Introduction
The expression of human Oct4, Sox2, c-Myc and Nanog
can turn a variety of differentiated cells into induced pluripotent
stem cells with embryonic stem cell-like properties (1–3).
Oct4 is a member of the POU transcription factor family, and is
significant in the pluripotency and self-renewal of embryonic stem
cells (4,5). Induced pluripotent stem cell
formation can be caused by Oct4 alone in mouse and human neural
stem cells (6,7).
Oct4 only exhibits these functions intracellularly
(8), whereas the human
immunodeficiency virus (HIV) Tat protein can be taken up by cells
where it activates viral genome transcription (9). In the late 1990s, the first use of
Tat as a delivery agent to introduce proteins into cells in
vitro (10) and in mice in
vivo (11) was reported,
followed by numerous studies concerning the application of Tat to
deliver various proteins into cells in the form of Tat-fusion
proteins or Tat-protein conjugates (12–14).
In the present study, fusing the Oct4 gene with Tat47–57
allowed Oct4 to penetrate the cell membrane.
Oct4 has been produced using Escherichia coli
(15) or mammalian cells (16). However, these expression systems
are limited by low yields, complex manipulations or high culture
costs. The methylotrophic yeast, Pichia pastoris, has the
advantage of eukaryotic and prokaryotic expression systems and has
widely been used to express a variety of biologically active
proteins in extremely high yields (17–20).
In the present study, the Oct4 gene was fused with a human serum
albumin (HSA) signal peptide and HIV Tat47–57, and then
inserted into the pPICZαC vector. The recombinant
Tat47–57-Oct4 fusion protein was expressed with P.
pastoris X-33 under the control of aldehyde oxidase 1 (AOX1).
Tat47–57-Oct4 was secreted into the growth medium,
yielding ~210 mg/l. The transmembrane transport ability of purified
Tat47–57-Oct4 fusion protein was confirmed by
immunofluorescence analysis.
Materials and methods
Acquisition of the Oct4 gene
To obtain the Oct4 gene, total RNA was extracted
from human livers and used as the template for reverse
transcription, conducted for 30 min at 42ºC followed by heat
treatment for 2 min at 94ºC to inactivate avian myeloblastosis
virus reverse transcriptase. The cDNA was then used as the template
for polymerase chain reaction (PCR) using the following primers:
P1, 5′-CCATGGCGGGACACCTGGCTTC-3′ and P2,
5′-TCAGTTTGAATGCATGGGTG-3′.
The PCR protocol consisted of initial pre-heating at
94ºC for 5 min, followed by 32 cycles of 94ºC for 30 sec, 60ºC for
30 sec and 72ºC for 90 sec and a 5-min final elongation at 72ºC.
The PCR product was inserted into the PMD-18 vector to generate the
plasmid, pTOct4, which was verified by DNA sequencing.
Construction of expression vector,
pPICZαC-Tat-Oct4
Three primers were designed to construct a plasmid
containing the human serum albumin (HSA) signal peptide and HIV
Tat47–57 fused with Oct4. Partial DNA sequences of the
HSA signal peptide, HIV Tat47–57 and a 13-bp homologue
of the Oct4 DNA sequence were included in primer P3: Forward,
5′-TTATTCGC GAGGTGTGTTTCGTCGATACGGTAGAAAGAAGCGTCG
ACAGCGTCGACGAATGGCGGGACACC-3′. The homologue DNA
sequence of Oct4 is shown in bold, the DNA sequence of HIV
Tat47–57 is underlined and the partial DNA sequence of
the HSA signal peptide is shown in italics. The DNA sequences of
the Bspt1041 recognition sites and the HSA signal peptide were
included in P4: Forward, 5′-GGTTC
GAAACGATGAAGTGGGTAACCTTTATTTCCCTTCTTTTTC
TCTTTAGCTCGGCTTATTCGCGAGGTGTG-3′. The DNA sequence of the HSA
signal peptide is in italics, and the DNA sequence of the
recognition site for Bspt104I is underlined. A total of 17
bp of reverse homologue DNA sequences of Oct4 and the XbaI
recognition sites were included in P5: Forward, 5′-CCAGAATTCTCAGTTTGAATGCATGG-3′.
The DNA sequence of the recognition site for XbaI is
underlined and the stop codon is shown in bold.
Two rounds of PCR were performed. Firstly, the
plasmid pTOct4 was used as the template in the first PCR with
primers P3 and P2. The PCR reaction mixture was initially heat
denatured at 94ºC for 5 min, followed by 16 cycles with 30 sec of
denaturation at 94ºC, 30 sec of annealing at 60ºC and 90 sec of
extension at 72ºC, 30 sec of denaturation at 94ºC, 30 sec annealing
at 53ºC and 90 sec extension at 72ºC. An additional extension for 5
min at 72ºC was performed to ensure the completion of the PCR
products. Secondly, the PCR products were used as the template for
the second PCR with primers P4 and P5. The cycle program consisted
of 33 cycles with 30 sec of denaturation at 94ºC, 30 sec of
annealing at 59ºC and 90 sec of extension at 72ºC and a final
extension of 5 min at 72ºC. The product was digested with
Bspt104I and XbaI and ligated into pPICZαC at the
same sites to generate pPICZαC-pHSA-Tat-Oct4. The result was
confirmed by DNA sequencing.
Screening for high-level expression
colonies
The recombinant expression vector,
pPICZαC-pHSA-Tat-Oct4, was linearized by SacI and then
introduced into P. pastoris X-33 (Invitrogen Life
Technologies, Carlsbad, CA, USA) by electroporation with a
Micropulser (Bio-Rad, Hercules, CA, USA) according to the pPICZαC
vector manual. The transformants were screened on yeast extract
peptone dextrose (YPD) agar plates containing zeocin, and cultured
at 28ºC for at least three days. The positive clones were selected
and cultured in 5 ml buffered glycerol-complex (BMGY) medium at
28ºC for 24 h with agitation at 250 rpm in an orbital shaker
(Thermo Fisher Scientific, Boston, MA, USA). The genomic DNA was
extracted and amplified using 5′AOX1 and 3′AOX1 as primers to
verify whether the Tat-Oct4 gene was integrated into the genome
stably. Non-transformed yeast DNA was extracted as a control group.
To achieve a high yield of Tat-Oct4, the positive transformants
were cultured in 10 ml BMGY medium [1.0% yeast extract, 2.0%
peptone, 1.34% yeast nitrogen base, 0.5 mg/l biotin, 100 mM
potassium phosphate (pH 6.0) and 1.0% glycerol] and incubated at
28ºC for 24 h. Next, the cells were cultured in 10 ml buffered
methanol-complex medium in which 1.0% glycerol was replaced by 0.5%
methanol. Fresh methanol was added every 24 h to maintain the
concentration at 0.5% (v/v) for nine days. The expression level of
Tat-Oct4 in the supernatant was determined by SDS-PAGE and western
blotting.
Pilot-scale fermentation of
Tat47–57-Oct4
The clone with the highest level of Tat-Oct4
expression was cultured in 2 liters YPD medium in a 5-liter conical
flask in a shaking incubator (Thermo Fisher Scientific), at 28ºC
until the optical density (OD) of cultured P patoris at 600
nm reached 10. This culture was then added into a 80-liter NBS
Bioflo 5000 fermenter (New Brunswick Scientific, Enfield, CT, USA)
containing 40 liters of fermentation basal salt medium FM21,
supplemented with PTM1 trace salts (21) and biotin (0.04 ml stock solution).
The level of dissolved oxygen (DO) was maintained at 30–40% and the
stirring rate was 400 rpm in the 80-liter NBS Bioflo 5000 fermenter
(New Brunswick Scientific, Enfield, CT, USA). The medium was
maintained at pH 4.0 by automatic addition of 5M NH4OH
and 1M phosphoric acid and 5% antifoam was also delivered as
required. The temperature was controlled at 28ºC. The fermentation
was divided into three phases; the designated glycerol,
glycerol-fed and methanol-fed batch phases. The pH of the medium
was maintained at 4.0 during the glycerol phase and once the
glycerol was consumed at the end of the first phase, there was a
sharp increase in DO value, and the second phase was initiated.
During this phase, 50% glycerol feed, containing 1.2% (v/v) PTM1
trace salts, was added at an initial speed of 400 ml/h with
peristaltic pump (LongerPump, Baoding Hebei, China), with the speed
of addition gradually increasing to 720 ml/h. The glycerol was
supplied until a cell yield of 180–220 g/l wet weight was achieved.
The third phase was initiated by starting a 100% methanol feed
containing 1.2% (v/v) PTM1 trace salts. During the methanol
induction phase, the pH was adjusted to 8.0. Methanol was initially
added at 144 ml/h for 4 h to allow the culture to adapt to growth
on methanol, subsequent to which, the addition speed was gradually
increased to 440 ml/h. The samples of the culture medium were
collected every 4 h to analyze the wet cell weight, the
OD600 and the expression level of Tat-Oct4.
Purification of
Tat47–57-Oct4
The supernatant was harvested by centrifugation
(10,000 × g, 5 min) and the proteins were precipitated by 35%
saturated ammonium sulfate. As the molecular weight of
Tat47–57-Oct4 is ~40 kDa, all the proteins weighing
between 10 and 100 kDa were isolated and concentrated using
Vivaflow 200 PES with 50,000 and 10,000 molecular weight cut off
(Sartorius, Goettingen, Germany). To purify the sample further, the
supernatant was diluted three times with 50 mM NaAc-HAc and loaded
onto a SP Sepharose column (Amersham-Pharmacia, Piscataway, NJ,
USA), pre-equilibrated with 50 mM NaAc-HAc buffer and set at a flow
rate of 30 ml/min. The protein was eluted in a linear salt gradient
and monitored by measuring the UV absorbance at 280 nm. The
fractions containing Tat-Oct4 (from the SP Sepharose XL column)
were desalted and concentrated by ultrafiltration (10,000 molecular
weight cut off, Vivaflow 200) and filtered by a 0.22-μm filter. The
components were analyzed by SDS-PAGE and western blotting to
determine which contained Tat-Oct4. The purified Tat-Oct4 was
analyzed on a high-performance liquid chromatography (HPLC) system
(Waters, Milford, MA, USA) using a C4 reversed-phase column
(Waters, Milford, MA, USA). The protein concentration was
determined by the Bradford method using bovine serum albumin (BSA)
as the concentration standard (22). The purified Tat-Oct4 was
freeze-dried rapidly in a high vacuum freeze dryer, ALPHA-1–4
(Martin Christ Company, Harz, Germany). The concentration of
Tat-Oct4 at each step of the procedure was quantified by an
enzyme-linked immunosorbent assay and the product was stored under
sterile conditions at −80ºC.
SDS-PAGE and western blotting
The purified protein, Tat-Oct4 was analyzed by
SDS-PAGE performed with a 12% gel and stained with Coomassie
brilliant blue, according to the method of Sambrook and Russel
(23).
The proteins in the gel were transferred to a
polyvinylidene fluoride (PVDF) membrane for western blotting using
a semi-dry electroblotting apparatus (Bio-Rad) at 15 V for 30 min
in 25 mM Tris/192 mM glycine. The membrane was blocked by
incubating with Tris-buffered saline with Tween 20 (TBST)
containing 2% BSA over 12 h at 4ºC, then washed three times with
0.2% BSA in TBST and incubated with rabbit anti-human Oct4
polyclonal antibody (Abcam, Cambridge, UK) for 3 h at room
temperature, followed by a final three washes with TBST. The
membrane was then incubated with the secondary goat anti-rabbit
antibody (Abcam, Boston, MA, USA) for another 3 h, washed three
times with TBST and then washed with TBS for 15 min. The Tat-Oct4
fusion protein was detected using 3,3′-diaminobenzidine
tetrahydrochloride reagents (Beyotime, Jiangsu, China).
N-terminal amino acid sequence
analysis
To determine the N-terminal sequence, the purified
Tat-Oct4 was electrophoresed on a 12% SDS-PAGE gel and
electroblotted onto a PVDF membrane. Following blotting, the PVDF
membrane was stained with Amido black and the Tat-Oct4 band was cut
out. The N-terminal amino acid sequence analysis was conducted
using a PPSQ-21A protein sequencer (Shimadzu, Kyoto, Japan).
Detection of transmembrane transport
ability of Tat-Oct4
Fibroblasts were obtained from human foreskin
tissue, obtained from the first hospital of Jilin University and
the patient consent was signed by the patient himself. The
fibroblasts were inoculated in a 24-well plate at a density of
1×105 cells per well. After 24 h, the cells were treated
with serum-free minimum essential medium containing 1 μM Tat-Oct4
or saline water (negative control group), and cultured with
serum-free medium for 6 h only. The cells were then washed three
times with phosphate-buffered saline (PBS) for 10 min to remove
proteins from the outside of the cells, fixed with 4%
paraformaldehyde for 30 min, washed three times with PBS for 5 min
and then treated with 0.25% Triton X-100 in PBS and 5% BSA (to
block non-specific binding) for 30 min. The cells were then
incubated overnight at 4ºC with rabbit Oct4 polyclonal antibody
(Abcam, Cambridge, UK), then washed three times with PBS for 5 min
and incubated further with fluorescein isothiocyanate-conjugated
goat anti-rabbit immunoglobulin G antibody for 30 min at 37ºC. The
cells were rinsed three times and incubated with propidium iodide
solution. Finally, the cells were rinsed another three times and
examined using a fluorescence microscope (FA500; Olympus, Tokyo,
Japan).
Results and Discussion
Construction and transformation of
Tat-Oct4
The 1,086-bp gene fragment encoding human Oct4 was
amplified by PCR with primers P1 and P2, and subcloned into the
PMD-18 vector to generate the plasmid, pTOct4. The 1,200-bp gene
fragment encoding the HSA signal peptide, HIV Tat47–57,
Oct4 Bspt1041 and the XbaI recognition site, was amplified
by two-step PCR from pTOct4 and subcloned into pPICZαC to generate
the recombinant expression vector, pPICZαC-Tat-Oct4. Nucleotide
sequencing analysis confirmed that the two vectors contained the
correct gene.
Expression of P. pastoris and screening
for high-level expression colonies
To integrate the HSA signal peptide, HIV
Tat47–57 and Oct4 were incorporated into the genome of
P. pastoris by homologous recombination. pPICZαC-Tat-Oct4
was linearized with SacI and transformed into
electrocompetent P. pastoris cells. A successful integration
was confirmed by screening with zeocin and PCR with AOX1 universal
primers. The PCR results revealed that 95% zeocin-positive yeast
transformants had one specific band of ~1,471 bp.
The positive clones were inoculated in BMGY medium
for 24 h, the cells were collected and the expression of the
Tat47–57-Oct4 fusion protein was induced in the buffered
methanol-complex medium. Subsequent to a 168-h induction, each
strain was analyzed on SDS-PAGE to detect the
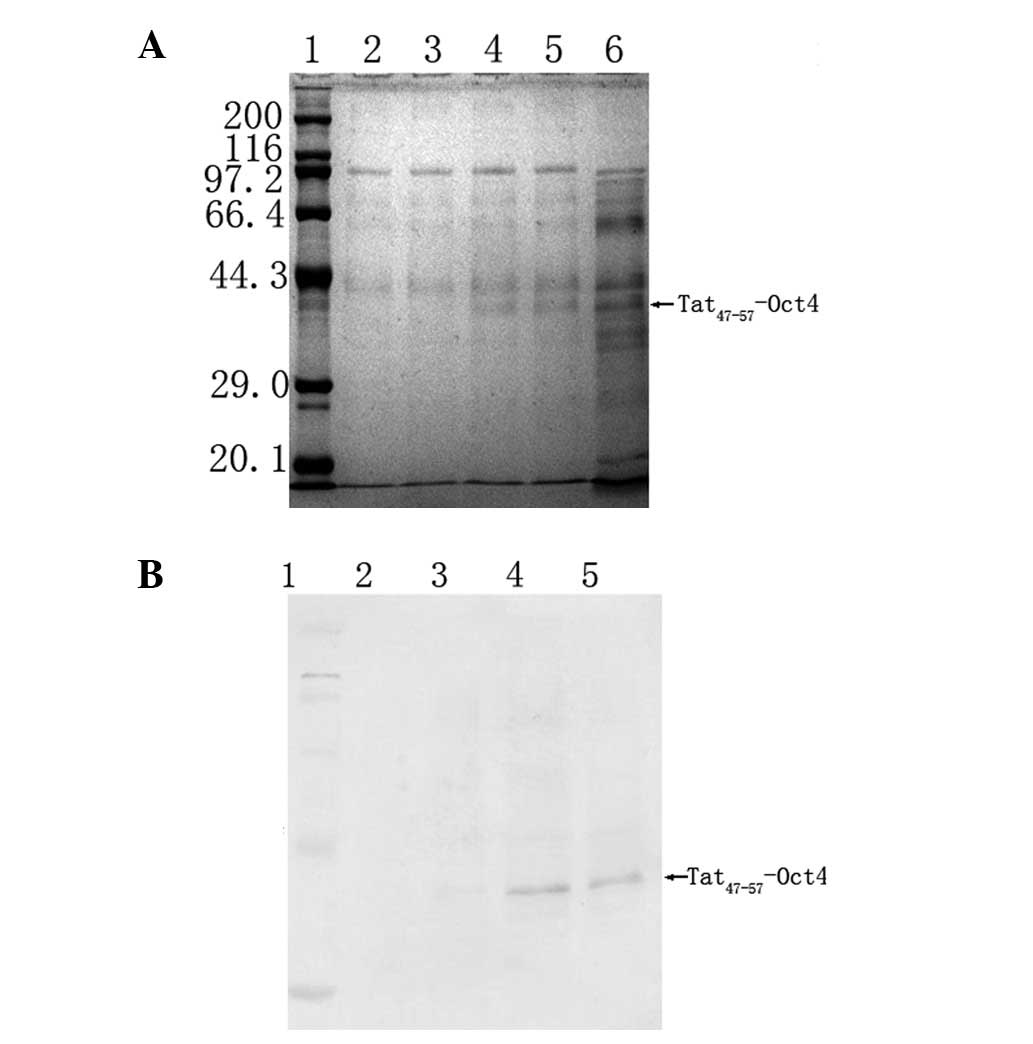
Tat47–57-Oct4 expression. According to the DNA sequence
of Tat-Oct4, the calculated molecular weight of Tat-Oct4 was ~40
kDa (Fig. 1).
Pilot-scale expression, purification and
characterization of purified Tat47–57-Oct4
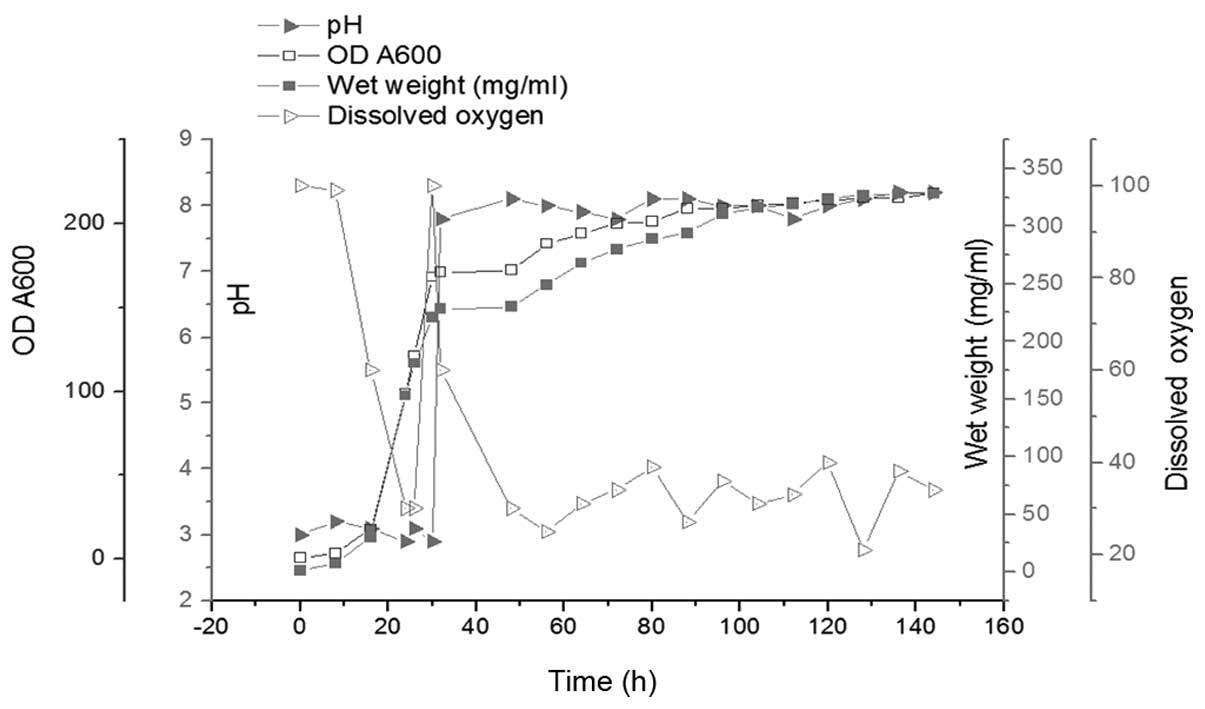
All the details of the batch fermentation are shown
in Fig. 2. The glycerol phase
lasted 30 h and the methanol induction phase lasted 112 h. At the
end of the glycerol phase, the OD600 of the culture
reached 168; the wet cell weight reached 221 mg/ml and the DO value
sharply increased from 30 to 100 mg/ml. In order to consume all the
glycerol, the cells were cultured for an additional 2 h without any
additions. Methanol was then added into the culture as the inducer
and the carbon source during P. pastoris fermentation. The
methanol feed rate was adapted according to the DO value, which was
maintained at 30–40%. The weight of wet cells and the corresponding
OD600 reached 328 and 218 mg/ml subsequent to a 112-h
induction. The expression level of Tat47–57-Oct4 during
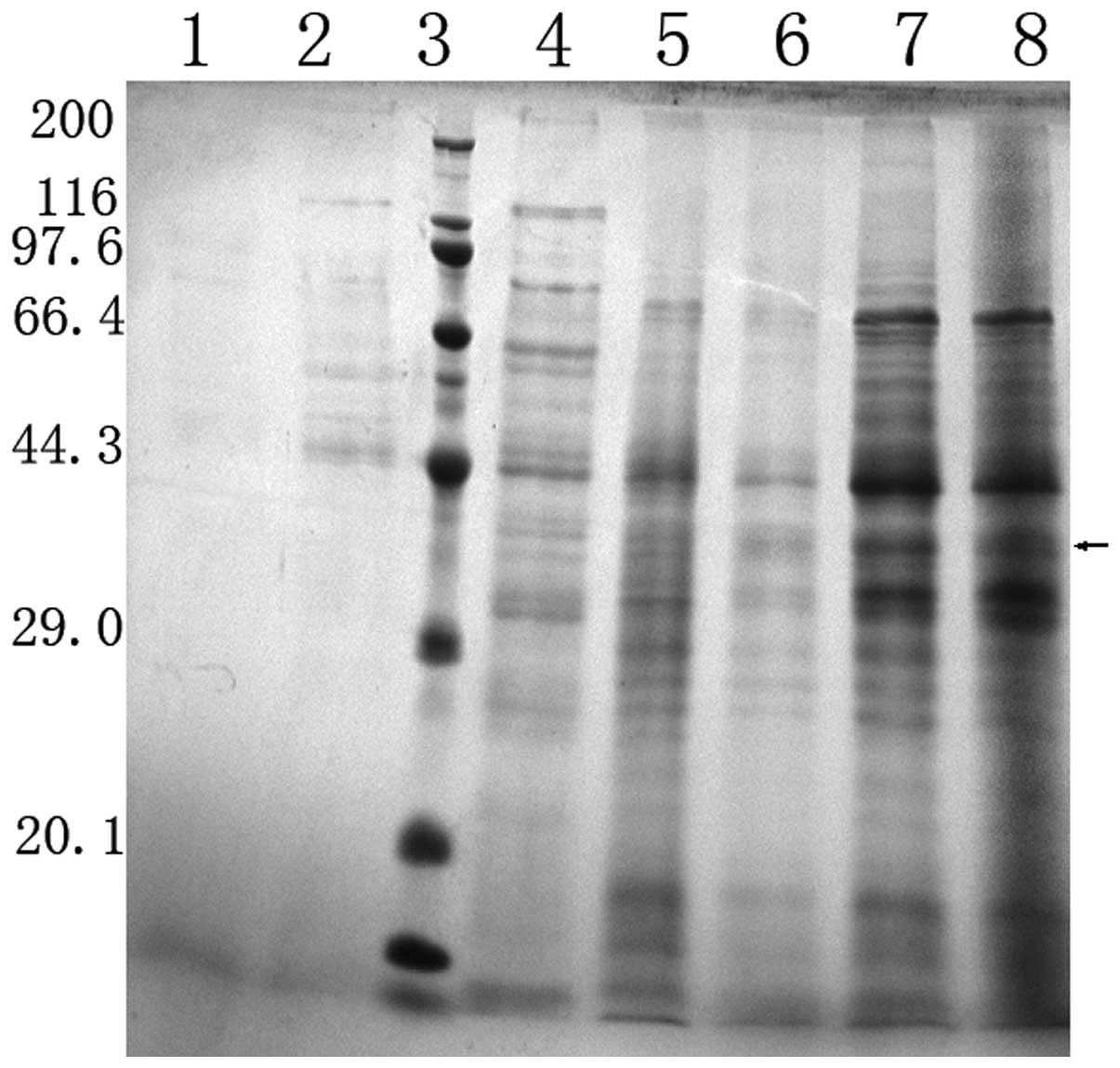
fermentation was revealed by Coomassie-stained SDS-PAGE (Fig. 3).
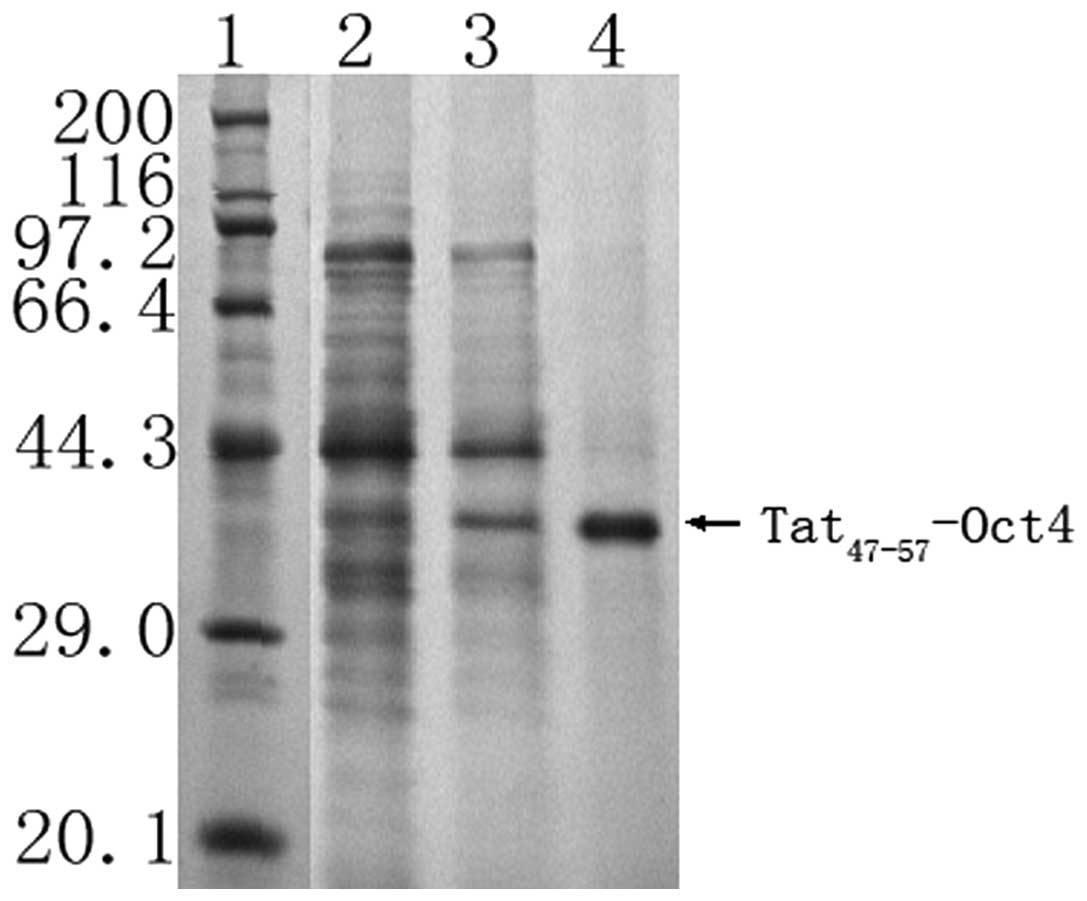
Purity and yield of
Tat47–57-Oct4
Following the fermentation and purification
processes, 601 mg purified Tat47–57-Oct4 was obtained
from 60 liters of culture medium. The Tat47–57-Oct4
purity was 95.6%, as revealed by SDS-PAGE (Fig. 4) and HPLC. The protein recovery
ratio and purity of Tat47–57-Oct4 at the various
purification steps are summarized in Table I.
 | Table ISummary of purification steps of
Tat47–57-Oct4 from P. pastoris. |
Table I
Summary of purification steps of
Tat47–57-Oct4 from P. pastoris.
| Purification
steps | Total proteins,
mg |
Tat47–57-Oct4, mg | Recovery, % | Purity, % |
|---|
| Supernatant | 252900 | 15100 | | 4.98 |
| Vivaflow 200 | 87100 | 12080 | 80.50 | 13.86 |
| SP Sepharose
XL | 7510 | 6864 | 56.00 | 91.34 |
| Vivaspin | 6010 | 5750 | 85.00 | 95.60 |
N-terminal amino acid sequence
analysis
The signal peptide and propeptide of HSA, which
consists of a signal sequence of 24 amino acids
(MKWVTFISLLFLFSSAYSRGVFRR), can be cleaved at the site after amino
acid residues SR or RR. The N-terminal sequencing of the purified
protein revealed the sequence of the first 15 amino acids as
GVFRRYGRKKRRQRR, indicating that the signal peptide was cleaved at
the site after amino acid residue SR.
Detection of the Tat-Oct4 transmembrane
transport ability
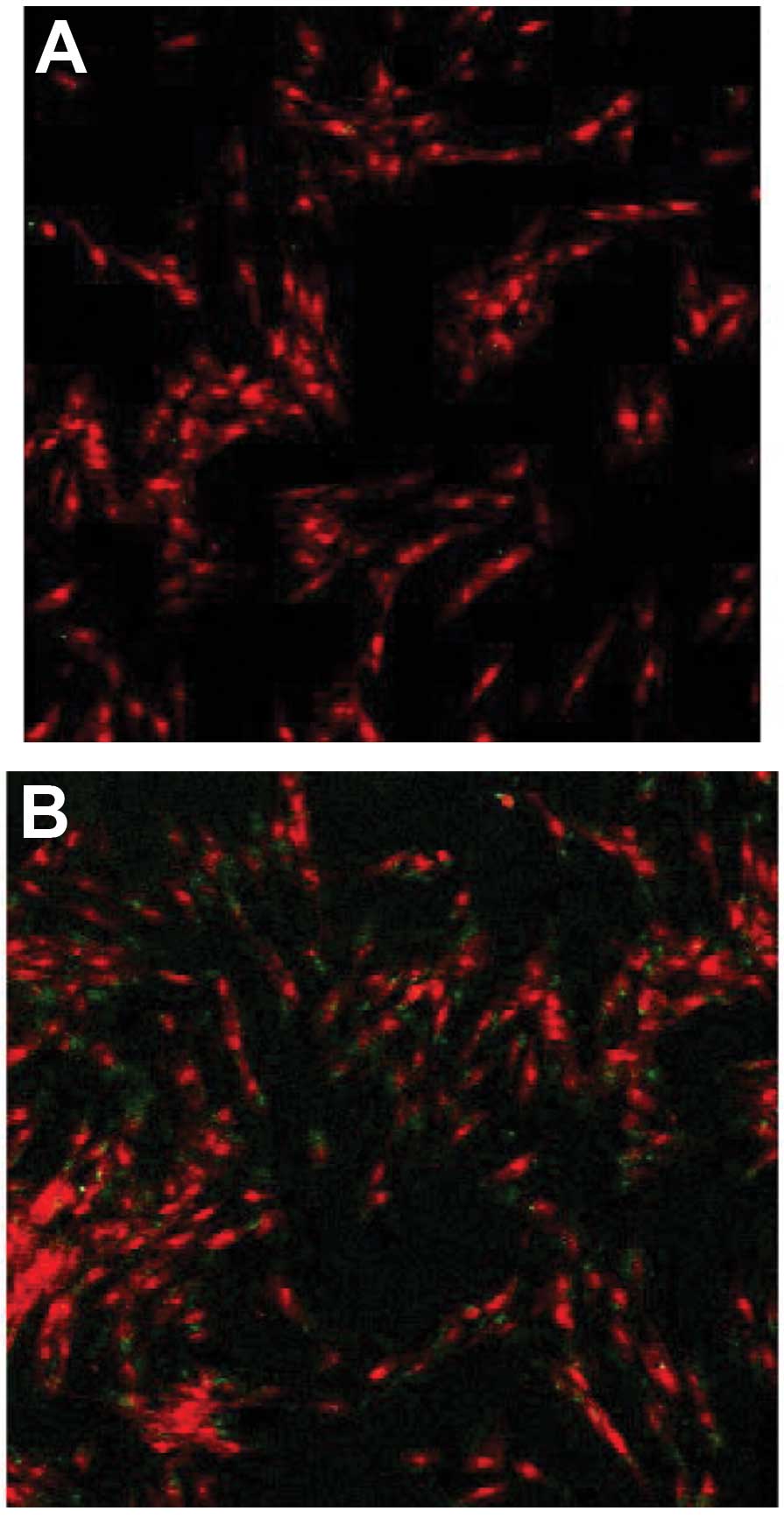
The transmembrane transport ability of Tat-Oct4 was
analyzed by indirect immunofluorescence. The results demonstrated
that visible green fluorescence signals appeared in the human
foreskin fibroblasts treated with serum-free medium containing
Tat-Oct4. The green fluorescence signal was absent in the cells
treated with saline (the negative control group), indicating that
Tat47–57-Oct4 has the ability to pass through the cell
membrane (Fig. 5).
Kim et al expressed Tat-SOD in E.coli
and tested the protectve effect against ischemic brain injury. They
observed that Tat-SOD was able to enter brain neurons and protect
them from ischemic insult and cell death (24). Liu et al used EPO and
EPO-Tat to treat a rat model of transient focal ischemia and
demonstrated that 1,000 U/kg EPO-TAT exhibited a comparable
neuroprotection to 5,000 U/kg EPO with no detectable side effects
(25). Furthermore Zhou et
al used E.coli. to produce human Oct4–11R-His fusion
protein and observed the membrane penetrating ability of the fusion
protein. They showed that the fusion protein was able to
effectively enter the BJ cells and locate around the nuclei, but
the authors did not investigate the bioactivity of the fusion
protein (26). In the present
study, the Tat47–58-Oct4 fusion protein was successfully expressed
using the P. pastoris expression system and purified
following pilot-scale fermentation by precipitation,
ultrafiltration and chromatography. The yield and purity of Tat47
57-Oct4 were 210 mg/l and 95.6%, respectively, and the fusion
protein revealed the ability to penetrate human foreskin
fibroblasts. Therefore, the yeast expression system described in
the present study is a useful tool to produce a large quantity of
active Tat47 57-Oct4 fusion protein, which is likely to facilitate
the mechanistic and potential clinical application studies of Oct4.
In the next step we will investigate the bioactivity of the Tat47
57-Oct4 fusion protein in induced pluripotent stem (iPS) cells.
Acknowledgements
This study was supported by the Jilin Kangrui
Regenerative Medicine Co., Ltd. Changchun, P.R. China.
References
|
1
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vierbuchen T, Ostermeier A, Pang ZP,
Kokubu Y, Südhof TC and Wernig M: Direct conversion of fibroblasts
to functional neurons by defined factors. Nature. 463:1035–1041.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang B, Dong H, Li Q, Yu Y and Zhang Z,
Zhang Y, Wang G and Zhang Z: Differentiation of reprogrammed mouse
cardiac fibroblasts into functional cardiomyocytes. Cell Biochem
Biophys. 66:309–318. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosner MH, Vigano MA, Ozato K, Timmons PM,
Poirier F, Rigby PW and Staudt LM: A POU-domain transcription
factor in early stem cells and germ cells of the mammalian embryo.
Nature. 345:686–692. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Niwa H, Miyazaki J and Smith AG:
Quantitative expression of Oct-3/4 defines differentiation,
dedifferentiation or self-renewal of ES cells. Nat Genet.
24:372–376. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marión RM, Strati K, Li H, Murga M, Blanco
R, Ortega S, Fernandez-Capetillo O, Serrano M and Blasco MA: A
p53-mediated DNA damage response limits reprogramming to ensure iPS
cell genomic integrity. Nature. 460:1149–1153. 2009.PubMed/NCBI
|
|
7
|
Kim JB, Sebastiano V, Wu G, Araúzo-Bravo
MJ, Sasse P, Gentile L, Ko K, Ruau D, Ehrich M, van den Boom D, et
al: Oct4-induced pluripotency in adult neural stem cells. Cell.
136:411–419. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lam CS, Mistri TK, Foo YH, Sudhaharan T,
Gan HT, Rodda D, Lim LH, Chou C, Robson P, Wohland T and Ahmed S:
DNA-dependent Oct4-Sox2 interaction and diffusion properties
characteristic of the pluripotent cell state revealed by
fluorescence spectroscopy. Biochem J. 448:21–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moy P, Daikh Y, Pepinsky B, Thomas D,
Fawell S and Barsoum J: Tat-mediated protein delivery can
facilitate MHC class I presentation of antigens. Mol Biotechnol.
6:105–113. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagahara H, Vocero-Akbani AM, Snyder EL,
Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA and Dowdy
SF: Transduction of full-length TAT fusion proteins into mammalian
cells: TAT-p27Kip1 induces cell migration. Nat Med. 4:1449–1452.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwarze SR, Ho A, Vocero-Akbani A and
Dowdy SF: In vivo protein transduction: delivery of a biologically
active protein into the mouse. Science. 285:1569–1572. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang Z, Ji M, Peng Z, Huang S, Xiao Q, Li
C, Zeng J, Gao M and Feng W: Purification of TAT-CC-HA protein
under native condition, and its transduction analysis and
biological effects on BCR-ABL positive cells. Biomed Pharmacother.
65:183–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JS, Park SY, Cho HI, Sohn HJ and Kim
TG: Enhanced induction of T cell immunity using dendritic cells
pulsed with HIV Tat and HCMV-pp65 fusion protein in vitro. Immune
Netw. 11:182–189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao B, Wang Y, Zhang Y, Li Y, Zhang X, Xu
Y, Chen L, Li C, Ju Y and Meng S: TAT-mediated gp96 transduction to
APCs enhances gp96-induced antiviral and antitumor T cell
responses. Vaccine. 31:545–552. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin
T, Trauger S, Bien G, Yao S, Zhu Y, et al: Generation of induced
pluripotent stem cells using recombinant proteins. Cell Stem Cell.
4:381–384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim D, Kim CH, Moon JI, Chung YG, Chang
MY, Han BS, Ko S, Yang E, Cha KY, Lanza R and Kim KS: Generation of
human induced pluripotent stem cells by direct delivery of
reprogramming proteins. Cell Stem Cell. 4:472–476. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sen Gupta C and Dighe RR: Hyperexpression
of biologically active human chorionic gonadotropin using the
methylotropic yeast, Pichia pastoris. J Mol Endocrinol.
22:273–283. 1999.
|
|
18
|
Damaso MC, Almeida MS, Kurtenbach E,
Martins OB, Pereira N Jr, Andrade CM and Albano RM: Optimized
expression of a thermostable xylanase from Thermomyces
lanuginosus in Pichia pastoris. Appl Environ Microbiol.
69:6064–6072. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murasugi A, Kido I, Kumai H and Asami Y:
Efficient production of recombinant human pleiotrophin in yeast,
Pichia pastoris. Biosci Biotechnol Biochem. 67:2288–2290.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laborde C, Chemardin P, Bigey F,
Combarnous Y, Moulin G and Boze H: Overexpression of ovine leptin
in Pichia pastoris: physiological yeast response to leptin
production and characterization of the recombinant hormone. Yeast.
21:249–263. 2004.PubMed/NCBI
|
|
21
|
Sreekrishna K, Brankamp RG, Kropp KE,
Blankenship DT, Tsay JT, Smith PL, Wierschke JD, Subramaniam A and
Birkenberger LA: Strategies for optimal synthesis and secretion of
heterologous proteins in the methylotrophic yeast Pichia
pastoris. Gene. 190:55–62. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sambrook J and Russell DW:
SDS-polyacrylamide gel electrophoresis of proteins. CSH Protoc.
2006. View Article : Google Scholar
|
|
24
|
Kim DW, Eum WS, Jang SH, Kim SY, Choi HS,
Choi SH, An JJ, Lee SH, Lee KS, Han K, Kang TC, Won MH, Kang JH,
Kwon OS, Cho SW, Kim TY, Park J and Choi SY: Transduced Tat-SOD
fusion protein protects against ischemic brain injury. Mol Cells.
19:88–96. 2005.PubMed/NCBI
|
|
25
|
Liu P, Liu X, Akf Liou EY, Xing J, Jing Z,
Ji X, Liu X, Zhao H, Yan F, Chen J, Cao G and Luo Y: The
Neuroprotective Mechanism of Erythropoietin-TAT Fusion Protein
Against Neurodegeneration from Ischemic Brain Injury. CNS Neurol
Disord Drug Targets. Aug 27–2013.(Epub ahead of print).
|
|
26
|
Chengliang Zhou, Fengqing Xu, Chunhong
Wang, Tao Liu, Xinrong Peng and Qijun Qian: Expression of human
Oct4 and cell penetrating peptide fusion protein. Academic Journal
of Second Military Medical University. 31:489–493. 2010.(In
Chinese).
|