Introduction
The use of neoadjuvant chemotherapy (NAC) or
pre-operative systemic therapy is being increasingly considered for
patients with operable breast cancer (1), since the survival rates are similar
to those in patients receiving standard post-operative chemotherapy
and the success of breast-conserving surgery is significantly
improved in patients treated with NAC. The aim of NAC is to achieve
a pathological complete response (pCR), i.e., the absence of
malignant cells at the tumor site, as pCR has been associated with
longer disease-free survival (DFS) and overall survival (OS) rates
(2). However, a small proportion
of patients are likely to fail to respond or their condition will
progress during primary chemotherapy. The identification of such
non-responding patients at an early stage may spare these
individuals from undertaking the toxicity of ineffective
chemotherapy and allow them to begin non-cross resistant regimes or
alternative treatment strategies. The identification of tumor
biological markers that accurately predict the response to
treatment may aid in optimizing NAC.
Second mitochondria-derived activator of caspases
(Smac) is a novel pro-apoptotic protein, also known as direct
inhibitor of apoptosis-binding protein with low pI (DIABLO), which
was initially identified independently by two groups in 2000
(3,4). SMAC/DIABLO is released from
mitochondria into the cytosol concurrently with cytochrome c
and eliminates the inhibitory effects of the inhibitor of
apopotosis proteins (IAPs) to promote apoptosis. SMAC/DIABLO has a
significant regulatory role in the sensitization of cancer cells to
immune- and drug-induced apoptosis (5). As a member of the IAP family,
survivin has been demonstrated to be involved in apoptosis
inhibition and cell cycle control. The formation of the
survivin-SMAC complex requires the N-terminus of mature
SMAC/DIABLO, and the distribution of survivin and SMAC/DIABLO
within the cell reveal that they co-localize within the cytosol
during interphase. Thus, SMAC/DIABLO promotes apoptosis by
interacting with survivin (6).
However, there are a number of studies concerning
the clinical values of Smac and survivin expression in advanced
breast cancer patients treated with NAC. To aid in our
understanding of the correlation between the expression of survivin
and Smac, and the clinical outcome of patients with advanced breast
cancer receiving NAC, survivin and Smac expression was measured in
the present study using immunohistochemistry prior to and following
NAC treatment in breast cancer tissue specimens. The expression
levels were analyzed for the correlation with clinicopathological
factors, the response to neoadjuvant chemotherapy and the survival
rate.
Patients and methods
Patients
The consecutive cohort comprised 98 patients who
presented with locally advanced primary breast cancer between
December 2005 and December 2009 at the Department of Breast
Surgery, Wuhu Second People’s Hospital, Wannan Medical College
(Wuhu, China) and were treated with anthracycline-based NAC. The
Research Ethics Committee of Wuhu Second People’s Hospital
affiliated to Wannan Medical College approved the study. The median
age of the patients was 49 years old (range, 32–69 years). The
patients were staged according to the Union Internationale Contre
le Cancer tumor node metastasis (TNM) classification (7), and a core biopsy was performed prior
to chemotherapy to allow pathological diagnosis and evaluation of
biological parameters. The patients were then treated with four
cycles of anthracycline-based therapy [500 mg/m2
5-fluorouracil (5-FU), 75–100 mg/m2 epirubicin and 500
mg/m2 cyclophosphamide on day one of a 21-day cycle]. No
further adjuvant chemotherapy was allowed in the patients included
in the present study and no patient received neoadjuvant or
adjuvant trastuzumab. The patients then underwent breast-conserving
surgery or mastectomy within 3–4 weeks of the final treatment, and
tumor tissues were obtained at surgery. Written informed consent
was obtained from all patients. Patients with metastatic carcinoma
to four or more axillary lymph nodes or with breast-conserving
surgery, received radiotherapy. All the estrogen/progesterone
receptor (ER/PR)-positive cases received adjuvant hormonal
treatment. A histological examination was used to confirm invasive
carcinoma by needle biopsy. The patients were followed up for 10–68
months (median, 57 months) during which, 28 patients (28.6%)
succumbed to the tumor and 10 (10.2%) developed local or distant
metastasis.
Evaluation of treatment response
An assessment of the tumor response was evaluated
prior to chemotherapy and following each cycle. A pathological
response to NAC was evaluated using histological examination of the
surgical specimens. The absence of invasive tumor in the final
surgical breast and axillary lymph node samples was defined as pCR,
while other conditions were regarded as residual disease (RD)
(8).
Immunohistochemistry
Immunohistochemistry was performed on core biopsies
and whole tumor sections. Formalin-fixed paraffin sections of tumor
tissues were subjected to immunohistochemical staining. The
sections were cleared, treated with 0.3% H2O2
for 10 min at room temperature, placed in 0.01 mmol/l sodium
citrate (pH 6.0) and heated in a microwave oven for antigen
retrieval. The slides were incubated with rabbit polyclonal
anti-Smac antibodies (1:200; Santa Cruz Biotechnology Inc., Santa
Cruz, CA, USA) or rabbit polyclonal anti-survivin antibodies
(1:100: Santa Cruz Biotechnology Inc.) at 4°C overnight in a
humidified atmosphere, rinsed three times in 0.1 mmol/l PBS for 2
min, incubated for 30 min at room temperature with goat anti-mouse
horseradish peroxidase (Boster, China) and stained with
3′3-diaminobenzidine. For the negative controls, the primary
antibody was replaced with non-specific rabbit immunoglobulin G
(Cell Signalling Technology, Inc., Beverly, MA, USA). Two
pathologists scored the results independently based on the
intensity of staining and the extent of expression. The intensity
of staining was scored as: 0, negative; 1, weak; or 2, strong. The
extent of expression was scored based on the percentage of positive
cells: 0, negative; 1, 1–25%; 2, 26–50%; 3, 51–75%; and 4, 76–100%.
The overall score of each case was a sum of the intensity of
staining and the extent of expression scores. Overall scores of ≥4
were defined as high staining, whereas those <4 represented low
staining.
Statistical analysis
The SPSS version 15.0 software package (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. Protein
expression versus clinicopathological criteria was assessed using
the Pearson χ2 test of association. A logistic
regression analysis was used to identify significant multivariate
predictors. A multivariate model was used to analyze all the
variables that were significant in the univariate analysis. The
Kaplan-Meier method was used to calculate the cumulative survival
(DFS and OS) time and was analyzed by the log-rank test. The
multivariate survival analysis was performed according to the Cox
proportional hazards model. P<0.05 was used to indicate a
statistically significant difference.
Results
Expression and correlation of Smac and
survivin in pre-treatment biopsies and post-operative
specimens
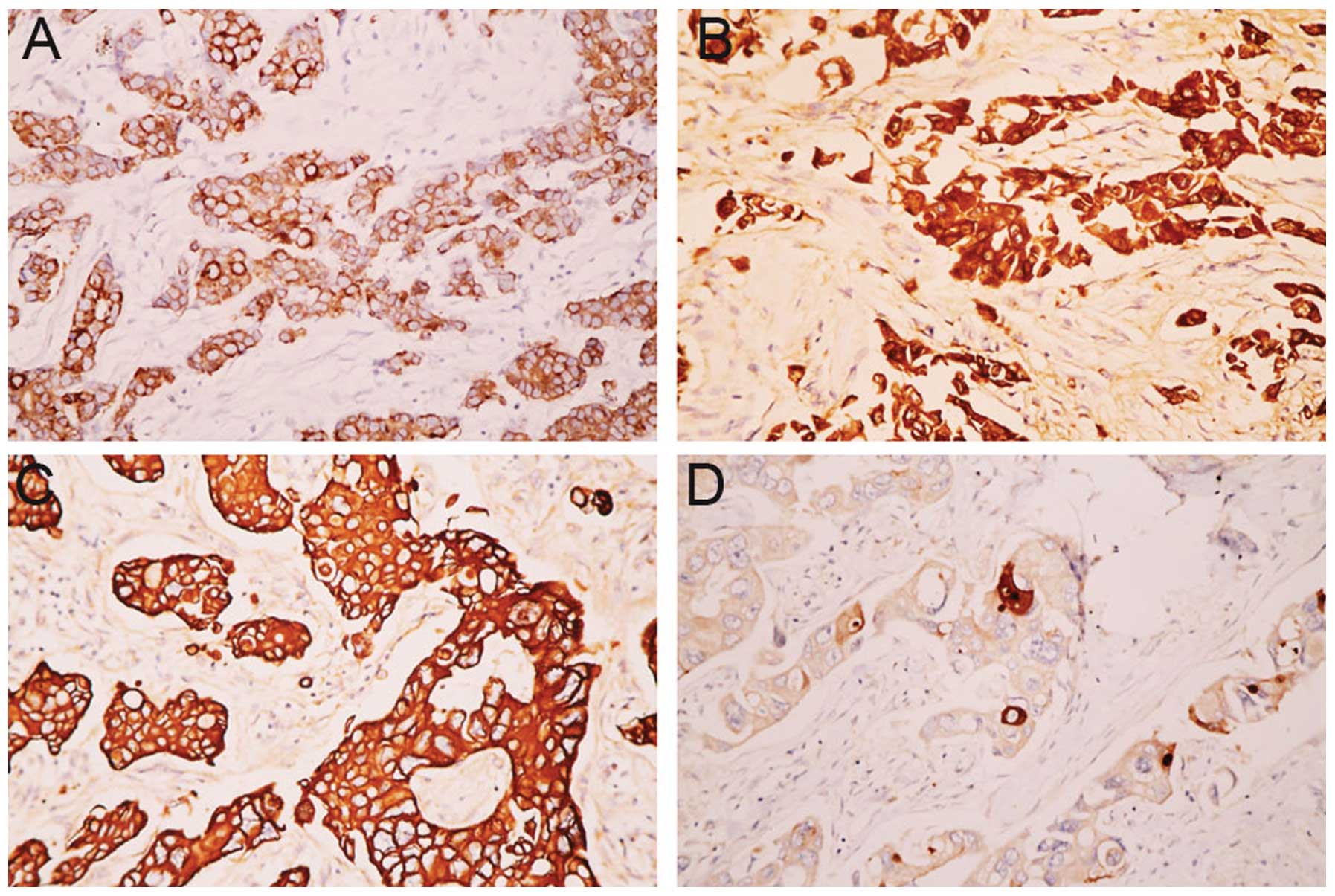
Figure 1 shows
representative images of Smac and survivin expression prior to and
following NAC. Smac and survivin were mainly detected in the
cytoplasm of breast cancer cells. Smac was expressed in 40.8%
(n=40/98) of biopsies and in 59.5% (n=47/79) of the resected
specimens. Smac expression was significantly upregulated following
NAC (P=0.013; Fig. 1A and B).
However, survivin was expressed in 53.1% (n=52/98) of biopsies and
in 36.7% (29/79) of the resected specimens. The expression of
survivin was significantly decreased (P=0.03) in the resected
specimens (Fig. 1C and D).
Moreover, in the biopsy and surgical specimens, Smac expression was
inversely correlated with survivin expression (r=−0.217, P=0.032;
and r=−0.335, P=0.003, respectively).
Correlation between Smac and survivin
expression in pre-treatment biopsies and clinicopathological
characteristics
Table I shows the
full clinicopathological characteristics of the patient cohort that
were assessable for Smac and survivin expression. No correlation
was observed between the expression levels of survivin and Smac and
the clinical factors, including age, tumor size, grade, lymph node
metastasis, clinical stage and ER, PR and human epidermal growth
factor receptor (Her)-2 expression (P>0.05).
 | Table ICorrelation between Smac and survivin
expression and clinicopathological characteristics of primary
breast cancer patients. |
Table I
Correlation between Smac and survivin
expression and clinicopathological characteristics of primary
breast cancer patients.
| | Smac, n | | Survivin, n | |
|---|
| |
| |
| |
|---|
| Characteristics | Number | High | Low | P-value | High | Low | P-value |
|---|
| Age, years |
| ≤50 | 46 | 22 | 24 | 0.184 | 28 | 18 | 0.145 |
| >50 | 52 | 18 | 34 | | 24 | 28 | |
| Tumor size, cm |
| ≤3 | 38 | 15 | 23 | 0.830 | 18 | 20 | 0.369 |
| >3 | 60 | 25 | 35 | | 34 | 26 | |
| Grade |
| I/II | 45 | 21 | 24 | 0.278 | 24 | 21 | 0.960 |
| III | 53 | 19 | 34 | | 28 | 25 | |
| Lymph node
metastasis |
| Positive | 62 | 26 | 36 | 0.809 | 34 | 28 | 0.644 |
| Negative | 36 | 16 | 20 | | 18 | 18 | |
| TNM stage |
| II | 43 | 21 | 22 | 0.153 | 19 | 24 | 0.120 |
| III | 55 | 19 | 36 | | 33 | 22 | |
| ER |
| Positive | 56 | 19 | 37 | 0.109 | 32 | 24 | 0.350 |
| Negative | 42 | 21 | 21 | | 20 | 22 | |
| PR |
| Positive | 35 | 13 | 22 | 0.511 | 22 | 13 | 0.148 |
| Negative | 63 | 27 | 36 | | 30 | 33 | |
| Her-2 |
| Positive | 42 | 15 | 27 | 0.113 | 21 | 21 | 0.599 |
| Negative | 56 | 25 | 31 | | 31 | 25 | |
Smac and survivin protein expression and
tumor response
Following anthracycline-based neoadjuvant
chemotherapy, 19 patients achieved pCR (19.4%), and RD was
identified in 79 patients (80.6%). Various clinicopathological
parameters, together with Smac and survivin expression, were
subjected to univariate and multivariate analyses for their
association with pCR (Table II).
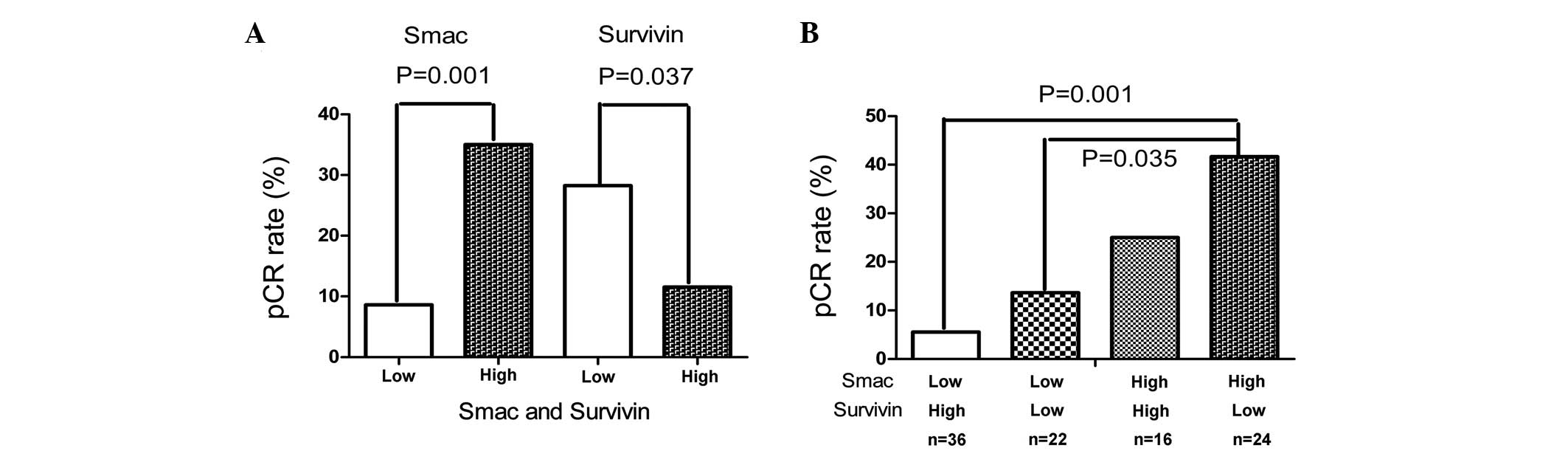
Univariate analysis indicated that a lack of ER (P=0.004), positive
Smac (P=0.001) and negative survivin (P=0.037) were significantly
associated with pCR (Fig. 2A). On
multivariate analysis, ER, Smac and survivin remained significantly
associated with pCR (P=0.001, P=0.031 and P=0.012, respectively).
Smac and survivin were significantly associated with pCR by
multivariate analysis; therefore, their combination was evaluated,
as shown in Figure 2B. Breast
tumors with no survivin expression and positive Smac expression
demonstrated the highest pCR rate (41.67%), and those with positive
survivin expression and a lack of Smac expression revealed the
lowest rate (5.56%). The breast tumors with negative Smac and
negative survivin and those with positive Smac and positive
survivin expression demonstrated intermediate pCR rates (13.64 and
25.0%, respectively).
 | Table IIUnivariate and multivariate analyses
(logistic regression) for tumor response. |
Table II
Univariate and multivariate analyses
(logistic regression) for tumor response.
| Univariate
analysis | | Multivariate
analysis | |
|---|
|
| |
| |
|---|
| Factors | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age, ≤50 vs. >50
years | 1.149 | 0.471–2.799 | 0.760 | - | - | - |
| Tumor size, ≤3 vs.
>3 cm | 1.107 | 0.444–2.763 | 0.828 | - | - | - |
| Node status,
cN− vs. cN+ | 0.994 | 0.352–2.808 | 0.991 | - | - | - |
| Tumor grade, I–II
vs. III | 2.112 | 0.730–6.117 | 0.168 | - | - | - |
| TNM stage, II vs.
III | 1.435 | 0.511–4.028 | 0.493 | - | - | - |
| ER, − vs. + | 5.102 | 1.664–15.625 | 0.004 | 12.502 | 3.012–52.630 | 0.001 |
| PR, − vs. + | 1.401 | 0.504–3.894 | 0.518 | - | - | - |
| Her-2, − vs. + | 1.255 | 0.459–3.429 | 0.658 | - | - | - |
| Smac, high vs.
low | 3.548 | 1.406–8.955 | 0.001 | 4.141 | 1.142–15.013 | 0.031 |
| Survivin, low vs.
high | 0.326 | 0.128–0.826 | 0.037 | 5.714 | 1.466–22.222 | 0.012 |
Smac and survivin protein expression and
survival
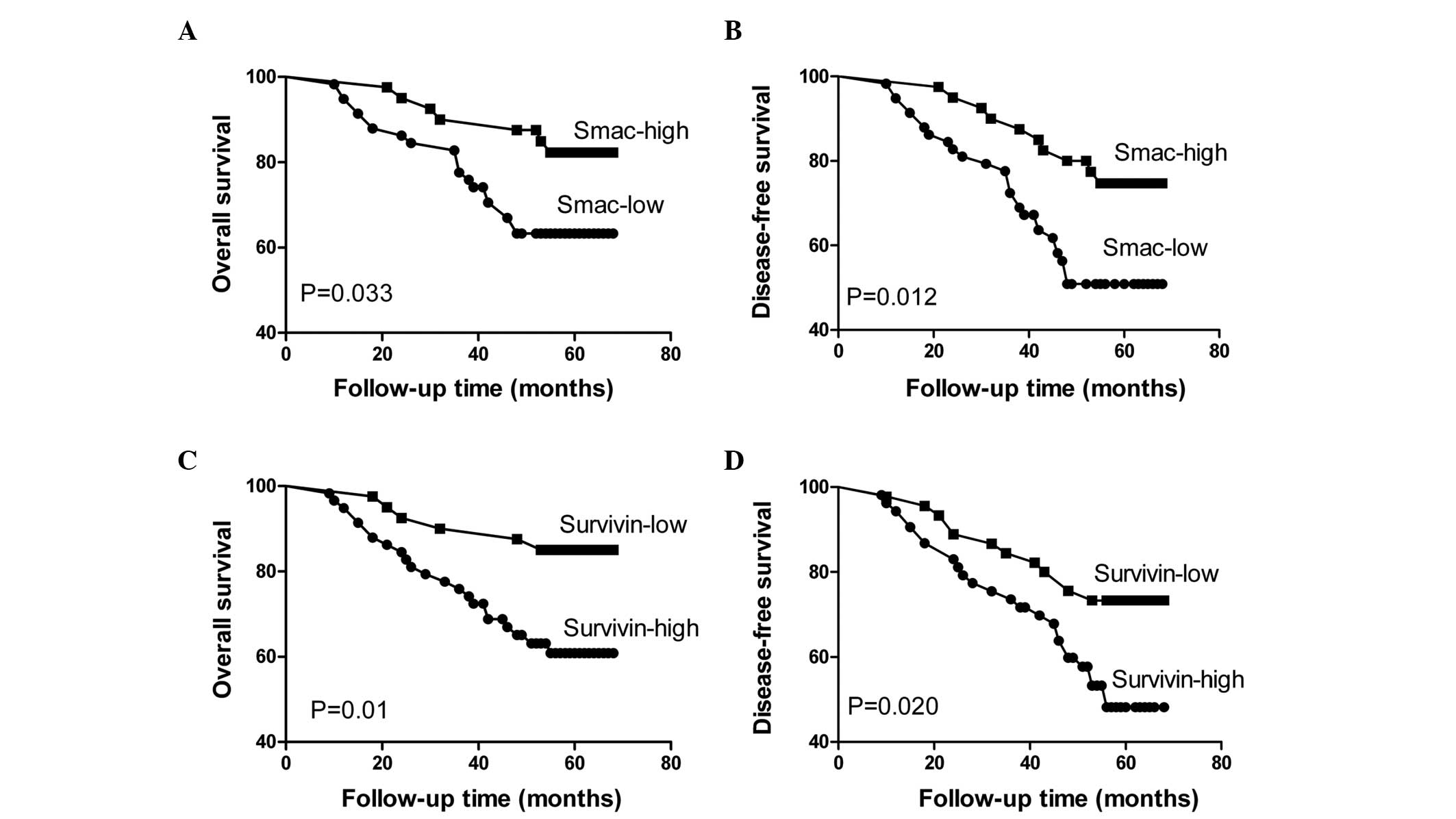
An analysis of the impact of Smac and survivin
status is shown in Figure 3; high
expression of Smac in the biopsy specimens was revealed to be a
favorable prognostic factor, as measured by OS (P=0.033) and DFS
(P=0.012) (Fig. 3A and B). By
contrast, the patients with positive survivin expression tended to
have a poorer prognosis than the patients with negative survivin
expression, as measured by OS (P=0.01) and DFS (P=0.020) (Fig. 3C and D).
In Cox regression, when analyzing patient age, lymph
node metastasis, histological grade, tumor size, TNM stage, Smac
expression, survivin expression, ER, PR and Her-2, only Smac
expression (P=0.029; hazard ratio (HR), 2.950; 95% confidence
interval (CI), 1.173–20.812 and P=0.044; HR, 3.831; 95% CI,
1.042–12.085), survivin expression (P=0.015; HR, 8.850; 95% CI,
1.587–32.682 and P=0.007; HR, 4.690; 95% CI, 1.511–14.556) and TNM
stage (P=0.017; HR, 9.497, 95% CI, 1.491–21.490 and P=0.038; HR,
4.020; 95% CI, 1.083–14.929) remained as independent prognostic
factors for OS and DFS, respectively (Table III).
 | Table IIIMultivariate Cox-regression analysis
for OS and DFS. |
Table III
Multivariate Cox-regression analysis
for OS and DFS.
| OS | | DFS | |
|---|
|
| |
| |
|---|
| Factors | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, ≤50 vs. >50
years | 0.621 | 0.272–3.933 | 0.613 | 0.473 | 0.105–2.132 | 0.330 |
| Tumor size, >3
vs. ≤3 cm | 1.022 | 0.196–8.416 | 0.485 | 3.145 | 0.469–11.739 | 0.235 |
| Node status,
cN− vs. cN+ | 6.993 | 0.833–18.825 | 0.073 | 3.362 | 0.549–12.602 | 0.190 |
| Tumor grade, III
vs. I–II | 1.230 | 0.678–4.414 | 0.252 | 1.010 | 0.247–3.977 | 0.891 |
| TNM stage, III vs.
I–II | 9.497 | 1.491–21.490 | 0.017 | 4.020 | 1.083–14.929 | 0.038 |
| ER, − vs. + | 1.323 | 0.531–3.300 | 0.548 | 1.159 | 0.154–8.735 | 0.786 |
| PR, − vs. + | 1.247 | 0.475–3.275 | 0.654 | 2.710 | 0.505–12.492 | 0.245 |
| Her-2, − vs. + | 1.171 | 0.465–2.950 | 0.738 | 1.021 | 0.303–3.273 | 0.895 |
| Smac, low vs.
high | 2.950 | 1.173–20.812 | 0.029 | 3.831 | 1.042–12.085 | 0.044 |
| Survivin, low vs.
high | 8.850 | 1.587–32.682 | 0.015 | 4.690 | 1.511–14.556 | 0.007 |
Discussion
The achievement of a pCR is a significant goal for
NAC. Despite intensive efforts to determine the correct regimen for
individual breast cancer patients, there is no reliable marker to
select the best treatment or to monitor response during therapy. To
the best of our knowledge, the present study is the first to
analyze the potential value of Smac and survivin protein expression
in predicting the response of breast cancer to NAC.
Smac may be considered a therapeutic target due to
its role as an IAP antagonist. Smac contains a mitochondrial
targeting signal that is required for translocation to the cytosol.
Upon cleavage of the targeting signal, Smac may enter the cytosol
where it may bind to the bacculovirus IAP repeat domain of the IAPs
(9). Smac, caspase-3 and -9 are
competitive substrates of IAPs, therefore, upon binding of Smac the
caspases are released and their substrates cleaved, which as a
consequence induces apoptosis. This is known to be significant for
normal development and for chemotherapy and radiotherapy
responsiveness. Previous studies have indicated that Smac-mediated
apoptosis is significant for the apoptotic responses induced by
several anticancer agents, including certain chemopreventive agents
(10,11). Xu et al indicated that the
upregulation of Smac is a chemosensitization mechanism in
esophageal squamous cell carcinoma. Smac-knockdown significantly
suppressed cisplatin-induced apoptosis, mitochondrial membrane
potential collapse, caspase activation and cytochrome c
release, leading to cisplatin resistance in vitro and in
vivo (12). Fandy et al
(13) transiently transfected
full-length or mature Smac into breast cancer cells and treated
them with anticancer drugs. It was observed that Smac enhanced the
antiproliferative and pro-apoptotic effects in MCF-7 cells. In
ovarian carcinoma cell lines, infection with an adenovirus encoding
Smac increased the sensitivity to chemotherapy and radiotherapy,
and increased the activation of caspase-3 and -9 (14). Similarly, the present study
revealed that breast cancer patients with high Smac expression had
a good response to NAC. Moreover, a high expression of Smac was
demonstrated to correlate with improved DFS and OS in patients with
advanced breast cancer treated with anthracycline-based
chemotherapy. These findings are in line with a study reported by
Mizutani et al (15), where
renal cell carcinoma patients with positive Smac expression had a
longer post-operative disease-specific survival when compared with
those with no Smac expression. These results are not unexpected,
since overexpression of Smac sensitizes tumor cells against
anticancer drug-induced apoptosis (16).
The survivin gene, BIRC 5, is a member of the IAP
family and has several cellular functions, including the inhibition
of apoptosis and the dysregulation of mitosis, cell cycle
progression, carcinogenesis and DNA repair (17). As an IAP, high levels of survivin
have been associated with a poor prognosis in various types of
human cancer. However, several studies that attempted to delineate
the clinical role of survivin expression demonstrated contradictory
results with regard to its subcellular localization and prognostic
significance (18,19). Certain studies indicated that high
levels of cytoplasmic survivin and low levels of nuclear survivin
are associated with a poor clinical outcome, whereas others
indicated the opposite or reported no correlation (20,21).
These discrepancies may reflect differences in the methods used to
detect survivin, the different antibodies with differing
sensitivities used in immunohistochemistry analyses and the
different types of patients selected in the studies. In the present
study, survivin was revealed to be most frequently expressed in the
cytoplasm, which was concurrent with the study by Span et al
(22), which stated that the
cytoplasmic form is the predominant cellular species of survivin.
Overexpression of survivin is associated with resistance to
chemotherapy, and decreased survivin expression is associated with
increased sensitivity to etopside and 5-FU (23). In the present study, survivin
expression was significantly negatively associated with the
response to chemotherapy and with DFS and OS; however, no
significant correlation was identified with other
clinicopathological factors, including clinical stage, age, grade
or lymph node metastasis. The results indicated that high survivin
expression in the tumor is predictive of a poorer response and
prognosis in patients with advanced breast cancer treated with
anthracycline-based NAC. Boidot et al (24) also demonstrated that increased
survivin transcript expression is significantly correlated with
resistance to an epirubicin-based combination regimen and reduced
DFS. However, Estevez et al (25) identified no correlation between
nuclear survivin expression and the response to NAC in patients
with breast cancer. Thus, future studies evaluating the predictive
role of survivin should include a thorough assessment of the
expression of different transcripts combined with ultrastructural
analyses to clarify whether a cytosolic or nuclear accumulation is
a better predictor of response.
In the present study, a significant negative
correlation was revealed between the expression of survivin and
Smac in the pre-treatment of breast carcinoma (P<0.01).
Following NAC, tumoral Smac expression was upregulated and survivin
expression was downregulated. Smac expression also had an inverse
correlation with survivin expression. This increase in Smac
expression may directly or indirectly result in the downregulation
of survivin expression in the sample analyzed, which may explain
the increased cell apoptosis during treatment with anticancer
agents. Furthermore, Smac-positive and survivin-negative tumors
were observed to demonstrate the highest pCR rate. These results
confirmed that Smac is an antagonist of survivin, preventing the
inhibition of caspases to sensitize cancer cells to NAC.
The growth, differentiation and survival of mammary
epithelial cells is directed by a synergy between estrogen and
progesterone. The response to hormonal therapy may be predicted by
the status of the aforementioned hormones. However, the tumor
response to chemotherapy and their role in its prediction remains
unclear. Darb-Esfahani et al (26) reported that hormone
receptor+/HER2+-coexpressing carcinomas tend
to respond well to NAC, indicating favorable prognoses, and that
hormone receptor+/HER2− tumors have good
prognoses, irrespective of pCR. Similarly, in a retrospective study
that included 1,118 patients treated with various NAC regimes,
Liedtke et al (27)
identified equally high pCR rates in
ER+/HER2+ and ER−/HER2−
carcinomas (21 vs. 22%). By contrast, two previous clinical studies
revealed a correlation between chemosensitivity and a negative
hormonal receptor status (28,29).
In the present study, the pCR rate of ER-positive tumors was
revealed to be significantly lower compared with that of
ER-negative tumors. Multivariate analysis demonstrated that an
ER-negative status was also a significant predictor for a good
response to NAC. One hypothesis states that ER may be a marker of
the differentiation of luminal epithelial cells and that
well-differentiated tumors are not as likely to be responsive to
chemotherapy compared with those that are less differentiated.
Furthermore, no statistically significant correlation was observed
between hormone receptor expression and Smac or survivin in breast
cancer. The present observations agree with studies reported by
Kennedy et al (30) and Xie
et al (31), which
demonstrated that no statistically significant correlation exists
between survivin and Smac expression and either ER or PR.
In conclusion, among primary breast cancer patients
receiving anthracycline-based NAC, patients with positive Smac
expression and a lack of survivin expression were more likely to
achieve pCR. By assessing the expression of Smac and survivin, the
prognosis of breast cancer patients receiving NAC may also be
estimated. However, in recent years, taxanes as a standard
treatment have been included in the majority of the neoadjuvant
study protocols. Furthermore, targeted therapies, including
lapatinib, trastuzumab, bevacizumab, pertuzumab, everolimus and
others, should be tested, particularly when resistance against a
standard chemotherapy is observed or anticipated. Therefore, this
may represent a limitation for interpreting the results with regard
to the clinical setting. Moreover, due to the retrospective
evaluation and the limited sample size, the results should be
confirmed in larger cohorts, preferentially in prospective
trials.
References
|
1
|
Goldhirsch A, Wood WC, Gelber RD, Coates
AS, Thürlimann B and Senn HJ; 10th St. Gallen conference. Progress
and promise: highlights of the international expert consensus on
the primary therapy of early breast cancer 2007. Ann Oncol.
18:1133–1144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kaufmann M, Hortobagyi GN, Goldhirsch A,
et al: Recommendations from an international expert panel on the
use of neoadjuvant (primary) systemic treatment of operable breast
cancer: an update. J Clin Oncol. 24:1940–1949. 2006. View Article : Google Scholar
|
|
3
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Verhagen AM, Ekert PG, Pakusch M, et al:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Goyal L: Cell death inhibition: keeping
caspases in check. Cell. 104:805–808. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song Z, Yao X and Wu M: Direct interaction
between survivin and Smac/DIABLO is essential for the
anti-apoptotic activity of survivin during taxol-induced apoptosis.
J Biol Chem. 278:23130–23140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sobin LH and Wittekind C: TNM
classification of malignant tumours. 6th edition. Wiley-Liss; New
York, NY: pp. 22–26. 2002
|
|
8
|
Mazouni C, Peintinger F, Wan-Kau S, et al:
Residual ductal carcinoma in situ in patients with complete
eradication of invasive breast cancer after neoadjuvant
chemotherapy does not adversely affect patient outcome. J Clin
Oncol. 25:2650–2655. 2007. View Article : Google Scholar
|
|
9
|
Martinez-Ruiz G, Maldonado V,
Ceballos-Cancino G, Grajeda JP and Melendez-Zajgla J: Role of
Smac/DIABLO in cancer progression. J Exp Clin Cancer Res.
27:482008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bank A, Wang P, Du C, Yu J and Zhang L:
SMAC mimetics sensitize nonsteroidal anti-inflammatory drug-induced
apoptosis by promoting caspase-3-mediated cytochrome c release.
Cancer Res. 68:276–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun Q, Zheng X, Zhang L and Yu J: Smac
modulates chemosensitivity in head and neck cancer cells through
the mitochondrial apoptotic pathway. Clin Cancer Res. 17:2361–2372.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Zhou L, Huang J, et al: Role of Smac
in determining the chemotherapeutic response of esophageal squamous
cell carcinoma. Clin Cancer Res. 17:5412–5422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fandy TE, Shankar S and Srivastava RK:
Smac/DIABLO enhances the therapeutic potential of chemotherapeutic
drugs and irradiation, and sensitizes TRAIL-resistant breast cancer
cells. Mol Cancer. 7:602008. View Article : Google Scholar
|
|
14
|
McNeish IA, Bell S, McKay T, Tenev T,
Marani M and Lemoine NR: Expression of Smac/DIABLO in ovarian
carcinoma cells induces apoptosis via a caspase-9-mediated pathway.
Exp Cell Res. 286:186–198. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mizutani Y, Nakanishi H, Yamamoto K, et
al: Downregulation of Smac/DIABLO expression in renal cell
carcinoma and its prognostic significance. J Clin Oncol.
23:448–454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fulda S, Wick W, Weller M and Debatin KM:
Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced
apoptosis and induce regression of malignant glioma in vivo. Nat
Med. 8:808–815. 2002.PubMed/NCBI
|
|
17
|
Blanc-Brude OP, Mesri M, Wall NR, Plescia
J, Dohi T and Altieri DC: Therapeutic targeting of the survivin
pathway in cancer: initiation of mitochondrial apoptosis and
suppression of tumor-associated angiogenesis. Clin Cancer Res.
9:2683–2692. 2003.
|
|
18
|
Shinohara ET, Gonzalez A, Massion PP, et
al: Nuclear survivin predicts recurrence and poor survival in
patients with resected nonsmall cell lung carcinoma. Cancer.
103:1685–1692. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Falleni M, Pellegrini C, Marchetti A, et
al: Survivin gene expression in early-stage non-small cell lung
cancer. J Pathol. 200:620–626. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rexhepaj E, Jirstrom K, O’Connor DP, et
al: Validation of cytoplasmic-to-nuclear ratio of survivin as an
indicator of improved prognosis in breast cancer. BMC Cancer.
10:6392010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chu JS, Shew JY and Huang CS:
Immunohistochemical analysis of survivin expression in primary
breast cancers. J Formos Med Assoc. 103:925–931. 2004.PubMed/NCBI
|
|
22
|
Span PN, Sweep FC, Wiegerinck ET, et al:
Survivin is an independent prognostic marker for risk
stratification of breast cancer patients. Clin Chem. 50:1986–1993.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hayashi N, Asano K, Suzuki H, et al:
Adenoviral infection of survivin antisense sensitizes prostate
cancer cells to etoposide in vivo. Prostate. 65:10–19. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boidot R, Vegran F and Lizard-Nacol S:
Predictive value of survivin alternative transcript expression in
locally advanced breast cancer patients treated with neoadjuvant
chemotherapy. Int J Mol Med. 23:285–291. 2009.
|
|
25
|
Estevez GL, Fortes JL, Adrover E, et al:
Doxorubicin and cyclophosphamide followed by weekly docetaxel as
neoadjuvant treatment of early breast cancer: analysis of
biological markers in a GEICAM phase II study. Clin Transl Oncol.
11:54–59. 2009. View Article : Google Scholar
|
|
26
|
Darb-Esfahani S, Loibl S, Müller BM, et
al: Identification of biology-based breast cancer types with
distinct predictive and prognostic features: role of steroid
hormone and HER2 receptor expression in patients treated with
neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer
Res. 11:R692009. View
Article : Google Scholar
|
|
27
|
Liedtke C, Mazouni C, Hess KR, et al:
Response to neoadjuvant therapy and long-term survival in patients
with triple-negative breast cancer. J Clin Oncol. 26:1275–1281.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bear HD, Anderson S, Brown A, et al: The
effect on tumor response of adding sequential preoperative
docetaxel to preoperative doxorubicin and cyclophosphamide:
preliminary results from National Surgical Adjuvant Breast and
Bowel Project Protocol B-27. J Clin Oncol. 21:4165–4174. 2003.
View Article : Google Scholar
|
|
29
|
Colleoni M, Viale G, Zahrieh D, et al:
Chemotherapy is more effective in patients with breast cancer not
expressing steroid hormone receptors: a study of preoperative
treatment. Clin Cancer Res. 10:6622–6628. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kennedy SM, O’Driscoll L, Purcell R, et
al: Prognostic importance of survivin in breast cancer. Br J
Cancer. 88:1077–1083. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie W, Jiang P, Miao L, et al: Novel link
between E2F1 and Smac/DIABLO: proapoptotic Smac/DIABLO is
transcriptionally upregulated by E2F1. Nucleic Acids Res.
34:2046–2055. 2006. View Article : Google Scholar : PubMed/NCBI
|