Introduction
A well-known hazard to human health is air
pollution. One significant environmental pollutant is ambient
airborne particular matter (PM), which has been linked to several
types of cancer and cardiopulmonary diseases (1). Over the last few decades, several
studies have shown that a significant parameter in determining the
potential to cause inflammatory injury, oxidative damage and other
biological effects is the size and surface area of PM (2). Fine particles (diameter <2.5 μm;
termed as PM2.5) had stronger effects due to their
ability to enter deeper into the airways of the respiratory tract.
As a consequence, PM2.5 can reach the alveoli whereby
50% are retained in the lung parenchyma (3). In recent years, the hazardous effects
of PM2.5 have captured increasingly more public
attention. However, whether there are alternative methods to
protect us from the effects of PM2.5, whether its
discharge into the atmosphere can be reduced and if certain herbal
additive intake can actively defend the body against the damaging
effects of PM2.5 remain to be elucidated.
Panax ginseng has been safely used in China
for >2000 years. In Asia, Panax ginseng is a general
tonic and an adaptogen to maintain the body’s resistance to adverse
factors and homeostasis, including enhanced physical functions,
general vitality, anti-stress and anti-aging (4,5).
Ginsenoside Rg1, a steroidal saponin abundantly contained in
Panax ginseng, is one of its most active components and
contributes to a number of its effects (6). A previous study reveals that Rg1 has
protective effects on glutamate-induced lung injury (7). Based upon this literature, we
hypothesize that Rg1 may offset the ill effects of PM2.5
on human A549 lung epithelial cells. To date, there are limited
studies on the protective effects of Rg1 on an organism exposed to
PM2.5 in vivo or in vitro. Only a recent
study shows that Rg1 reduces the toxicity of PM2.5 on
human umbilical vein endothelial cells by upregulating the
intracellular antioxidative state (8). However, whether Rg1 has similar
protective effects on lung cells remains unclear considering the
lung is a major target organ attacked by PM2.5 (9). This preliminary study was designed to
examine the toxic effects of PM2.5 and the protective
effects of Rg1 on the A549 cells.
Materials and methods
Reagents
The following reagents were used: Dulbecco’s
modified Eagle’s medium (DMEM), fetal bovine serum (FBS; Gibco-BRL,
New York, NY, USA), Ginsenoside Rg1 with purity >98% was
purchased from Shanghai Winwerb Medical Science Co., Ltd) and
dissolved in double distilled water and dimethyl sulfoxide (DMSO)
and penicillin-streptomycin (both from Invitrogen, Carlsbad, CA,
USA). An assay kit of MDA was purchased from Nanjing Jiancheng
Biological Engineering Co., Ltd. (Nanjing, China).
Cell culture
The A549 human alveolar type II epithelial cells
were obtained from the Cell Bank of Peking Union Medical College
(Beijing, China) and were maintained in low-glucose DMEM
supplemented with 10% heated-inactivated FBS (Hyclone, Atlanta, GA,
USA), 100 U/ml penicillin and 100 μg/ml streptomycin in a
humidified atmosphere of 5% CO2 and 95% air at 37°C. The
cells were serum-starved for 24 h prior to being treated with the
PM2.5. The Rg1 were added to the cells 1 h prior to the
PM2.5 treatment.
PM2.5 sampling and
preparation
Urban atmospheric PM2.5 was kindly
provided by Professor Xiaohong Zhao from the College of Arts and
Sciences of Beijing Union University (Beijing, China).
PM2.5 was collected on 150-mm diameter nitrocellulose
filters (HAWP, Sartorius, La Ferté-sous-Jouarre, France) with a
high volume sampler machine (DA-80 Digitel, Cugy, Switzerland;
flowrate, 30 m3/h) on the roof of a five story building
on Xueyuan Road (Beijing, China) which was taller than surrounding
buildings. The particles were processed as described previously
(10). Briefly, the
PM2.5 samples were extracted from sampled filter strips
by immersing them in deionized water and then sonicating them for
30 min in a water bath sonicator (KQ-700 V and 700 W; Asha
Analytical Instruments Pvt. Ltd., Secunderabad, India). The
PM2.5 samples were then stored at −80°C until further
use.
Cell viability
A
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay was used to measure cell viability. Following treatment with
PM2.5 or Rg1, medium was discharged and the cells were
rinsed with PBS and the MTT (final concentration, 0.5 mg/ml) was
added for 4 h (11). The medium
was removed and the MTT reduction product dissolved in 1 ml DMSO.
The absorbance of each sample was assessed by Multiskan Ascent
(Thermo Scientific Inc., Waltham, MA, USA) at 565 nm. The test was
replicated three times and the cell viability was calculated as
follows: % cell viability = [(ODexperiment −
ODblank)/(ODcontrol − ODblank)] ×
100.
Cell toxicity
The cytotoxicity of the PM2.5 was
measured by the lactate dehydrogenase (LDH) activity in the cell
medium using ELISA. The absorbance was read using an ELISA reader
(Bio-Tek, Colmar, France) at 450 nm. An increase in the number of
dead or cell membrane-damaged cells increases the LDH activity in
the cell culture supernatant. In these experiments, the A549 cells
were exposed to PM2.5 for 24 h after pretreatment of Rg1
for 1 h. This incubation period is required to obtain reliable
measurements of the cytotoxicity.
Measurement of MDA
MDA was quantitated by spectrophotometry. Following
removal of the media, the membranes were solubilized in 400 μl of
8% SDS, 25 μl of 4% butylated hydroxy toluene in ethanol was added
and 500 μl of 10% phosphotungstic acid in 0.5 M sulfuric acid in a
serial manner. Following addition of 250 μl of 0.7% thiobarbituric
acid, the tubes were placed in a boiling bath for 50 min. Next, 1
ml of 1-butanol was added, the tubes were centrifuged and the
supernatant containing thiobarbituric acid reactants was collected
to measure the absorbance at 532 nm. Thiobarbituric acid reactants
were quantitated using a standard curve prepared with a 1 mM
solution of tetrahydroxypropane hydrolyzed in 1% sulfuric acid.
Statistical analysis
Statistical analysis of the data was performed using
one-way analysis of variance with the Newman-Keuls multiple
comparison post-hoc tests by using SPSS 13.0 software (SPSS, Inc.,
Chicago, IL, USA). All the data are expressed as the mean ±
standard deviation. P<0.05 was used to indicate a statistically
significant difference.
Results
Effect of PM2.5 on viability
of A549 cells
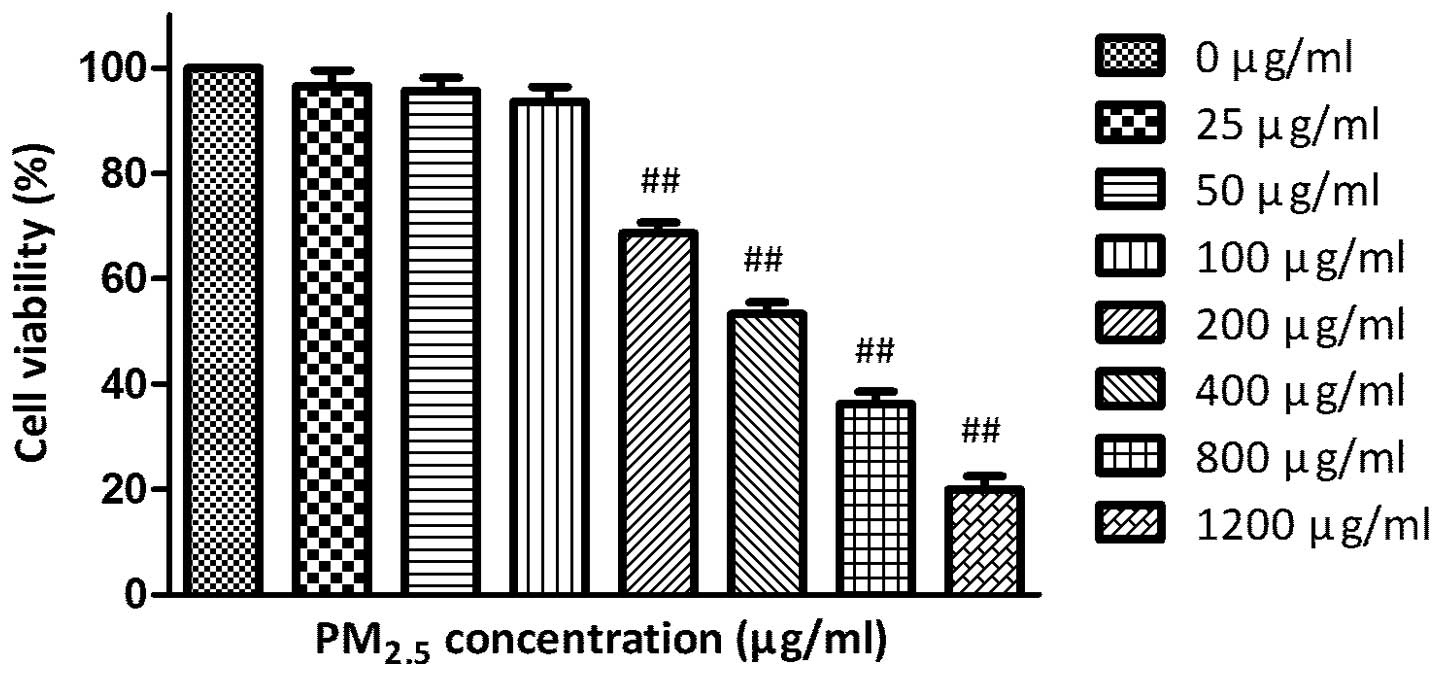
The MTT assay demonstrated that incubation with
0–1,200 μg/ml PM2.5 for 24 h decreased the A549 cells
viability in a concentration-dependent manner (Fig. 1).
Effect of Rg1 on viability of A549
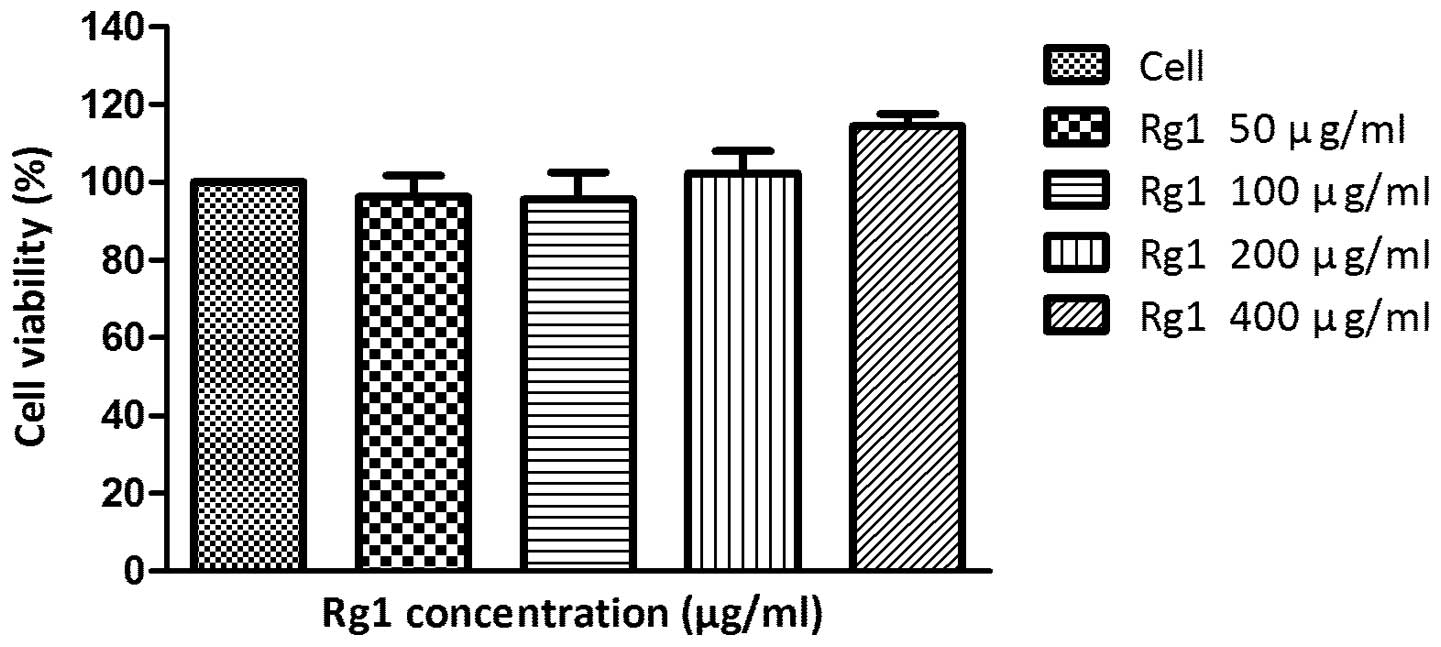
cells
The MTT assay revealed that treatments with 50–800
μg/ml Rg1 for 24 h did not change the A549 cells viability
significantly (Fig. 2).
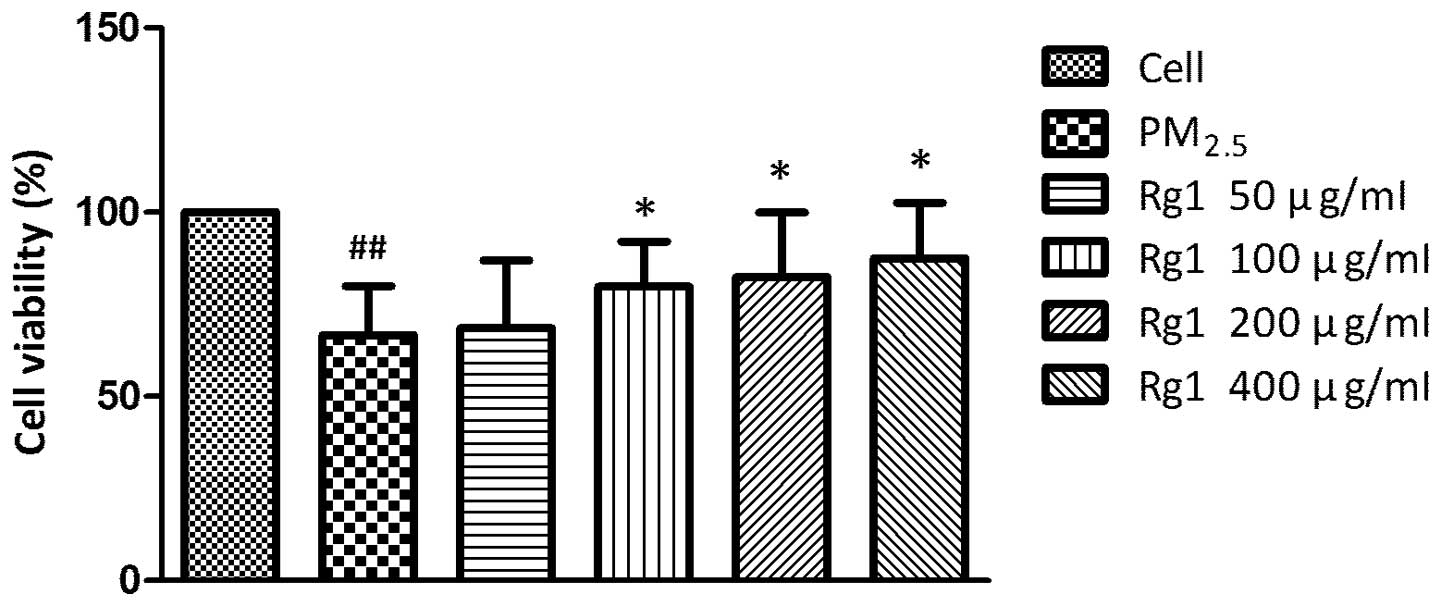
Effect of Rg1 on viability of A549 cells
exposed to 200 μg/ml PM2.5
The MTT assay revealed that pretreatments with
50–400 μg/ml Rg1 for 1 h increased the viability of A549 cells
exposed to 200 μg/ml PM2.5 in a dose-dependent manner
(Fig. 3).
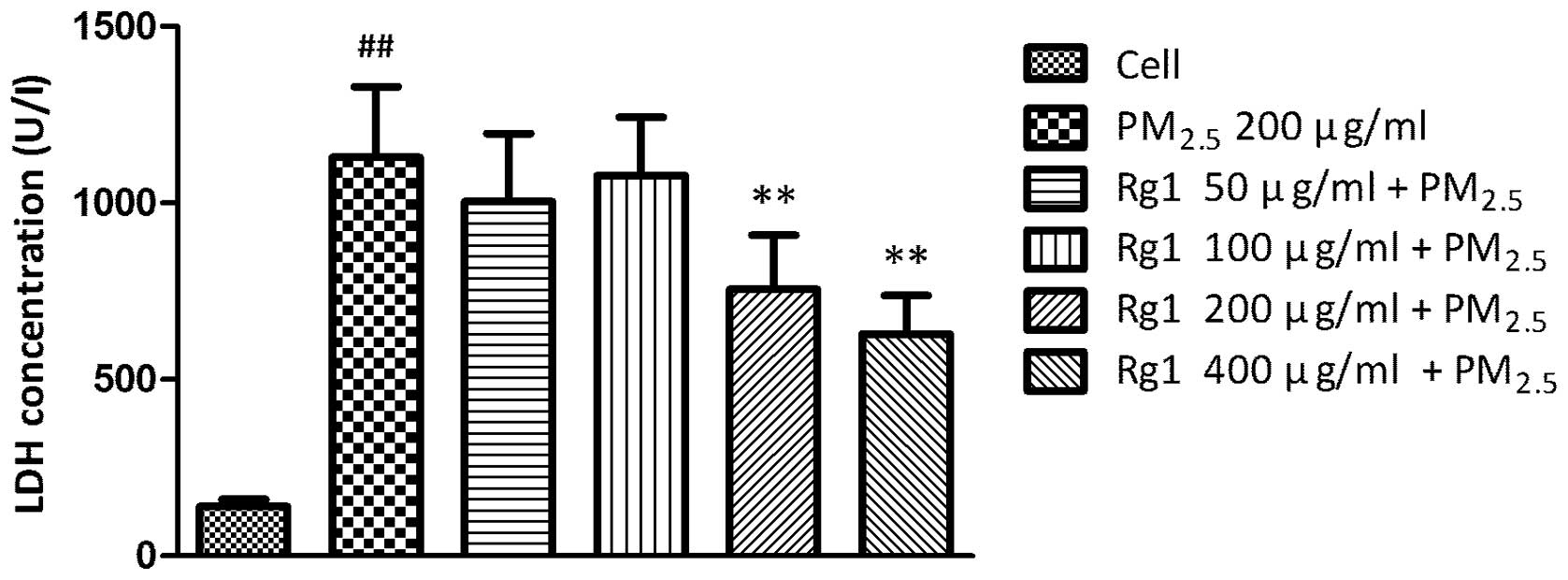
Effect of Rg1 on LDH concentration in
culture supernatants of A549 cells exposed to 200 μg/ml
PM2.5
The level of LDH in cell-free culture supernatants
significantly increased in untreated A549 cells exposed to
PM2.5 (1129.944±199.428 U/l; P<0.01) compared with
untreated control cells (140.248±20.771 U/l). Co-culture with 200
and 400 μg/ml Rg1 decreased PM2.5-stimulated LDH leakage
to 755.858±153.423 U/l (P<0.01) and 629.738±108.065 U/l
(P<0.01), respectively (Fig.
4).
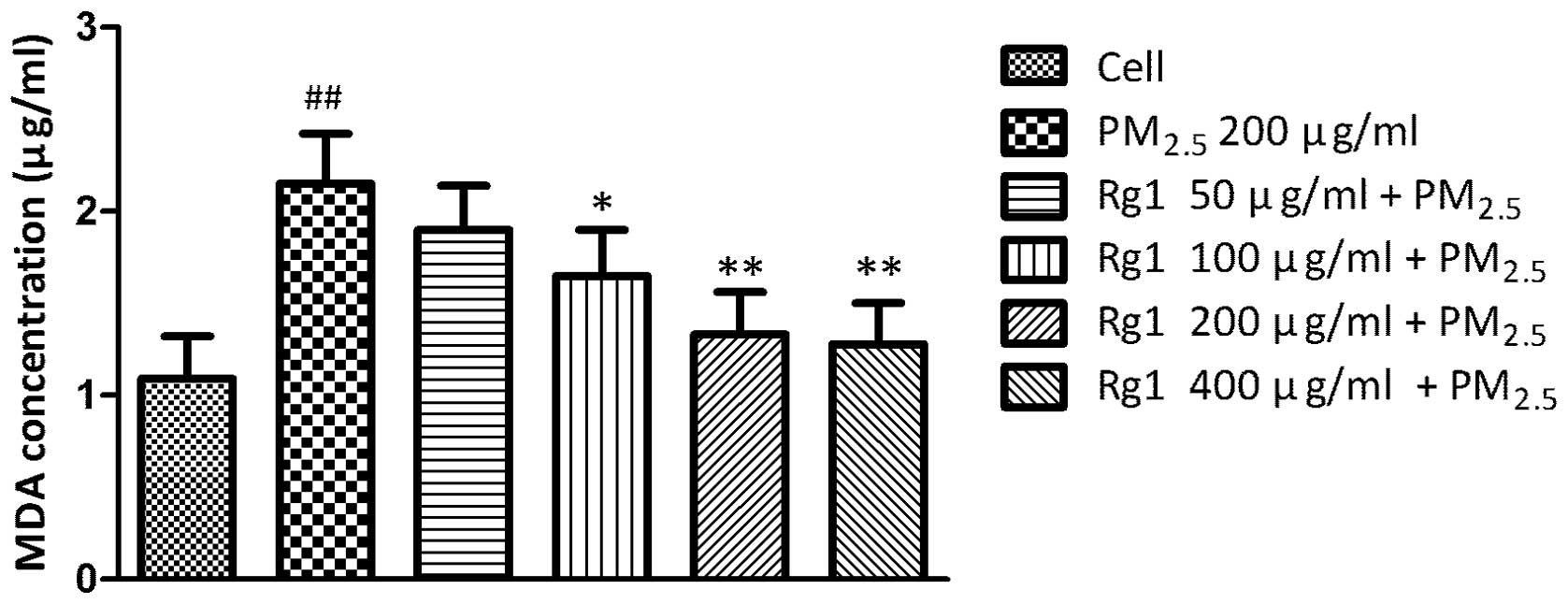
Effect of Rg1 on MDA concentration in
A549 cells exposed to 200 μg/ml PM2.5
The level of the oxidative stress based on the MDA
assay significantly increased in untreated A549 cells exposed to
PM2.5 (2.15±0.27 μg/ml; P<0.01) compared with
untreated control cells (1.09±0.23 μg/ml). Co-culture with 100, 200
and 400 μg/ml Rg1 decreased PM2.5-induced MDA production
to 1.65±0.25 μg/ml (P<0.05), 1.33±0.23 μg/ml (P<0.01) and
1.28±0.22 μg/ml (P<0.01), respectively (Fig. 5).
Discussion
In the present study, the 25–1,200 μg/ml of
PM2.5 decreased the A549 cells viability in a
dose-dependent manner, which was in accordance with previous
studies (12,13). Since 200 μg/ml was the minimum
effective dose of PM2.5, this dose was selected as the
exposure concentration in the following assays. Ginsenoside Rg1 was
a compound extracted from Panax ginseng and had been studied
extensively. The MTT assay demonstrated that 50–400 μg/ml Rg1 did
not alter the cell viability in A549 cells significantly. Although
there were no previous studies on the viability of A549 cells
co-incubated with Rg1, numerous studies indicated that Rg1 had no
marked cytotoxic effect on fibroblasts (14) or neuroblasts (15). Doses of 50–400 μg/ml were selected
as pretreatment concentrations of Rg1 in the following assays.
While the A549 cells were pretreated with Rg1 for 1 h at
concentrations of 50–400 μg/ml, followed by exposure to 200 μg/ml
PM2.5, Rg1 at three concentrations of 100, 200 and 400
μg/ml was observed to be capable of increasing the cells viability
significantly, which implied that Rg1 possessed a cytoprotective
effect in order to antagonize the lesion from PM2.5.
The measurement of LDH in the culture supernatant as
a result of leakage is an indicator of cell membrane integrity
(16). In the present study, 200
μg/ml PM2.5 increased the level of LDH in the A549 cell
culture supernatant compared with the control cells. However, 200
and 400 μg/ml Rg1 could decrease the LDH generation significantly
in a concentration-dependent manner. These results indicated that
Rg1 had inhibitory effects on the cytotoxicity induced by
PM2.5.
The cellular level of MDA is a sensitive marker for
oxidative damage, particularly lipid peroxidation and has been
widely used (17). In the present
study, the A549 cells exposed to PM2.5 produced more MDA
than the control cells indicating that PM2.5 could
induce more oxidative stress. This result was in accordance with a
previous study (18). Rg1 had been
proven to have an effect on antioxidative stress by inhibiting MDA
production (19,20). To the best of our knowledge, the
present study, for the first time, observed that 100, 200 and 400
μg/ml Rg1 could reduce the MDA level of the A549 cells exposed to
PM2.5 in a concentration-dependent manner. These results
indicated that Rg1 had the potential to protect the alveolar
epithelium from oxidative damage induced by PM2.5.
The present study preliminarily implies that
ginsenoside Rg1 is a promising candidate drug to antagonize the
harmful effects of PM2.5 on alveolar epithelium cells,
but the usefulness of Rg1 for this purpose requires further
investigation, in particular, clinical studies.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no.81102680) and the China
Postdoctoral Science Foundation (no. 20100470524 and
20110490548).
References
|
1
|
Valavanidis A, Fiotakis K and Vlachogianni
T: Airborne particulate matter and human health: toxicological
assessment and importance of size and composition of particles for
oxidative damage and carcinogenic mechanisms. J Environ Sci Health
C Environ Carcinog Ecotoxicol Rev. 26:339–362. 2008. View Article : Google Scholar
|
|
2
|
Sacks JD, Stanek LW, Luben TJ, et al:
Particulate matter-induced health effects: who is susceptible?
Environ Health Perspect. 119:446–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Polichetti G, Cocco S, Spinali A, Trimarco
V and Nunziata A: Effects of particulate matter (PM(10), PM(2.5)
and PM(1)) on the cardiovascular system. Toxicology. 261:1–8. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen CF, Chiou WF and Zhang JT: Comparison
of the pharmacological effects of Panax ginseng and Panax
quinquefolium. Acta Pharmacol Sin. 29:1103–1108. 2008.
View Article : Google Scholar
|
|
5
|
Xiang YZ, Shang HC, Gao XM and Zhang BL: A
comparison of the ancient use of ginseng in traditional Chinese
medicine with modern pharmacological experiments and clinical
trials. Phytother Res. 22:851–858. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu JM, Yao Q and Chen C: Ginseng
compounds: an update on their molecular mechanisms and medical
applications. Curr Vasc Pharmacol. 7:293–302. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shen L, Han JZ, Li C, et al: Protective
effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta
Pharmacol Sin. 28:392–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li CP, Qin G, Shi RZ, Zhang MS and Lv JY:
Ginsenoside Rg1 reduces toxicity of PM(2.5) on human umbilical vein
endothelial cells by upregulating intracellular antioxidative
state. Environ Toxicol Pharmacol. 35:21–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kouassi KS, Billet S, Garcon G, et al:
Oxidative damage induced in A549 cells by physically and chemically
characterized air particulate matter (PM2.5) collected in Abidjan,
Côte d’Ivoire. J Appl Toxicol. 30:310–320. 2010.PubMed/NCBI
|
|
10
|
Imrich A, Ning Y and Kobzik L: Insoluble
components of concentrated air particles mediate alveolar
macrophage responses in vitro. Toxicol Appl Pharmacol. 167:140–150.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
12
|
Akhtar US, McWhinney RD, Rastogi N, Abbatt
JP, Evans GJ and Scott JA: Cytotoxic and proinflammatory effects of
ambient and source-related particulate matter (PM) in relation to
the production of reactive oxygen species (ROS) and cytokine
adsorption by particles. Inhal Toxicol. 22(Suppl 2): 37–47.
2010.PubMed/NCBI
|
|
13
|
Gualtieri M, Mantecca P, Corvaja V, et al:
Winter fine particulate matter from Milan induces morphological and
functional alterations in human pulmonary epithelial cells (A549).
Toxicol Lett. 188:52–62. 2009. View Article : Google Scholar
|
|
14
|
Park S, Ahn IS, Kwon DY, Ko BS and Jun WK:
Ginsenosides Rb1 and Rg1 suppress triglyceride accumulation in
3T3-L1 adipocytes and enhance beta-cell insulin secretion and
viability in Min6 cells via PKA-dependent pathways. Biosci
Biotechnol Biochem. 72:2815–2823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Du X, Xu H, Jiang H and Xie J: Akt/Nrf2
activated upregulation of heme oxygenase-1 involves in the role of
Rg1 against ferrous iron-induced neurotoxicity in SK-N-SH cells.
Neurotox Res. 24:71–79. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fautz R, Husein B and Hechenberger C:
Application of the neutral red assay (NR assay) to monolayer
cultures of primary hepatocytes: rapid colorimetric viability
determination for the unscheduled DNA synthesis test (UDS). Mutat
Res. 253:173–179. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Zwart LL, Meerman JH, Commandeur JN and
Vermeulen NP: Biomarkers of free radical damage applications in
experimental animals and in humans. Free Radic Biol Med.
26:202–226. 1999.PubMed/NCBI
|
|
18
|
Aust AE, Ball JC, Hu AA, et al: Particle
characteristics responsible for effects on human lung epithelial
cells. Res Rep Health Eff Inst. 110:1–65; discussion 67–76.
2002.PubMed/NCBI
|
|
19
|
Deng HL and Zhang JT: Anti-lipid
peroxilative effect of ginsenoside Rb1 and Rg1. Chin Med J (Engl).
104:395–398. 1991.PubMed/NCBI
|
|
20
|
Yu SH, Huang HY, Korivi M, et al: Oral Rg1
supplementation strengthens antioxidant defense system against
exercise-induced oxidative stress in rat skeletal muscles. J Int
Soc Sports Nutr. 9:232012. View Article : Google Scholar
|