Introduction
The blood-brain barrier (BBB) is a diffusion barrier
essential for the normal function of the central nervous system
(CNS). The BBB has a key role in maintaining homeostasis within the
CNS, preserving the composition of the internal milieu, despite
variations in the periphery, and protecting the brain against
toxins, bacteria and viruses. Moreover, it regulates the uptake of
endogenous molecules and xenobiotics into the brain (1). Drug delivery to the CNS is one of the
major hurdles in the development of novel therapeutics for
neuropsychiatric disorders. In particular, there is a requirement
for transport across the BBB, which separates the circulating blood
from the CNS, for centrally acting drugs to achieve therapeutic
concentrations at their site of action. Of note, >98% of
potential CNS drugs are unable to cross the BBB to reach target
sites within the brain (2). The
BBB is the bottleneck in brain drug development and is the single
most important factor limiting future development of
neurotherapeutics (3).
P-glycoprotein is a plasma membrane glycoprotein
that is able to exclude a wide range of chemotherapeutic drugs and
other hydrophobic compounds from cells (4). It is also expressed at high levels in
non-cancerous tissues, such as the endothelial cells of BBB
capillaries in humans, as well as animals. P-glycoprotein may be
involved in the exclusion of various drugs from the capillary
endothelial cells, blocking their entry into the brain (5). Verapamil is the most extensively
characterized P-gp inhibitor and multidrug resistance-associated
protein (MDR) reversal agent (6).
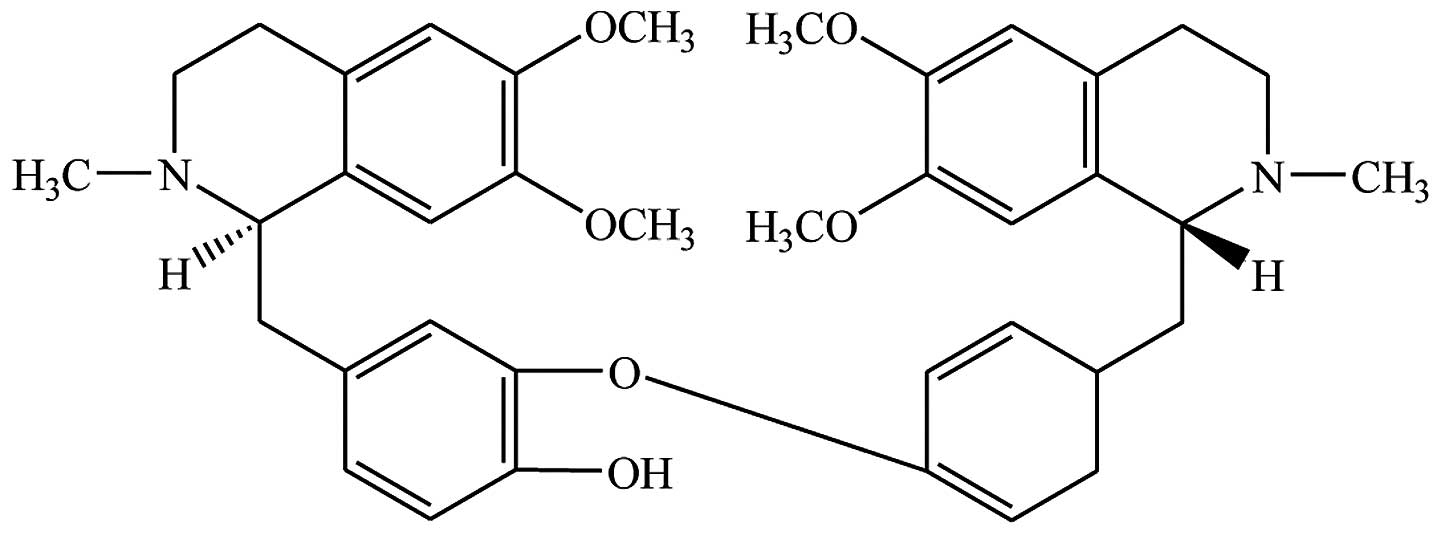
Dauricine (Fig. 1)
is a bisbenzylisoquinoline alkaloid isolated from the root of
Menispermum dauricum D.C. Dauricine has been suggested for
the treatment of various diseases, including cardiac ischemia,
angina and inflammation (7,8).
Previous studies have demonstrated that dauricine inhibits
angiogenesis in tumors (9) and
promotes apoptosis in tumor cells (10). A study by Li and Gong (11) suggested that dauricine exhibits
neuroprotective action in rats subjected to transient focal
cerebral ischemia by attenuating apoptosis in the ischemic penumbra
(12). However, it has yet to be
elucidated whether dauricine is capable of crossing the BBB. The
main purpose of the present study was thus to investigate whether
dauricine is able to cross the BBB by assessing dauricine levels in
the plasma and brain tissue of rats.
Materials and methods
Drugs and chemicals
Verapamil, a P-glycoprotein inhibitor, was obtained
from Shanghai Hefeng Pharmaceutical Co., Ltd. (Shanghai, China).
Tetrandrine was obtained from the National Institute for the
Control of Pharmaceutical and Biological Products. AB-8
cross-linked polystyrene and silica gel (SiO2) were
obtained from Nankai University (Professor Li, Ministry of
Education, Heilongjiang Key Laboratory of TCM Pharmacodynamic
Material Bases, Tianjin, China). Acetonitrile and methanol
[high-performance liquid chromatography (HPLC) grade] were obtained
from Merck KGaA (Darmstadt, Germany). Triethylamine, phosphoric
acid, and dichloromethane (analytical grade) were purchased from
Tianjin Bodi Chemical Co., Ltd. (Tianjin, China).
Dauricine preparation
The dried roots (5 kg) of Menispermum
dauricum D.C were extracted with 70% EtOH under reflux (2× 50
liters) for 2.5 h. The combined solution was filtered and
concentrated under vacuum to give a syrup, followed by resuspension
in distilled water. The suspension was passed through AB-8
cross-linked polystyrene, and sequentially eluted with distilled
water and 50% EtOH. The extraction eluted with 50% EtOH was
concentrated under vacuum to yield a syrup (56.0 g). This crude
residue was subjected to column chromatography (CC) on
SiO2 with a mobile phase of CHCl3/MeOH
(10:1→5:1), which gained five fractions (Fr. 1–5). Fr. 3–4 (10.4 g)
were subjected to CC on SiO2 (CHCl3/MeOH,
8→1; then octadecylsilyl (ODS), MeOH/H2O, 1:1) to yield
three substances. Each substance was purified by preparative HPLC
on a Hypersil-ODS II column (10 μm, 20×300 mm, flow rate 8 ml/min;
DIMKA Co., Beijing, China), with MeOH/H2O (70:30) as the
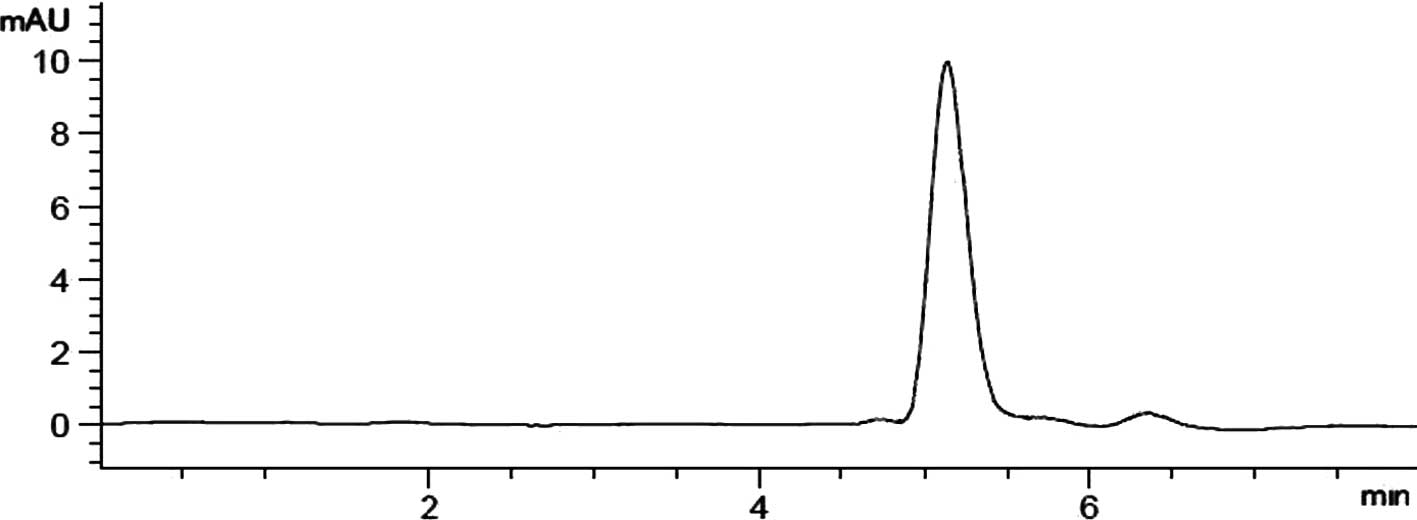
eluent, with a retention time of tR=15.0 min. Fig. 2 shows the typical HPLC chromatogram
of dauricine with a purity >96%.
Animals and treatment
Forty-eight male Sprague Dawley rats weighing 220±20
g were provided by the Experimental Animal Center of Heilongjiang
University of Chinese Medicine (Harbin, China). The certificate
number was 2008004. All experimental procedures conducted in this
study were performed in accordance with the guidelines for the Care
and Use of Laboratory Animals of Heilongjiang University of Chinese
Medicine. The rats were kept with free access to food and water and
a 12 h light/dark cycle. They were housed in plastic cages and
randomly divided into two groups: The control and verapamil groups,
containing 24 animals each. The rats in the verapamil group were
administered intraperitoneally (i.p.) with verapamil at a dose of
20 mg/kg. The rats in the control group were treated with an equal
volume of normal saline. After 90 min, all rats were injected with
dauricine (15 mg/kg) through the tail vein. At 15, 30 and 60 min
after dauricine treatment, the animals were anesthetized with
chloral hydrate (300 mg/kg, i.p.) and then 5 ml heparinized blood
[add 100 μl of 1% heparin (Tianjin Biochem Pharmaceutical Company,
Tianjin, China ) into the blood] was collected from the abdominal
aorta. The blood samples were centrifuged (3,500 × g, 4ºC) for 10
min and the plasma was separated. A 600-μl aliquot of plasma was
added to 1,200 μl methanol. Subsequent to vortexing, 3 ml
dichloromethane was added, and the mixture was vortexed for 3 min
and centrifuged at 3,500 × g for 10 min. The organic layer was
removed and evaporated to dryness at 40ºC under a stream of
nitrogen. The residue was dissolved in 600 μl mobile phase, and 10
μl supernatant liquid was injected into the HPLC system. The rats
were perfused with 100 ml ice-cold normal saline each. The brain
was instantly removed from the cranium and weighed. The brain was
then homogenized in four aliquots of 0.1 mol/l ice-cold phosphate
buffer (pH 7.4). Dichloromethane (3 ml) was added to 600 μl
homogenate. Following vortexing for 3 min and centrifuging (3,500 ×
g, 4ºC) for 10 min, the supernatants were evaporated to dryness
under a stream of nitrogen at 40ºC. The residue was analyzed
according to the method outlined in the proceeding section.
Chromatographic conditions
The chromatographic separation was performed using
an Agilent 1260 Series HPLC system (Agilent Technologies, Santa
Clara, CA, USA). The HPLC system consisted of a quaternary pump, an
ultraviolet detector operated at 281 nm and a Diamonsil C18 column
(250×4.6 mm, 5 μm) protected with a guard column packed with the
same material. The column was kept at room temperature. The mobile
phase was acetonitrile-0.4% phosphate buffer (20:80, v/v). The pump
was operated at a flow rate of 1 ml/min.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The statistical significances of the data were determined using
one-way analysis of variance followed by Least Significant
Difference testing. P<0.05 was considered to indicate a
statistically significant difference.
Results
HPLC of dauricine
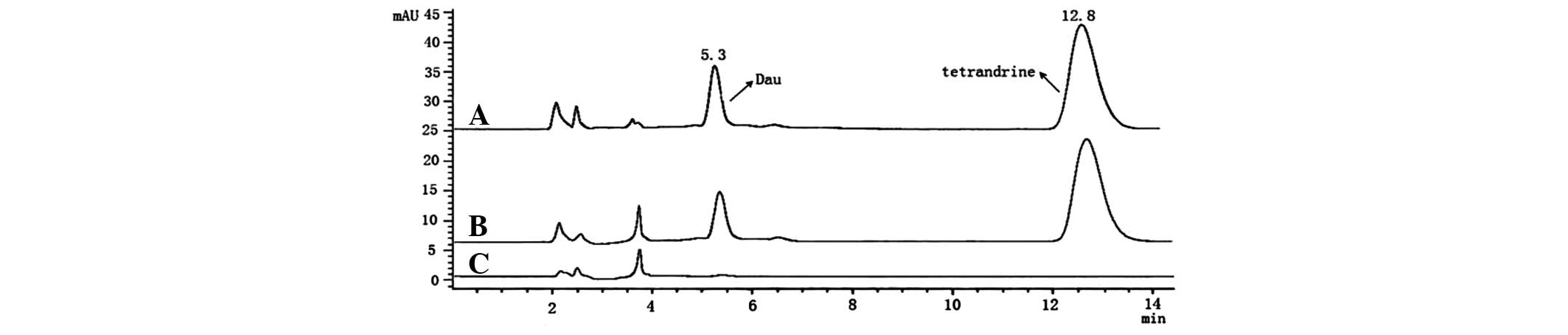
Fig. 3 shows
typical HPLC chromatograms of the brains of untreated rats, brain
spiked with dauricine (standard) and tetrandrine (internal
standard, No. 110711; National Institutes for Food and Drug
Control, Beijing, China), and brain of rats treated with dauricine
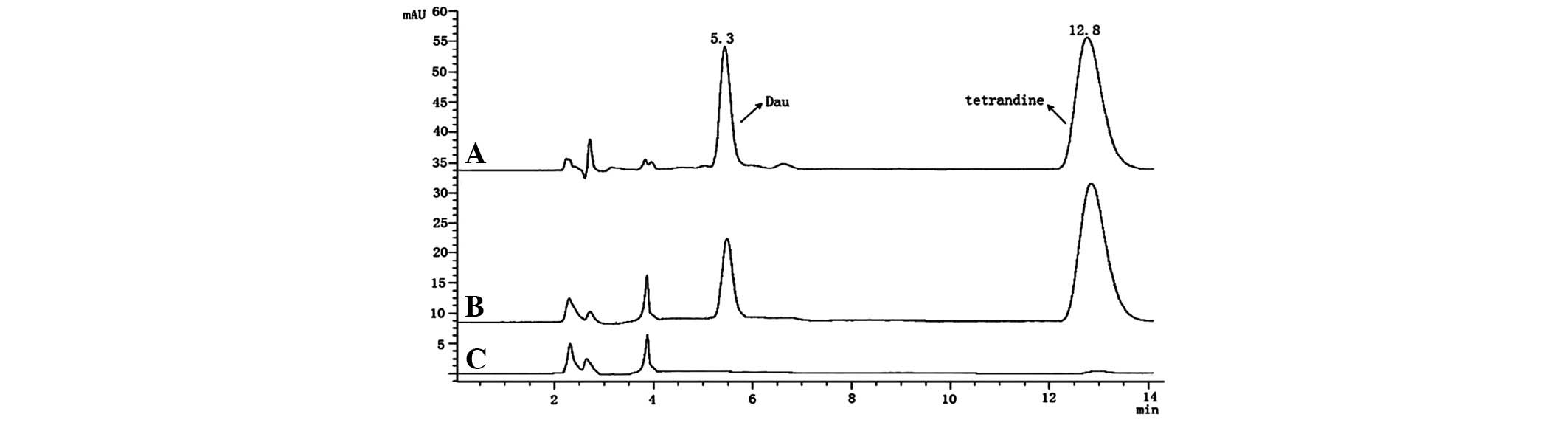
and spiked with tetrandrine (internal standard). Fig. 4 shows typical HPLC chromatograms of
plasma from untreated rats, plasma spiked with dauricine and
tetrandrine, and plasma of rats treated with dauricine and spiked
with tetrandrine. The retention times of dauricine and tetrandrine
were 5.3 and 12.8 min, respectively, where tetrandrine was the
internal standard.
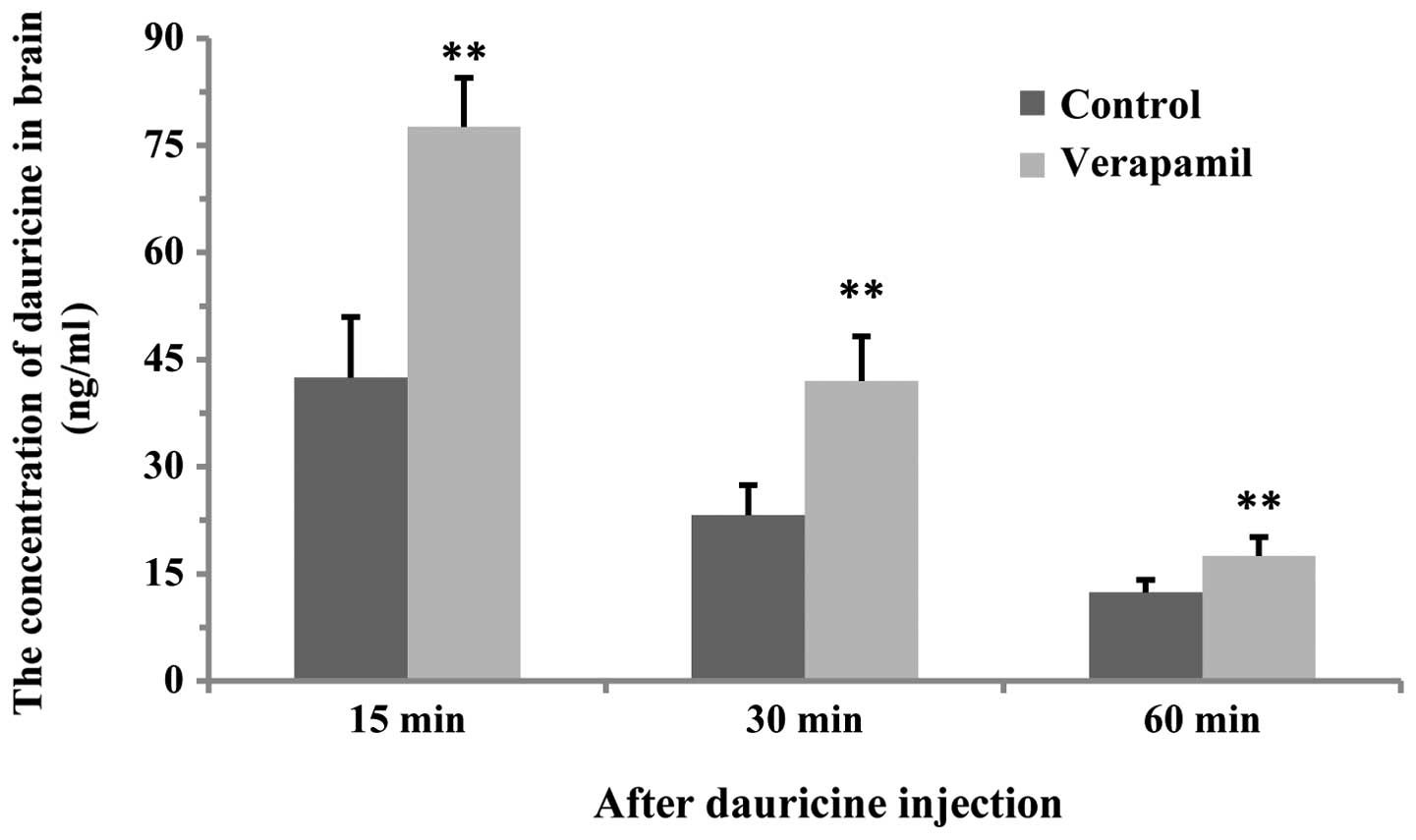
Effect of verapamil on the dauricine
concentration in the brain
At 15, 30 and 60 min after dauricine treatment,
dauricine levels in the brain of the verapamil group was
significantly increased compared with those of the control group
(P<0.01, Fig. 5).
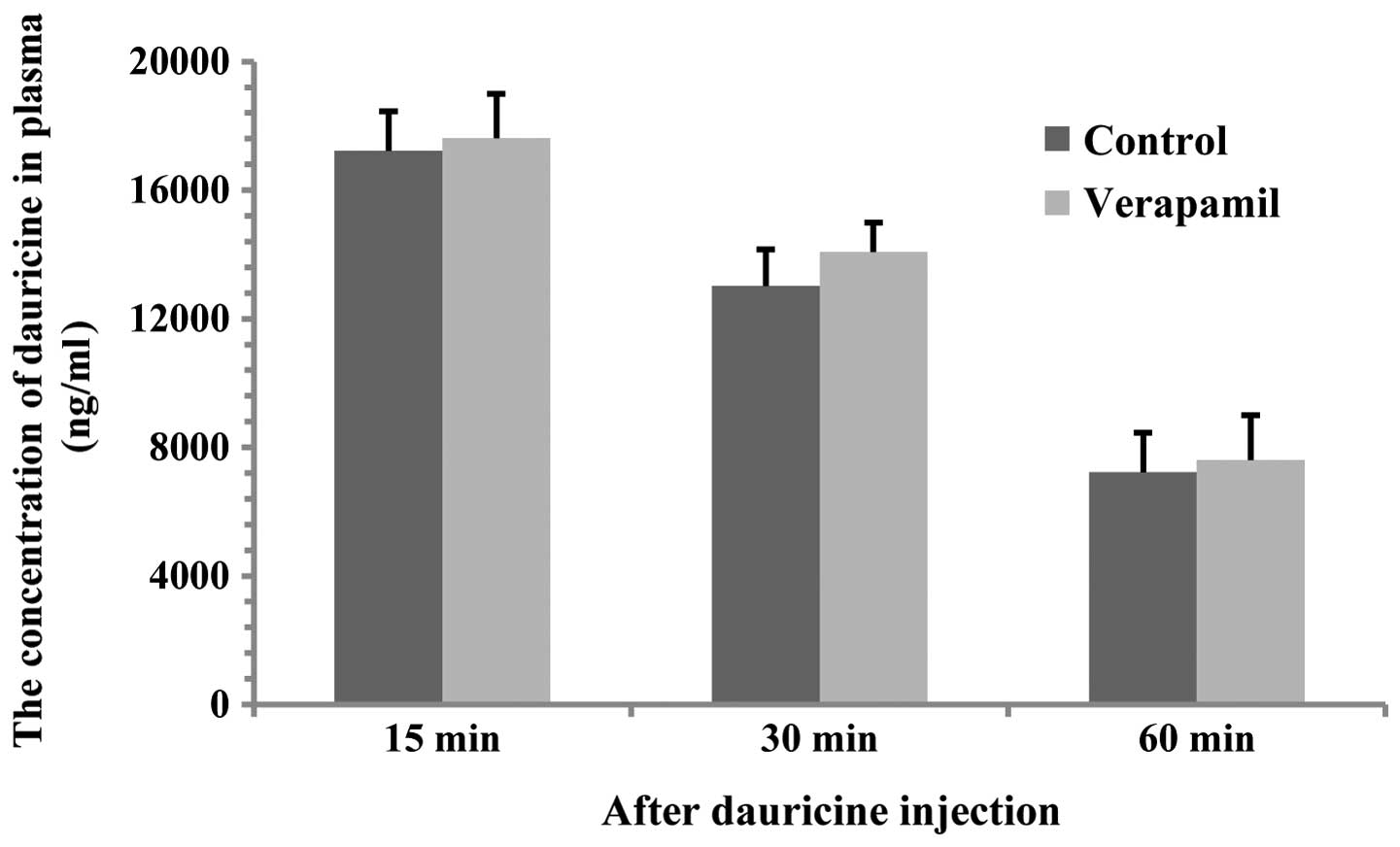
Effect of verapamil on dauricine levels
in plasma
Compared with the control, pretreatment with
verapamil exerted no significant effect on dauricine levels in
plasma (Fig. 6).
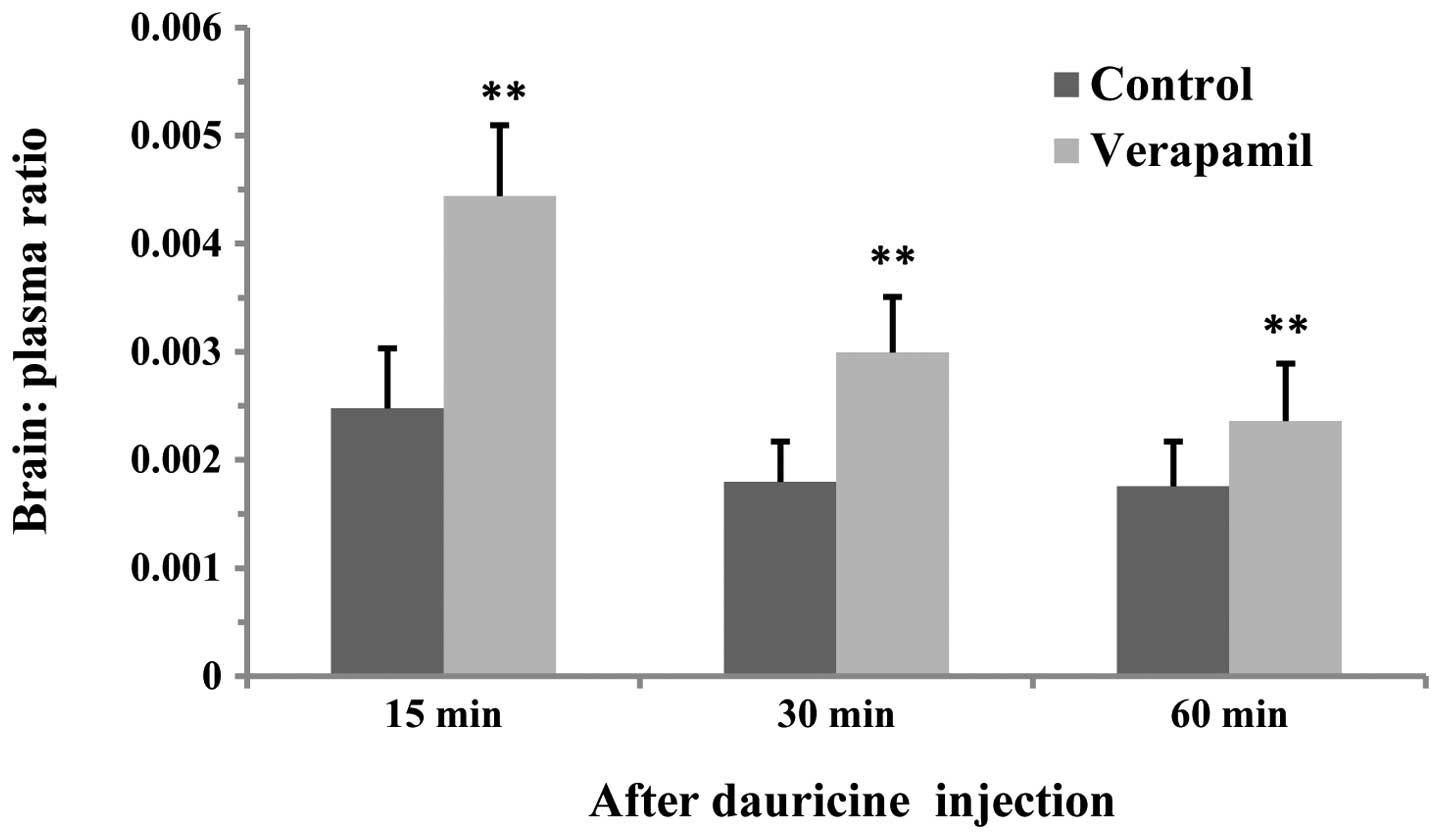
Effect of verapamil on the dauricine
brain:plasma ratio
At 15, 30 and 60 min after dauricine treatment, the
dauricine brain:plasma ratio in the verapamil group was
significantly wincreased compared with that of the control group
(P<0.01, Fig. 7).
Discussion
The BBB, which is situated between the blood and the
brain tissue, regulates the entry of drugs into the brain. The
therapeutic effect or the toxicity of drugs to the CNS is dependent
on the integrity of the BBB. The barrier function of the BBB
originates from two major aspects: Firstly, the physiological
structure, comprising brain microvessel endothelial cells,
astrocytes and tight junctions between the endothelial cells, is
the basis of the barrier function (12). Secondly, various types of transport
proteins (13) at the BBB mediate
the adsorption, excretion, distribution and elimination of
drugs.
The results of this study demonstrated that at 15
min after dauricine administration, dauricine concentration in the
brain reached a high level in the control and verapamil groups,
which indicated that dauricine is able to cross the BBB.
Furthermore, dauricine levels in the brain of the verapamil group
were significantly higher than those in the control; however,
verapamil did not affect dauricine levels in plasma, which
suggested that the effect of verapamil on dauricine levels in the
brain did not depend on the interference with the elimination of
dauricine from the blood. In turn, it may be deduced that
P-glycoprotein has an important role in the elimination of
dauricine from the brain, as verapamil, an inhibitor of
P-glycoprotein, was capable of increasing the levels of dauricine
in the brain.
This study only evaluated the influence of
P-glycoprotein on the levels of dauricine, and the effect of
dauricine on P-glycoprotein expression was not taken into
consideration. As a result, further studies are required to focus
on whether dauricine is able to modulate the function or expression
of P-glycoprotein.
In conclusion, the present study demonstrated that
dauricine is able to pass the BBB. It was also shown that
inhibiting P-glycoprotein was able to enhance dauricine levels in
the brain. The results suggest that dauricine, originating from
Traditional Chinese Medicine, may be a promising candidate for the
treatment of CNS disorders.
Acknowledgements
The study was supported by the Natural Science
Foundation of Heilongjiang Province of China (grant no.
D201038).
References
|
1
|
Abbott NJ, Patabendige AA, Dolman DE,
Yusof SR and Begley DJ: Structure and function of the blood-brain
barrier. Neurobiol Dis. 37:13–25. 2010. View Article : Google Scholar
|
|
2
|
Bellavance MA, Blanchette M and Fortin D:
Recent advances in blood-brain barrier disruption as a CNS delivery
strategy. AAPS J. 10:166–177. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mehdipour AR and Hamidi M: Brain drug
targeting: a computational approach for overcoming blood-brain
barrier. Drug Discov Today. 14:1030–1036. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He L and Liu GQ: Interaction of multidrug
resistance reversal agents with P-glycoprotein ATPase activity on
blood-brain barrier. Acta Pharmacol Sin. 23:423–429.
2002.PubMed/NCBI
|
|
5
|
Liu JY, Thom M, Catarino CB, Martinian L,
Figarella-Branger D, Bartolomei F, Koepp M and Sisodiya SM:
Neuropathology of the blood-brain barrier and pharmaco-resistance
in human epilepsy. Brain. 135:3115–3133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pérez-Tomás R: Multidrug resistance:
retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006.PubMed/NCBI
|
|
7
|
Qian JQ: Cardiovascular pharmacological
effects of bisbenzylisoquinoline alkaloid derivatives. Acta
Pharmacol Sin. 23:1086–1092. 2002.PubMed/NCBI
|
|
8
|
Xia JS, Li Z, Dong JW, Tu H and Zeng FD:
Dauricine-induced changes in monophasic action potentials and
effective refractory period of rabbit left ventricle in situ. Acta
Pharmacol Sin. 23:371–375. 2002.PubMed/NCBI
|
|
9
|
Tang XD, Zhou X and Zhou KY: Dauricine
inhibits insulin-like growth factor-I-induced hypoxia inducible
factor 1alpha protein accumulation and vascular endothelial growth
factor expression in human breast cancer cells. Acta Pharmacol Sin.
30:605–616. 2009. View Article : Google Scholar
|
|
10
|
Yang Z, Li C, Wang X, et al: Dauricine
induces apoptosis, inhibits proliferation and invasion through
inhibiting NF-kappaB signaling pathway in colon cancer cells. J
Cell Physiol. 225:266–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li YH and Gong PL: Neuroprotective effects
of dauricine against apoptosis induced by transient focal cerebral
ischaemia in rats via a mitochondrial pathway. Clin Exp Pharmacol
Physiol. 34:177–184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Begley DJ: Delivery of therapeutic agents
to the central nervous system: the problems and the possibilities.
Pharmacol Ther. 104:29–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Megard I, Garrigues A, Orlowski S, et al:
A co-culture-based model of human blood-brain barrier: application
to active transport of indinavir and in vivo-in vitro correlation.
Brain Res. 927:153–167. 2002. View Article : Google Scholar : PubMed/NCBI
|