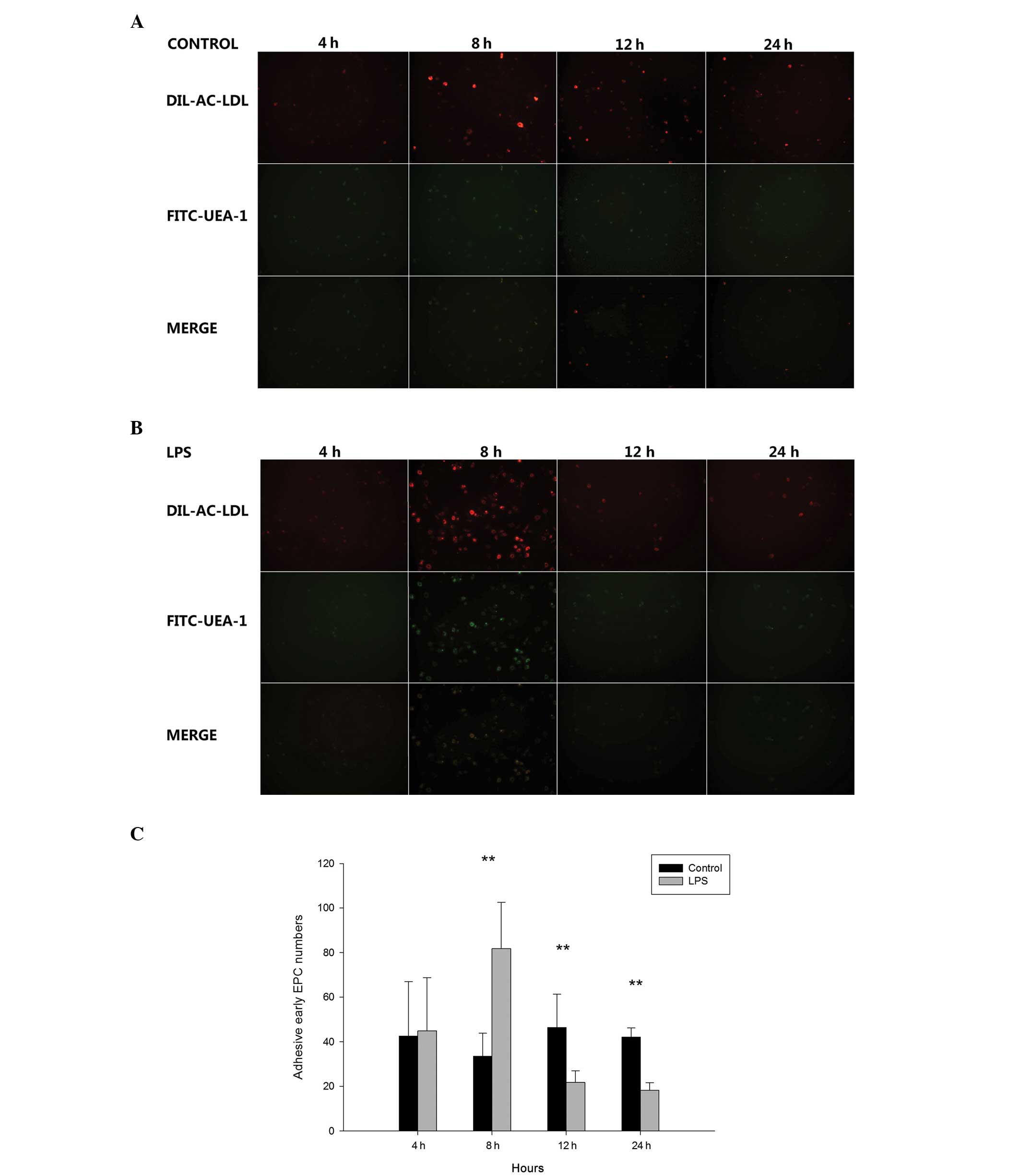
identified as early EPCs (Fig. 1).
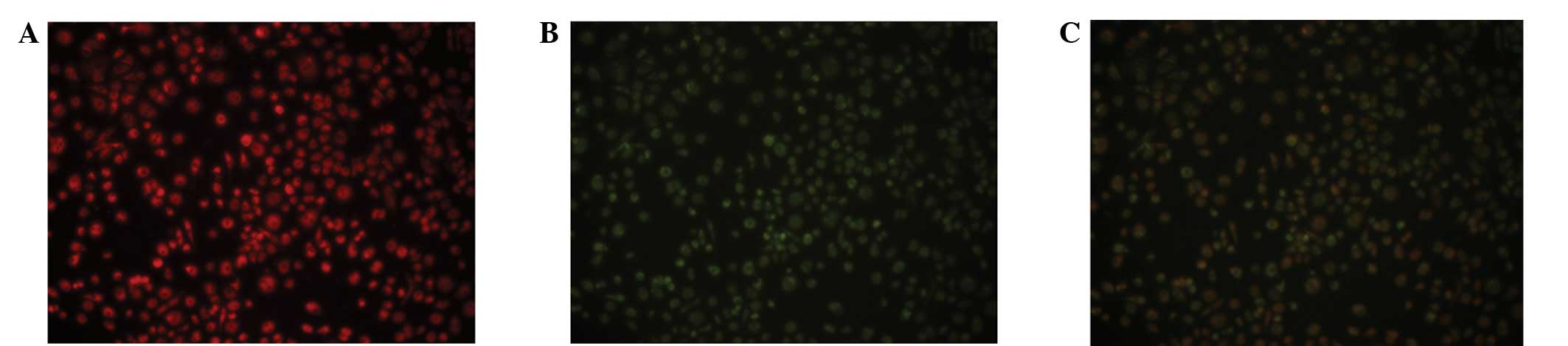
EPCs were characterized as double-positive for Dil-ac-LDL and
FITC-UEA-1 staining, which was observed using inverted fluorescent
microscopy (Fig. 2). EPCs also
exhibited a number of other endothelial characteristics, including
expression of CD34, von Willebrand factor (vWF) and VEGFR-2 (data
not shown).
CFU assay
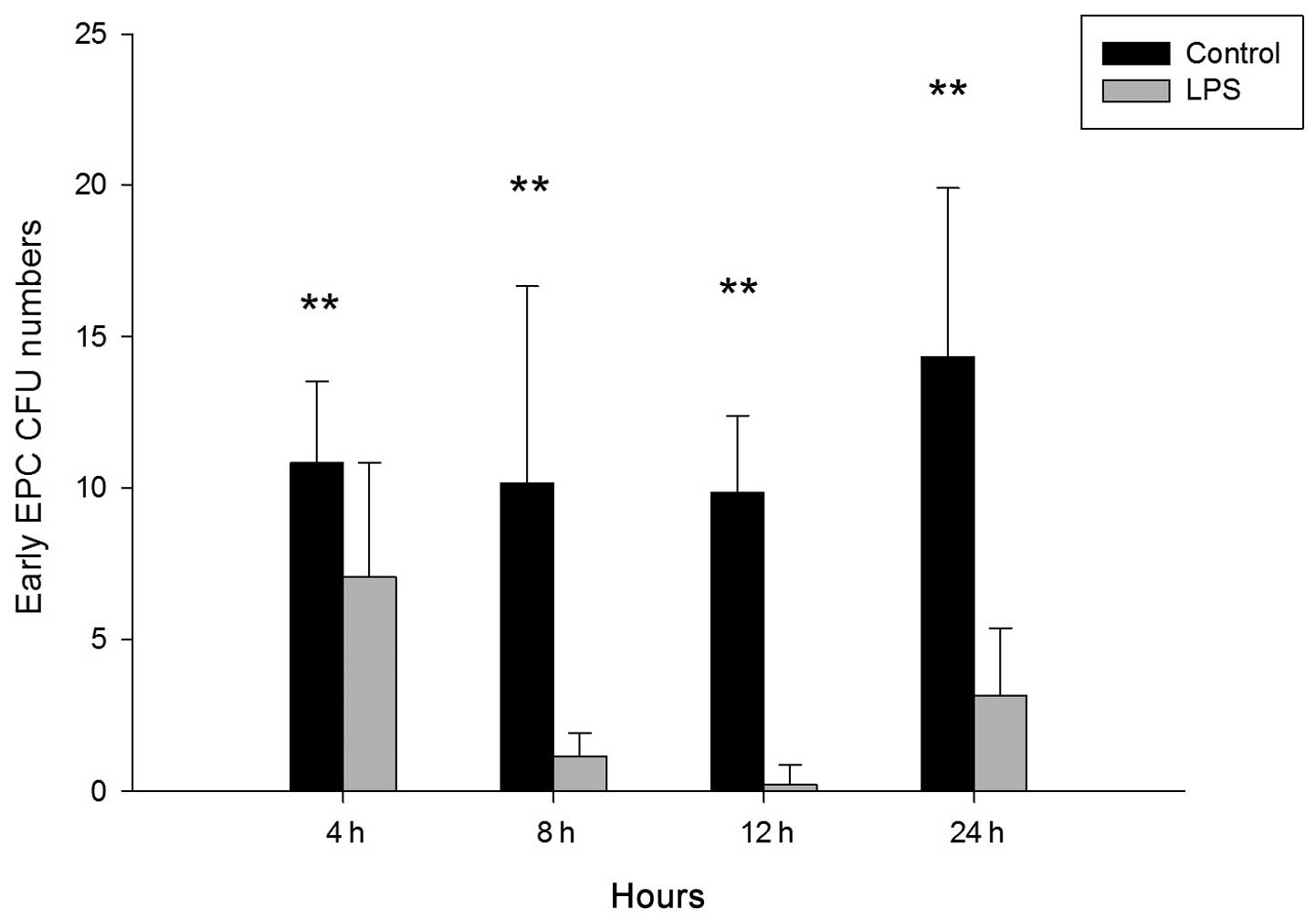
To investigate whether LPS influences the number of
CFUs of cultured EPCs from bone marrow in mice, LPS (2.5 mg/ml) was
administered via the trachea. After 4, 8, 12, and 24 h, monocytes
were isolated and cultured for seven days, and early EPCs were
harvested for analysis. As shown in Fig. 3, the number of CFUs was
significantly reduced in the LPS-treated group compared with the
control group at 4 h (control vs. LPS, 10.833±2.691 vs.
7.071±3.772; P<0.01), 8 h (control vs. LPS, 10.167±6.494 vs.
1.167±0.753; P<0.01), 12 h (control vs. LPS, 9.856±2.545 vs.
0.2222±0.667; P<0.01) and 24 h (control vs. LPS, 14.333±5.574
vs. 3.167±2.229; P<0.01).
Proliferation of EPCs
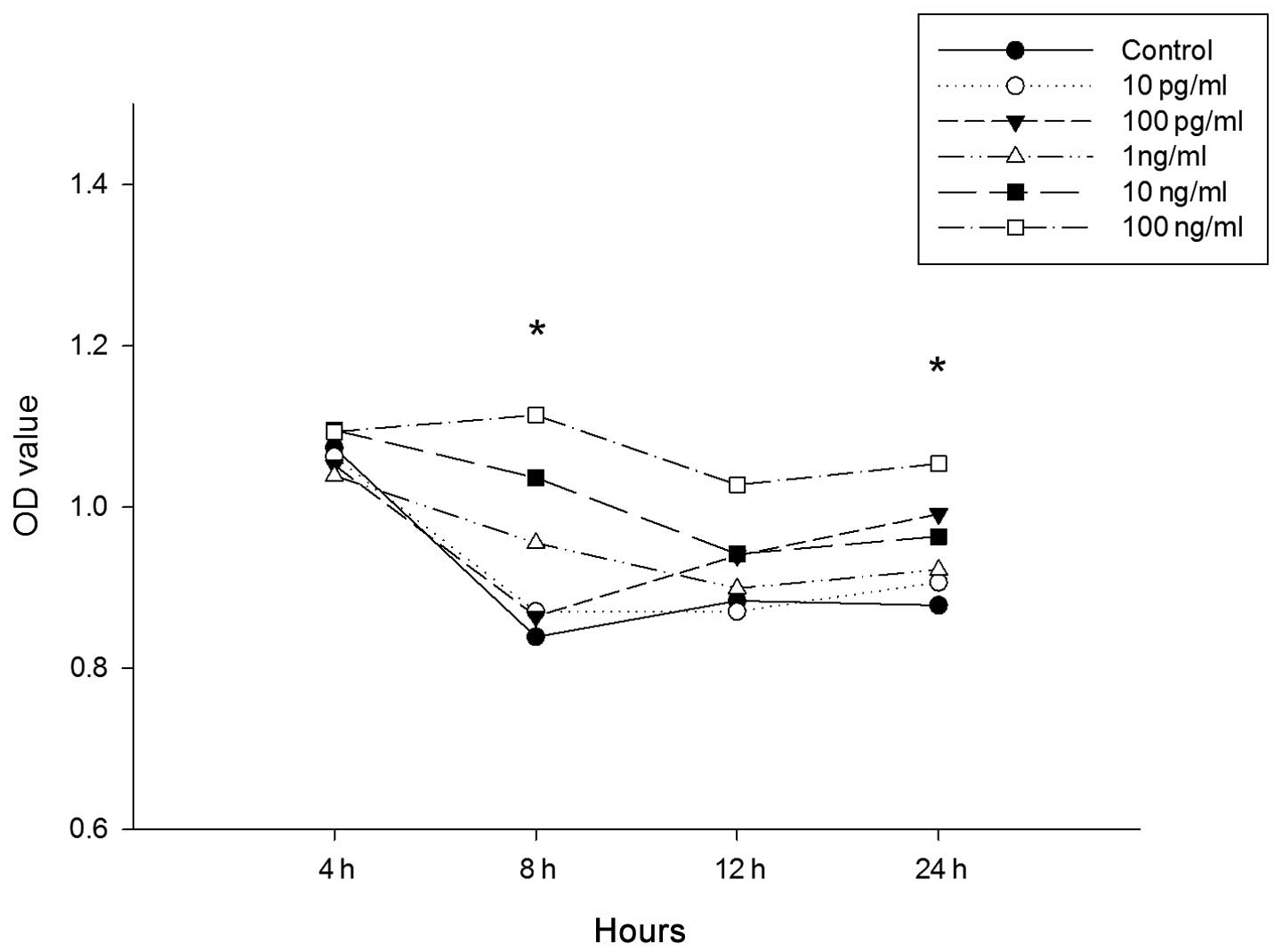
To investigate the possibility that LPS influences
the proliferation of the cultured EPCs from bone marrow in mice,
the CCK-8 assay was used. EPCs were cultured for seven days prior
to incubation with increasing concentrations of LPS (10 pg/ml, 100
pg/ml, 1 ng/ml, 10 ng/ml and 100ng/ml) for 4, 8, 12, and 24 h.
Cells treated with 10 pg/ml, 100 pg/ml and 1 ng/ml, showed no
significant difference in EPC proliferation at all time-points
(Fig. 4). For cells treated with
10 ng/ml for 8 h (control vs. LPS, 0.839±0.046 vs. 1.036±0.107;
P<0.01; Fig. 4) and cells
treated with 100 ng/m for 8 h (control vs. LPS, 0.839±0.046 vs.
1.154±0.047; P<0.01; Fig. 4)
and 24 h (control vs. LPS, 0.878±0.091 vs. 1.053±0.068; P<0.01;
Fig. 4), there was an increase in
EPC proliferation. As shown in Fig.
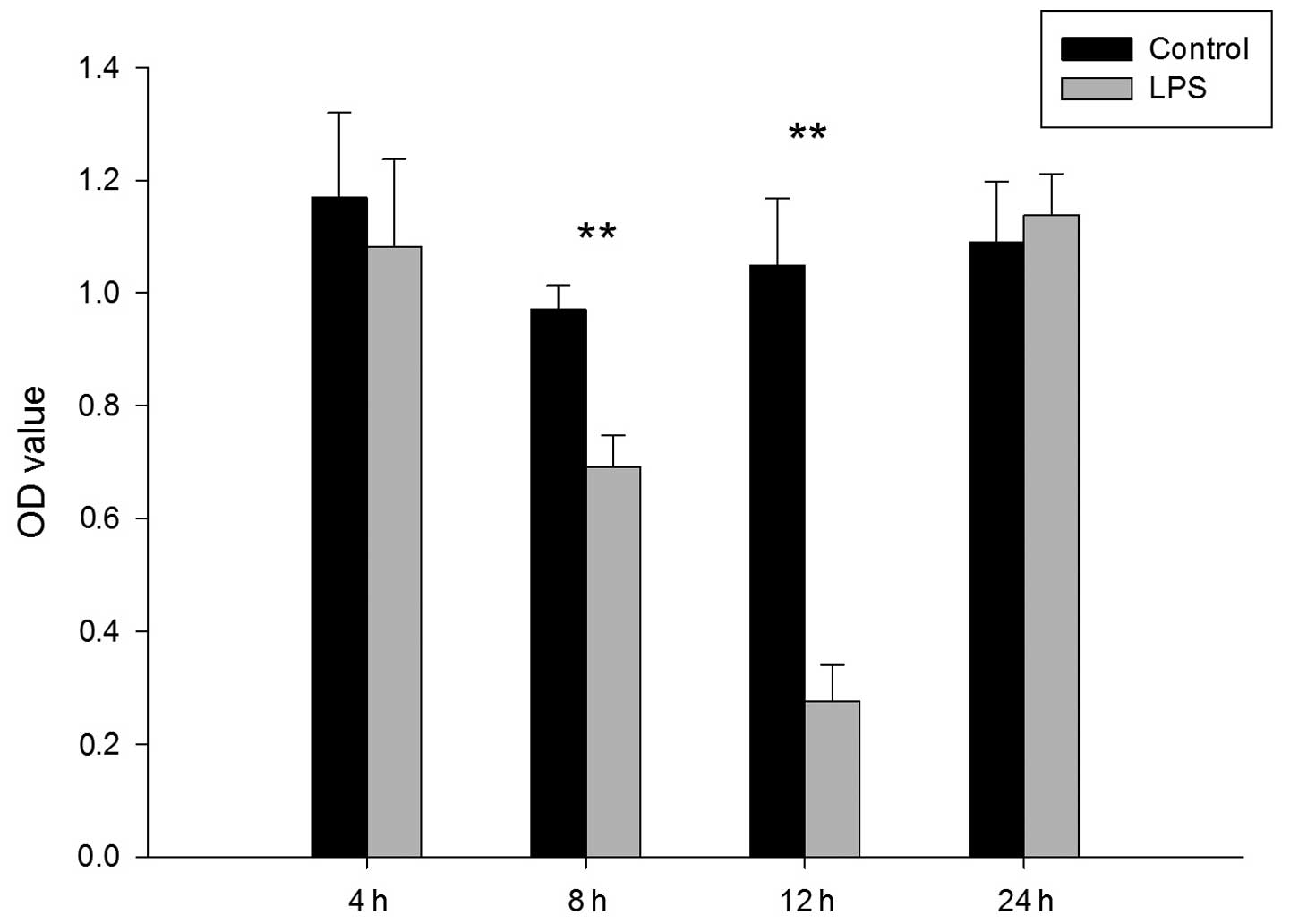
5, early EPC proliferation decreased significantly at 8 h
(control vs. LPS, 0.970±0.043 vs. 0.691±0.056; P<0.01) and 12 h
(control vs. LPS, 1.048±0.120 vs. 0.275±0.065; P<0.01) following
administration of LPS (2.5 mg/kg) via the trachea.
Senescence of EPCs
To investigate the possibility that LPS influences
the senescence of cultured EPCs from bone marrow in vitro in
mice, EPCs were cultured for seven days prior to incubation with
increasing concentrations of LPS for 4, 8, 12, and 24 h. No
significant differences were observed at any time-points for cells
incubated with 10 pg/ml, 100 pg/ml and 1 ng/ml LPS. Cells cultured
with 10 ng/ml LPS, however, showed a significant decrease in
senescence 8 h following LPS administration (control vs. LPS,
0.348±0.051 vs. 0.236±0.079; P<0.05; Fig. 6). In cells treated with 100 ng/ml
LPS, the decrease was significant at 4 h (control vs. LPS,
0.346±0.083 vs. 0.241±0.045; P<0.05; Fig. 6) and 8 h (control vs. LPS,
0.348±0.061 vs. 0.285±0.057; P<0.05; Fig. 6) following LPS treatment. To
investigate the influence of LPS on the senescence rate of EPCs in
mice in vivo, LPS (2.5 mg/kg) was administered via the
trachea. After 4, 8, 12, and 24 h, monocytes were isolated and
cultured for seven days, and the senescence cell assay performed.
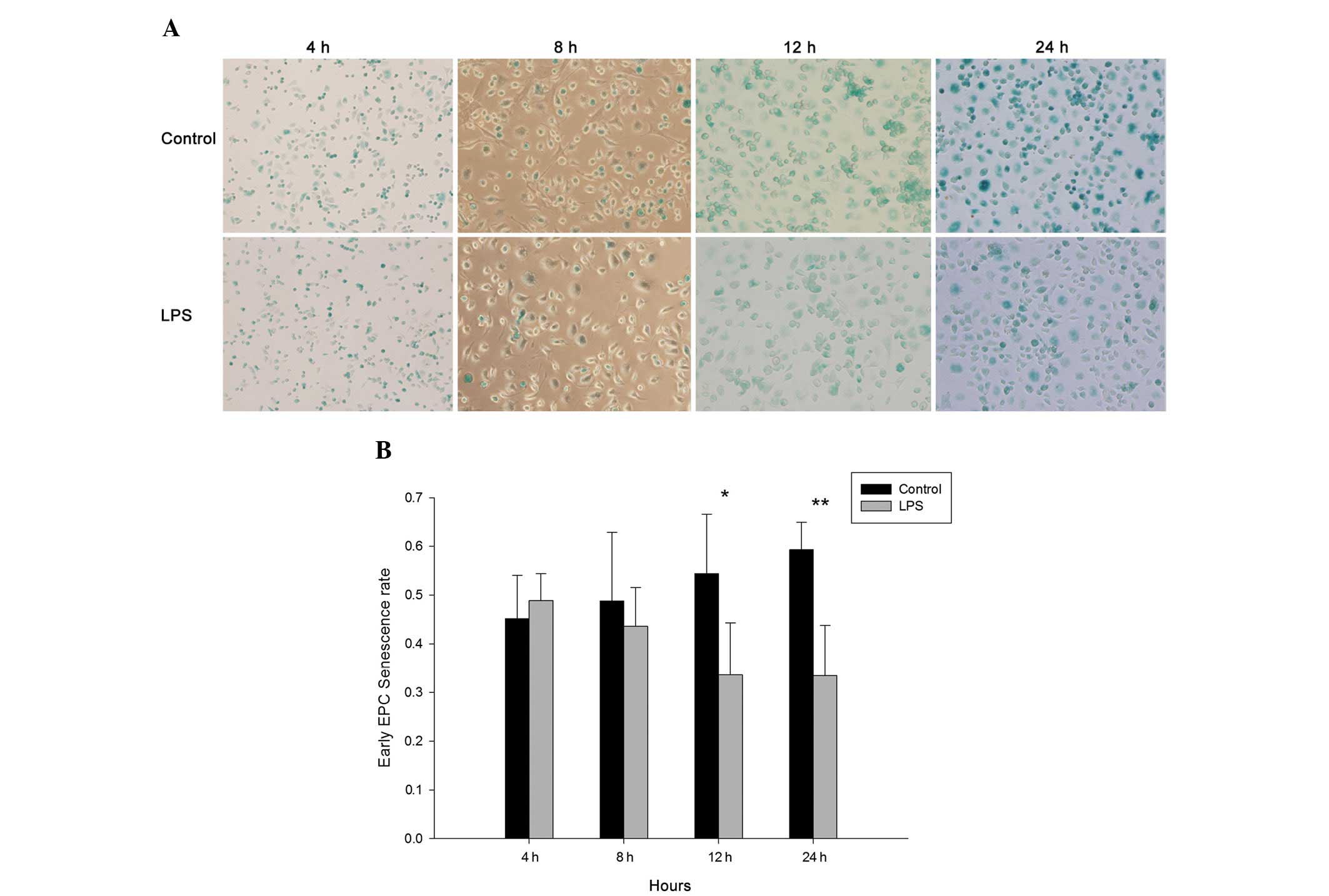
As shown in Fig. 7, the rate of
senescence was significantly reduced after 12 h (control vs. LPS,
0.544±0.122 vs. 0.336±0.107; P<0.05) and 24 h (control vs. LPS,
0.593±0.056 vs. 0.335±0.103; P<0.01).
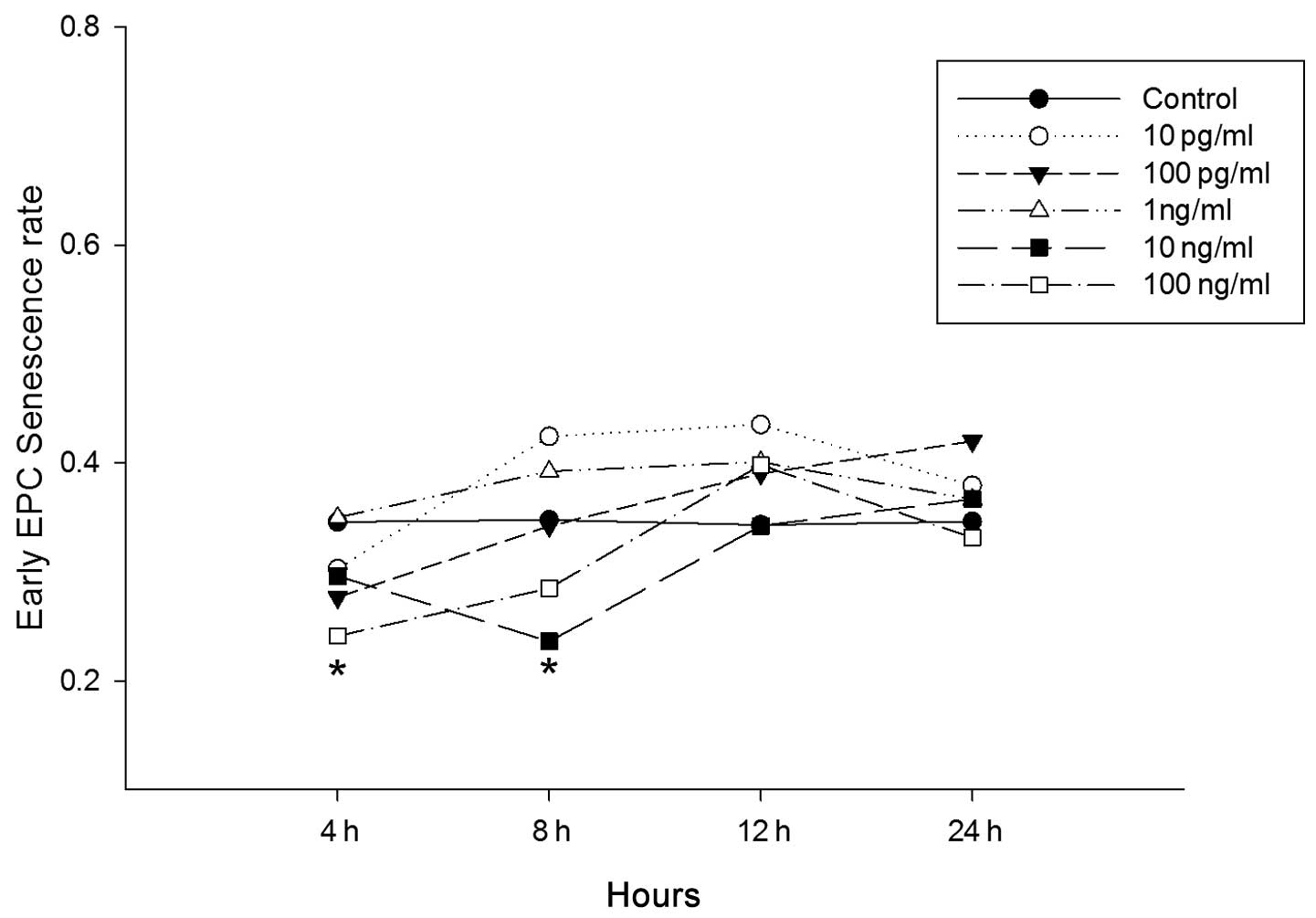 | Figure 6Effect of lipopolysaccharide (LPS) on
endothelial progenitor cell (EPC) senescence in vitro. Early
EPCs were incubated with varying concentrations of LPS (10pg/ml,
100pg/ml, 1ng/ml, 10ng/ml and 100ng/ml) for 4, 8, 12 and 24 h.
Control cells were treated with phosphate-buffered saline, the EPC
senescence cell assay was performed using a Senescence Cell
Staining kit (Sigma, St. Louis, MO, USA). The early EPC senescence
rates were counted in six randomly observed visual fields
(magnification, ×200). *P<0.05 compared with the
control group (n=6). |
Adhesion of EPCs
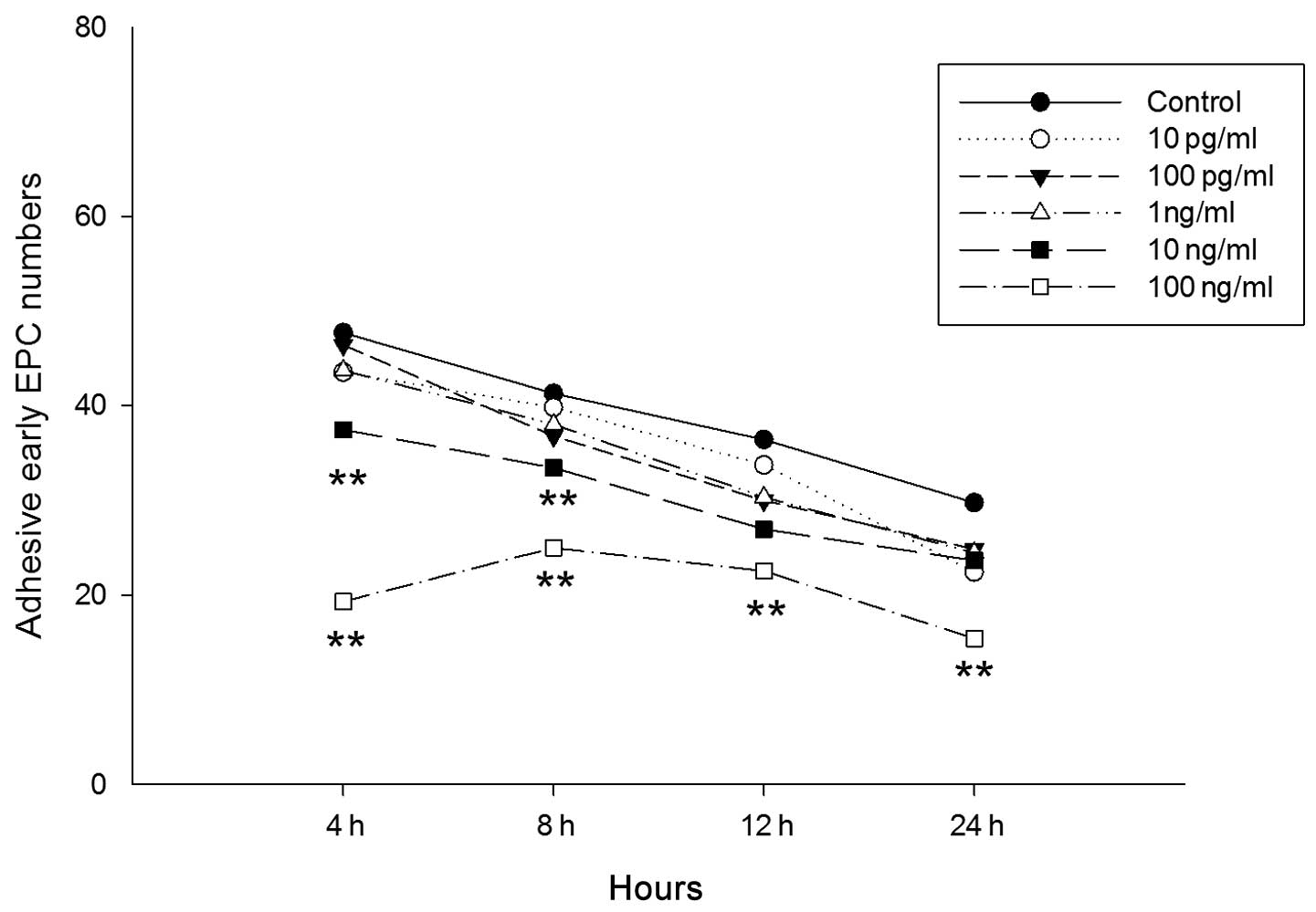
To investigate the possibility that LPS influences
the adhesion of cultured EPCs from bone marrow in mice, cultured
EPCs were incubated with varying concentrations of LPS (10 pg/ml,
100 pg/ml, 1 ng/ml, 10 ng/ml and 100 ng/ml) for 4, 8, 12 and 24 h,
prior to being cultured on fibronectin-coated plates for 30 min. It
was observed that EPCs treated with 10 and 100 ng/ml LPS exhibited
a significant decrease in adhesion, with the greatest decrease
observed in cells treated with 100 ng/ml (Fig. 8). However, no significant decrease
in adhesion was observed in cells treated with 10 pg/ml, 100 pg/ml
and 1 ng/ml. In the 10 ng/ml group, the decrease in adhesion was
significant at 4 h (control vs. LPS, 47.718±4.979 vs. 37.462±2.724;
P<0.01) and 8 h (control vs. LPS, 41.308±3.549 vs. 33.461±4.181;
P<0.01). In the 100 ng/ml group, the decrease in adhesion was
significant at 4 h (control vs. LPS, 47.718±4.979 vs. 27.000±2.062;
P<0.01), 8 h (control vs. LPS, 41.308±3.549 vs. 24.974±4.586;
P<0.01), 12 h (control vs. LPS 36.436±9.475 vs. 22.538±4.757;
P<0.01) and 24 h (control vs. LPS, 29.769±8.258 vs.
15.410±4.183; P<0.01). To determine the effect of LPS on EPC
adhesion in mice in vivo, LPS (2.5 mg/kg) was administered
via the trachea. After 4, 8, 12, and 24 h, EPCs were isolated and
cultured for seven days prior to the adhesion assay. As shown in
Fig. 9, the adhesion of the cells
was greatest at 8 h (control vs. LPS, 33.500±10.349 vs.
81.833±20.677; P<0.01) and then decreased at 12 h (control vs.
LPS, 46.500±14.830 vs. 21.833±5.099; P<0.01) and 24 h (control
vs. LPS, 42.117±4.135 vs. 18.288±3.44; P<0.01).
Discussion
ALI is a prevalent disease, particularly in
intensive care units. Previous studies have focused on cells
involved in ALI, including endothelial (15), macrophage, mononuclear and natural
killer T cells (16). Numerous
drugs have been found to exert a protective effect against
LPS-induced ALI, including matrine, which inhibits the inflammatory
response (17), heme oxygenase-1,
which negatively regulates the interleukin (IL)-33 and Toll-like
receptor (TLR)-4-mediated inflammatory response (18), and resolvin D1 which selectively
reacts with a lipoxin A4 receptor, inhibiting mitogen-activated
protein kinases and the nuclear factor-κ B pathway (19).
Previous studies investigating ALI have focused on
inflammatory cells, including macrophages and leukocytes. At
present, studies are targeting the endothelial repair pathway,
using stem cells to attenuate the organ inflammatory response. Such
stem cells have included EPCs, since their identification by
Asahara et al (5). EPCs
regenerate vascular endothelial cells and maintain the integrity of
the vascular endothelium (20).
The number and function of circulating EPCs has been shown to be
reduced in patients afflicted with certain diseases, including
cerebral aneurysm (21).
LPS is also known as endotoxin and is a key molecule
involved in the initiation of sepsis syndrome (22). The LPS-induced ALI model is widely
used as a disease model as it is capable of stimulating the
characteristics of human ALI/ARDS (23). Local exposure to LPS may induce
ALI, characterized by increased levels of neutrophils, protein
content and cytokines in the bronchoalveolar lavage fluid
associated with the severity of the disease (24). While it is well known that LPS
induces inflammation in the lung, the present study focused
specifically on the direct influence of LPS on EPCs in mice.
LPS directly influences endothelial cells through a
complex signaling pathway (25).
EPCs are progenitor cells that transform into endothelial cells,
meaning that the effect of LPS is not the same as it is for
endothelial cells. Previous data have shown that EPCs are
vulnerable to LPS and have demonstrated that expression of TLR4 is
the basis for this vulnerability (26). In ALI, endothelial cells and other
cells are injured (1). LPS damages
the constructive tissue, facilitating stem-like cells, such as
EPCs, to participate in tissue repair. The tissues injured by LPS,
however, are not the same as those targeted by other toxins.
The present study showed that the proliferation of
EPCs induced by LPS increased in 10 and 100 ng/ml LPS in
vitro, indicating that LPS activates EPCs rather than
inhibiting them. A previous study also showed that LPS treatment
failed to induce EPC apoptosis, instead promoting cell EPC
proliferation (27).
The characteristics of EPCs and bone marrow-derived
monocytes are associated with age-related changes (28–30).
In the EPC senescence assay, it was found that the senescence rate
decreased in 10 and 100 ng/ml LPS in vitro and also at 12
and 24 h in vivo. Di Stefano et al (31) found that LPS induces the expression
of procoagulant activity in EPCs, and that the effect of LPS was
dose-dependent, with statistical significance achieved at 100
ng/ml, which is consistent with the present result (31).
In the present in vivo study, 2.5 mg/ml (0.5
ml/kg body weight) LPS was used as previously described (32). In the course of 24 h it was found
that the number of CFUs was significantly reduced at all
time-points, particularly at 8 and 12 h. The rate of proliferation
was also reduced significantly at 8 and 12 h; however, this was not
significant at 4 and 24 h. This suggests that at 8 h ALI is most
severe and gradually recovers afterwards. A previous study revealed
that the number of EPCs was significantly reduced in patients
treated with LPS and was lowest 6 h after LPS treatment, but after
24 h the number of cells returned to baseline levels (33). This was almost consistent with the
present in vivo experiment. However, contradictory results
exist; certain evidence has suggested that the early phase of acute
low-grade inflammation is associated with a decrease in peripheral
EPCs (33), whilst increased
numbers of circulating EPCs have been observed in patients with
bacterial pneumonia prior to treatment (12). In the present in vitro study
the increased proliferation indicated that LPS directly influences
the proliferation of EPCs. The increase in proliferation peaked 8 h
after treatment with LPS.
The adhesive capacity of EPCs was impaired by LPS
and showed dose- and time-dependence. Adhesion was particularly
reduced at an LPS concentration of 100 ng/ml for all time-points.
In the adhesion assay in vivo, it was found that adhesion
reached a peak 8 h following LPS treatment and then gradually
decreased. This indicates that, directly following treatment with
LPS, the adhesion of EPCs was increased in order to repair injured
endothelial cells, and then reduced in order for progenitor cells
to be released from the bone marrow. The decreased adhesion at 12
and 24 h in the in vivo study suggests that this contributes
to the release of the EPCs or the precursors of EPCs from the bone
marrow and the targeting of injured tissue.
The results of the in vitro and in
vivo studies were distinct, possibly due to the complex
internal environment of ALI induced by LPS. The in vitro
environment was simplified whilst the in vivo environment
was complex, with other factors, such as cytokines, hormones and
inflammatory factors, having an effect. Overall, proliferation was
found to decrease in vivo. Decreased senescence rates and
increased proliferation in vitro promoted the number and
function of EPCs for tissue repair. The exact mechanism, however,
has yet to be elucidated.
In the present study, the number of the monocytes
isolated from the bone marrow was found to be decreased in the
LPS-treated group compared with the control group. This suggests
that the reduced numbers of EPCs and CFUs observed were partly due
to the reduced number of monocytes isolated from the LPS group. LPS
may directly reduce the number of monocytes, but the exact
mechanism is not yet known.
The present study also demonstrated that 10 and 100
ng/ml LPS are suitable for cell culture in vitro due to the
sensitivity of EPCs to LPS. The detailed mechanism of LPS in ALI,
including the mechanism underlying its effect on EPCs, requires
further investigation. The proliferation and adhesion activity of
the EPCs was activated in 8 h and then gradually decreased with
time. The exact mechanism has yet to elucidated, but further
investigation in this area may enable more effective drugs to be
developed that are capable of promoting the number and function of
EPCs for participation in tissue repair.
Acknowledgements
This study was supported by research grants from the
Natural Science Foundation of China (NSFC).
References
|
1
|
Dushianthan A, Grocott MP, Postle AD and
Cusack R: Acute respiratory distress syndrome and acute lung
injury. Postgrad Med J. 87:612–622. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Frutos-Vivar F, Nin N and Esteban A:
Epidemiology of acute lung injury and acute respiratory distress
syndrome. Curr Opin Crit Care. 10:1–6. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rubenfeld GD, Caldwell E, Peabody E, et
al: Incidence and outcomes of acute lung injury. N Engl J Med.
353:1685–1693. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobson JR: Pharmacologic therapies on
the horizon for acute lung injury/acute respiratory distress
syndrome. J Investig Med. 57:870–873. 2009.PubMed/NCBI
|
|
5
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asahara T, Takahashi T, Masuda H, et al:
VEGF contributes to postnatal neovascularization by mobilizing bone
marrow-derived endothelial progenitor cells. EMBO J. 18:3964–3972.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burnham EL, Taylor WR, Quyyumi AA, Rojas
M, Brigham KL and Moss M: Increased circulating endothelial
progenitor cells are associated with survival in acute lung injury.
Am J Respir Crit Care Med. 172:854–860. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam CF, Liu YC, Hsu JK, et al: Autologous
transplantation of endothelial progenitor cells attenuates acute
lung injury in rabbits. Anesthesiology. 108:392–401. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kähler CM, Wechselberger J, Hilbe W, et
al: Peripheral infusion of rat bone marrow derived endothelial
progenitor cells leads to homing in acute lung injury. Respir Res.
8:502007.PubMed/NCBI
|
|
10
|
Mao M, Wang SN, Lv XJ, Wang Y and Xu JC:
Intravenous delivery of bone marrow-derived endothelial progentor
cells improves survival and attenuates lipopolysaccharde-induced
lung injury in rats. Shock. 34:196–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafat N, Dacho C, Kowanetz G, et al:
Therapeutic effects of endothelial progenitor cells in LPS-induced
ARDS. Anasthesiologie & Intensivmedizin. 53:196–208. 2012.(In
German).
|
|
12
|
Yamada M, Kubo H, Ishizawa K, Kobayashi S,
Shinkawa M and Sasaki H: Increased circulating endothelial
progenitor cells in patients with bacterial pneumonia: evidence
that bone marrow derived cells contribute to lung repair. Thorax.
60:410–413. 2005. View Article : Google Scholar
|
|
13
|
Alm AS, Li K, Yang D, Andersson R, Lu Y
and Wang X: Varying susceptibility of pulmonary cytokine production
to lipopolysaccharide in mice. Cytokine. 49:256–263. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang PH, Tsai HY, Wang CH, et al:
Moderate intake of red wine improves ischemia-induced
neovascularization in diabetic mice - roles of endothelial
progenitor cells and nitric oxide. Atherosclerosis. 212:426–435.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao Y, Gorshkova IA, Berdyshev E, et al:
Protection of LPS-induced murine acute lunginjury by
sphingosine-1-phosphate lyase suppression. Am J Respir Cell Mol
Biol. 45:426–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aoyagi T, Yamamoto N, Hatta M, et al:
Activation of pulmonary invariant NKT cells leads to exacerbation
of acute lung injury caused by LPS through local production of
IFN-γ and TNF-α by Gr-1+monocytes. Int Immunol.
23:97–108. 2011.PubMed/NCBI
|
|
17
|
Zhang B, Liu ZY, Li YY, et al:
Antiinflammatory effects of matrine in LPS-induced acute lung
injury in mice. Eur J Pharm Sci. 44:573–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yin H, Li X, Yuan B, et al: Heme
oxygenase-1 ameliorates LPS-induced acute lung injury correlated
with downregulation of interleukin-33. Int Immunopharmacol.
11:2112–2117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang B, Gong X, Wan JY, et al: Resolvin D1
protects mice from LPS-induced acute lung injury. Pulm Pharmacol
Ther. 24:434–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Cao F, Yin T, et al: Moderate dose
insulin promotes function of endothelial progenitor cells. Cell
Biol Int. 35:215–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wei H, Mao Q, Liu L, et al: Changes and
function of circulating endothelial progenitor cells in patients
with cerebral aneurysm. J Neurosci Res. 89:1822–1828. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Medzhitov R: Toll-like receptors and
innate immunity. Nat Rev Immunol. 1:135–145. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen H, Bai C and Wang X: The value of the
lipopolysaccharide-induced acute lung injury model in respiratory
medicine. Expert Rev Respir Med. 4:773–783. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chatchavalvanich S, Nonas S, Miller I, et
al: Oxidized phospholipids reduce vascular leak and inflammation in
a rat model of acute lung injury. J Invest Med. 54:S3462006.
|
|
25
|
Dauphinee SM and Karsan A:
Lipopolysaccharide signaling in endothelial cells. Lab Invest.
86:9–22. 2006. View Article : Google Scholar
|
|
26
|
Ghaly T, Rabadi MM, Weber M, et al:
Hydrogel-embedded endothelial progenitor cells evade LPS and
mitigate endotoxemia. Am J Renal Physiol. 301:F802–F812. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He J, Xiao Z, Chen X, et al: The
expression of functional Toll-like receptor 4 is associated with
proliferation and maintenance of stem cell phenotype in endothelial
progenitor cells (EPCs). J Cell Biochem. 111:179–186. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoetzer GL, Van Guilder GP, Irmiger HM,
Keith RS, Stauffer BL and DeSouza CA: Aging, exercise, and
endothelial progenitor cell clonogenic and migratory capacity in
men. J Appl Physiol 1985. 102:847–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dimmeler S and Vasa-Nicotera M: Aging of
progenitor cells: limitation for regenerative capacity? J Am Coll
Cardiol. 42:2081–2082. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Widmann TA, Willmann B, Pfreundschuh M and
Beelen DW: Influence of telomere length on short-term recovery
after allogeneic stem cell transplantation. Exp Hematol.
33:1257–1261. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Stefano R, Barsotti MC, Armani C, et
al: Human peripheral blood endothelial progenitor cells synthesize
and express functionally active tissue factor. Thromb Res.
123:925–930. 2009.PubMed/NCBI
|
|
32
|
Alm AS, Li K, Chen H, Wang D, Andersson R
and Wang X: Variation of lipopolysaccharide-induced acute lung
injury in eight strains of mice. Respir Physiol Neurobiol.
171:157–164. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mayr FB, Spiel AO, Leitner JM, Firbas C,
Sieghart W and Jilma B: Effects of low dose endotoxemia on
endothelial progenitor cells in humans. Atherosclerosis.
195:e202–e206. 2007. View Article : Google Scholar : PubMed/NCBI
|