Introduction
Patients with diabetes mellitus (DM) present with
various pathological conditions, including accelerating
atherosclerosis, cardiac autonomic neuropathy, myocardial protein
changes (1), myocardial oxidation
(2) and intrinsic cardiomyopathy
(3,4). These cardiac diseases associated with
DM (5) are known as diabetic
cardiomyopathy (DCM). Previous studies on the pathogenesis of DCM
mainly focused on factors such as myocardial metabolism, calcium
transit and abnormal free oxygen radicals. The mechanisms of
intracellular signal transduction and cell function regulation in
DCM are of increasing interest and are the subject of current
studies. The excessive activation of the diacylglycerol-protein
kinase C (DAG-PKC) pathway has a pivotal role in intracellular
signal transduction systems. The activation of the DAG-PKC pathway
has been shown to be an important mechanism in the early
pathological changes during the formation of diabetic myocardial
cells. This study, based on previous investigations, used
streptozotocin (STZ)-induced diabetic rats as a model to
investigate the myocardial pathological changes of early diabetes,
and the correlation between PKC-β2 and c-Jun N-terminal kinase 1
(JNK1) was analyzed. The role of the enhancement of signaling
agents, such as JNK1 and the insulin receptor substrate 1 (IRS1),
in the occurrence and development of DCM was observed. The
intervention in the expression of signaling agents, such as JNK1
and IRS1, provided a theoretical basis for signal transduction
inhibition for the prevention and treatment of DCM.
Materials and methods
Modeling of diabetes and grouping
A total of 60 specific pathogen-free Sprague Dawley
rats provided by the Experimental Animals Ministry of China Medical
University (Shenyang, Liaoning, China) were randomly divided into
control, diabetic model and breviscapine-treated diabetes (20
μg/kg/day, intraperitoneal injection) groups, with 20 rats in each
group. Following normal feeding and subsequent fasting for 24 h
with free access to water, the rats of the experimental group were
treated with 60 mg/kg STZ (Sigma, St. Louis, MO, USA) dissolved in
0.1 mol/l citrate buffer (pH 4.5) by left intraperitoneal injection
with moderate action to avoid damaging internal organs and blood
vessels. The rats in the control group were injected with an
equivalent volume of citrate buffer. Urine and blood glucose levels
were assessed after 72 h. The rats with blood glucose >11.1
mmol/l with fasting, blood glucose >16.7 mmol/l without fasting,
qualitative urine glucose ≥+++, polydipsia polyphagia and
increasing urine output were defined as the DM model. The rats in
the intervention group were treated with breviscapine (20
μg/kg/day; Yunnan Natural Medicine Pharmaceutical Co., China)
through intraperitoneal injection. The rats in the control and the
model groups were injected with an equivalent volume of solvent.
All rats were fed a standard diet and provided with freely
available drinking water during the entire experiment, which had a
duration of eight weeks. There was no insulin therapy throughout
the entire experiment, and the rats were sacrificed at appropriate
time-points. This study was performed in strict accordance with the
recommendations from the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (NIH). The protocol
for the animal use was reviewed and approved by the Institutional
Animal Care and Use Committee (IACUC) of China Medical
University.
Determination of blood glucose and body
weight
Following fasting for 12 h, the entire blood of the
rat tail peripheral capillary was extracted to detect the fasting
blood sugar using the Roche Accu-CHEK active blood glucose meter
(Roche Diagnostics, GmbH, Mannheim, Germany). The body weight of
the rats was measured using scales once per week.
Determination of the entire heart and
left ventricular weights
Ten rats were randomly selected from the three
groups during the eight-week treatment. All rats were anesthetized
with 2 ml 3% sodium pentobarbital by intraperitoneal injection
following being weighed. The sternum was opened to expose the
heart, which was separated from the aortic roots, dried on filter
paper following rinsing with cold saline, and then weighed. The
left ventricle was then separated and weighed. The weight ratio of
heart and body (H/W; entire heart weight/body weight) and the left
ventricular mass index (LVMI; left ventricular weight/body weight)
were calculated, respectively.
Hematoxylin and eosin (HE) and Masson
staining
The pathological specimens were fixed for 24 h with
10% neutral formalin by the conventional method, dehydrated with
ethanol gradients, embedded in paraffin and stained with HE.
Positive areas of the stained collagen were quantified using
Image-Pro® Plus 6.0 medical image analysis software
(Media Cybernetics, Inc., Rockville, MD, USA) and were blindly
selected, and five fields of view were selected for each slice. The
myocardial collagen volume fractions (CVFs) were calculated using
the following formula: CVF (%) = collagen area/full field area
×100, and the averages were calculated.
Observation by electron microscopy
A total of 1 mm3 myocardial tissue was
fixed in 2.5% glutaraldehyde, rinsed with phosphate-buffered saline
(PBS), fixed with 1% osmium tetroxide, dehydrated with ethanol by
the conventional method and embedded in epoxy resin. Tissue was
then sectioned (70 nm), dyed with uranyl acetate and lead citrate,
and observed and filmed using JME-1200EX electron microscopy (Japan
Electron Optics laboratory Co., Ltd., Tokyo, Japan).
Immunohistochemistry
Sections of rat left ventricles (0.5×0.5 cm) were
fixed in 4% paraformaldehyde for 24 h, embedded in conventional
paraffin, and sectioned into 4 pm slices. PKC-β2, JNK1 and IRS-1
were immunohistochemically quantified. The expression levels of
JNK1, IRS-1 and PKC-β2 were detected using the StreptAvidin-Biotin
Complex (SABC) method. The morphological image was analyzed using
systems software (Xiamen City Bao Technology Co., Ltd., Xiamen,
China), five fields of view were randomly tested for each slice,
and then the average optical density value (MOD) was calculated for
each sample. The MOD values were used as semi-quantitative
parameters for the expression levels of JNK1, IRS-1 and PKC-β2.
Quantitative polymerase chain reaction
(qPCR)
Myocardial left ventricular free wall (100 mg) was
placed in diethylpyrocarbonate (DEPC) water prepared in advance,
prior to being numbered and frozen in liquid nitrogen, and stored
at −80ºC to measure the expression of JNK1 mRNA and IRS-1 mRNA in
the left ventricle. Total RNA was extracted using a one-step method
according to the instructions for the TRIzol® reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). In order to
determine RNA concentration and purity, 1 μl RNA sample was
obtained and 79 μl DEPC water was added to detect OD260 and OD280
values using an ultraviolet spectrophotometer. A ratio between the
two of 1.8 to 2.0 suggested that the RNA purity of the sample was
sufficient. cDNA was then synthesized using reverse transcription,
in accordance with the kits’ instructions (cDNA synthesis kits;
Beijing Genomics Corporation, Beijing, China). The qPCR reaction
was performed using an ABI 7500 thermal cycler (Biometra,
Göttingen, Germany). cDNA was then synthesized using reverse
transcription with mRNA.
Statistical analysis
The experimental data were processed using SPSS 13.0
statistical software (SPSS, Inc., Chicago, IL, USA). The test
results are expressed as the mean ± standard deviation, and
analyzed using analysis of variance or the Student’s t-test.
P<0.05 indicated a significant difference. P<0.01 indicated a
highly significant difference.
Results
General indexes
Blood glucose levels were significantly increased in
the diabetic model group and the breviscapine-treated diabetes
group compared with the control group (P<0.01) and the body
weight was significantly reduced (P<0.01), while blood glucose
levels were not significantly different between the diabetic model
group and the breviscapine-treated diabetes group, and the body
weight in the breviscapine-treated diabetes group was higher than
that of the diabetic model group (P<0.05). The left ventricular
weight, the ratio of heart and body weight and the left ventricular
mass of the diabetic model and breviscapine-treated diabetes group
were significantly higher than those of the control group (all
P<0.01), and these differences between the diabetic model and
breviscapine-treated diabetes groups were also significant (all
P<0.01) (Table I).
 | Table IIndex comparison of rat blood glucose,
body weight, entire heart weight, left ventricular weight, ratio of
heart and body weight and left ventricular mass among the treatment
groups. |
Table I
Index comparison of rat blood glucose,
body weight, entire heart weight, left ventricular weight, ratio of
heart and body weight and left ventricular mass among the treatment
groups.
| Group | Cases (n) | Fasting blood-glucose
(mmol/l) | Body weight (g) | Entire heart weight
(g) | Left ventricular
weight (g) | Ratio of heart and
body weight (10−3) | Left ventricular mass
(10−3) |
|---|
| Control | 10 | 7.00±1.1 | 312.5±17.08 | 0.84±0.17 | 0.74±0.04 | 2.7±0.06 | 2.37±0.02 |
| Diabetic | 10 | 26.8±2.9a | 203.8±4.79a,b | 0.79±0.39a,b | 0.59±0.02a,b | 3.6±0.17a,b | 2.94±0.07a,b |
| Intervention | 10 | 27.9±1.2 | 247.5±18.9 | 0.73±0.18 | 0.61±0.05 | 3.2±0.13 | 2.49±0.01 |
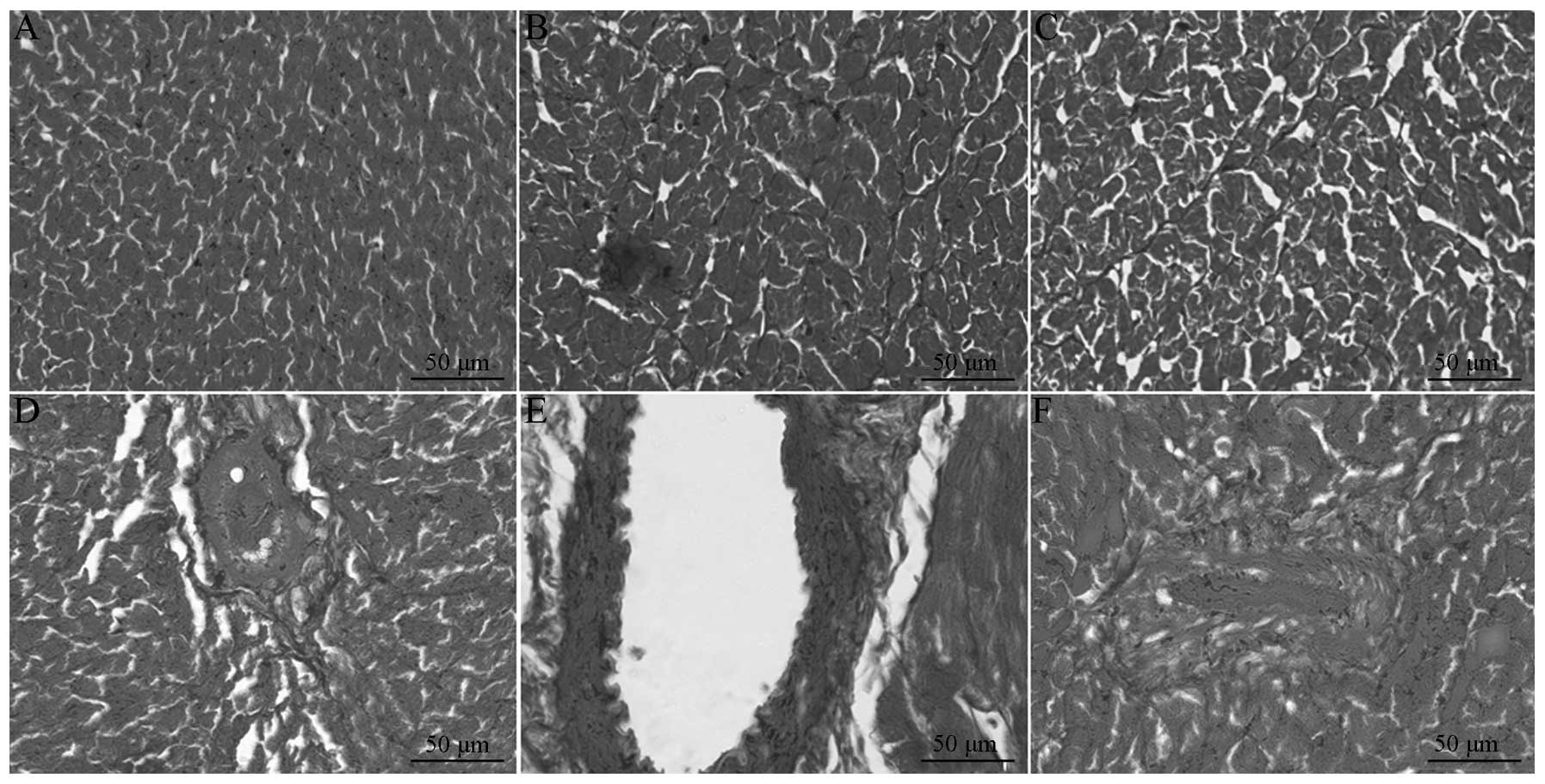
Results of myocardium interstitial
fibrosis
According to the HE staining, the myocardium was
clearly striated and regularly arrayed in the normal rats, while
the diabetic model group featured disordered cell arrays and focal
necrosis, which was improved following treatment with breviscapine.
The collagenous fibers were blue-green in the Masson staining, and
the muscle fibers were red. The collagen was evenly distributed in
the normal rats. In the diabetes group, the myocardial collagenous
fibers were significantly increased, disordered, unevenly
distributed and closely arranged around the myocardial cells, and
were observed to surround the small vessels. The myocardial
collagenous fibers were significantly decreased in the
breviscapine-treated diabetes group. The myocardial CVF and
perivascular collagen areas (PVCA) were significantly increased,
with the increase in PVCA being more marked, ~2.5-fold that of the
normal amount, and the CVF was about two-fold that of the normal
amount (Table II; Fig. 1).
 | Table IIComparison of rat myocardial collagen
volume fraction and perivascular collagen areas among the treatment
groups. |
Table II
Comparison of rat myocardial collagen
volume fraction and perivascular collagen areas among the treatment
groups.
| Group | Cases (n) | CVF (%) | PCVA (%) |
|---|
| Control | 10 | 3.19±0.57 | 7.15±1.06 |
| Diabetic | 10 | 9.57±0.94a,b | 18.32±4.41a,b |
| Intervention | 10 | 6.41±1.58 | 12.14±0.22 |
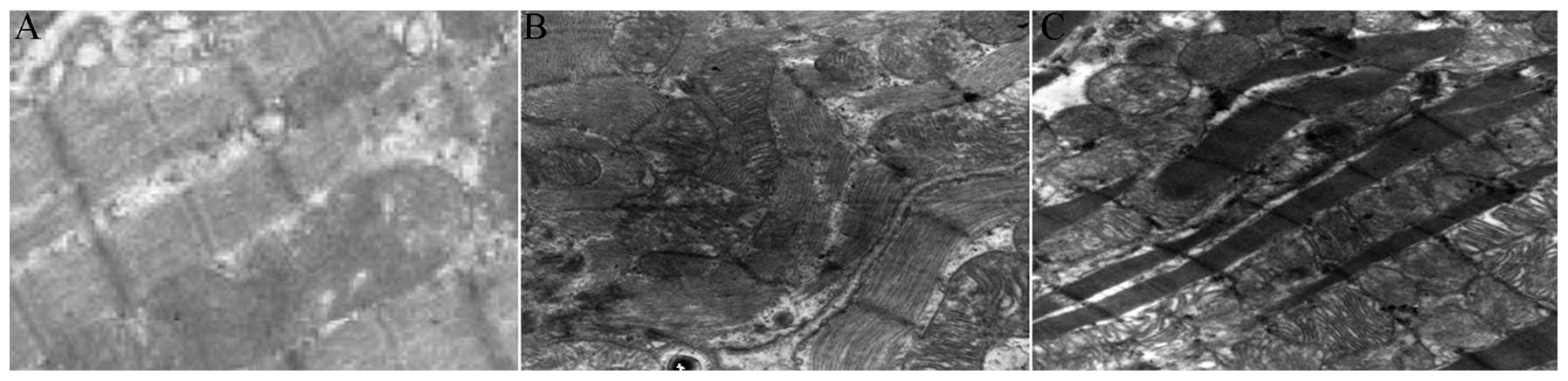
Observation by electron microscopy
The rat myocardial cells in the normal group
exhibited numerous regularly arranged myofilaments with clear
bright-dark zones. The mitochondria were normal, round or oval,
contained obviously advanced ridges and were concentrated within
the cell. Regularly arranged intercalated disc connections were
found in the myocardial cells. The muscle fibrils of the myocardial
cells in the diabetes model group were significantly reduced, the
myofilaments were arranged in a disordered and sparse manner, and
certain myofilaments were fragmented, tortuous, locally dissolved
and contained missing and unclear bright-dark zones. The
mitochondria were arranged in a disordered manner, and certain
mitochondria were obviously swollen, and contained ridges that were
widened, fragmented or even missing. In addition, the density of
the stroma in these mitochondria was reduced and vacuoles had
formed in the stroma. Following the intervention, the number of the
muscle fibrils was increased compared with the diabetes model
group, the myofilaments were arranged regularly, the mitochondria
were slightly swollen, the intercristal spaces were slightly
widened, and amalgamated and fractured vacuole structures were
reduced (Fig. 2).
Immunohistochemistry
The images of three groups were captured using the
micro-imaging analytical system (MetaMorph/DP10/BX41) to obtain the
optical density values. The MODs of PKC-β2, JNK1, p-JNK and IRS1 in
the diabetes model groups were significantly higher than those in
the control group, and the expression levels in the
breviscapine-treated diabetes group were reduced compared with the
diabetes model group, suggesting statistical significance
(P<0.05) (Table III, Fig. 3).
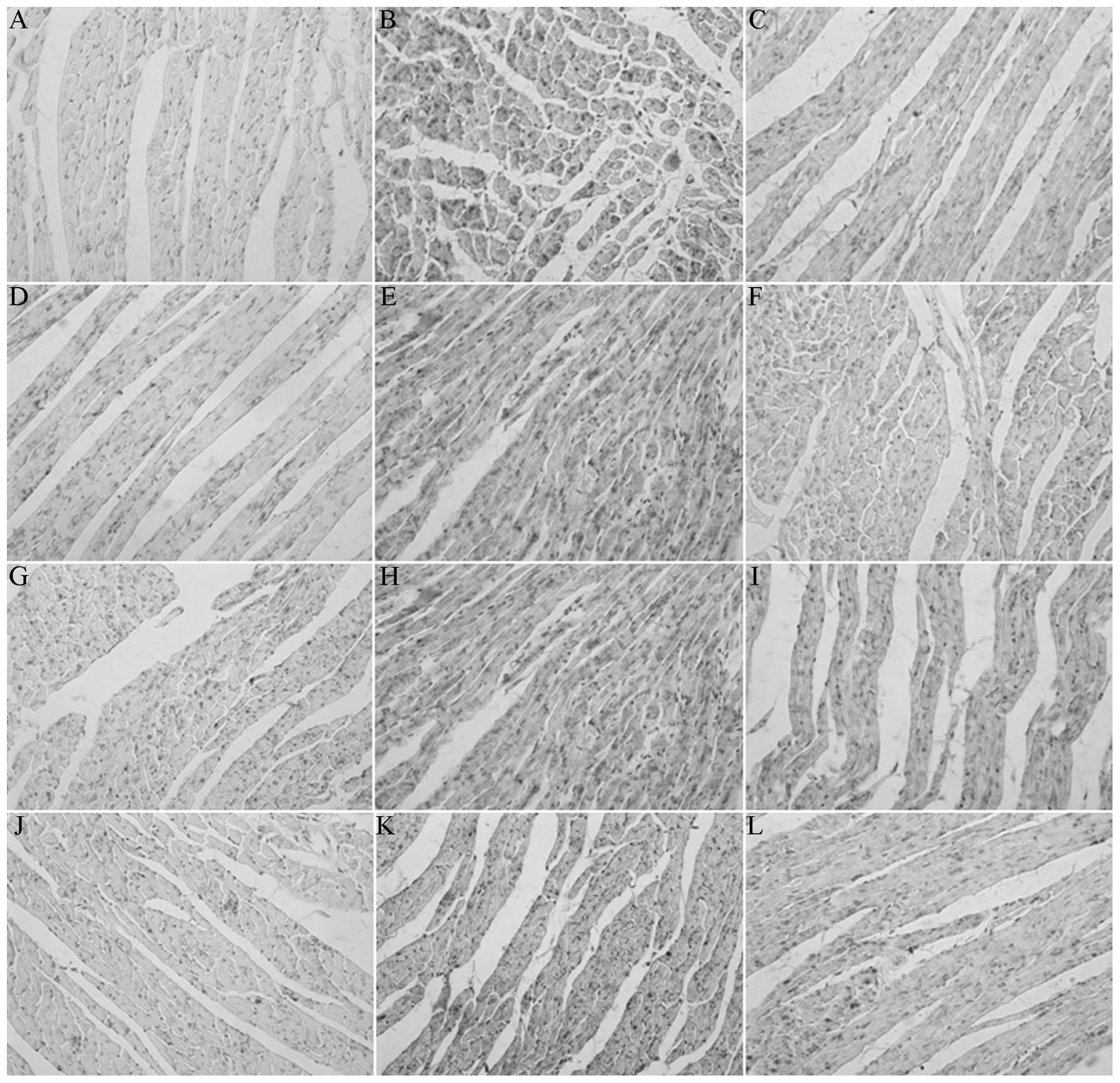 | Figure 3Expression of PKCII, JNK1, p-JNK1 and
IRS1 proteins in rat myocardial tissues in each group
(immunohistochemistry; magnification, ×200). (A–C) Positive
expression of PKCII protein for myocardial tissue in the (A)
normal, (B) diabetic and (C) intervention groups. (D–F) Positive
expression of JNK1 protein for myocardial tissue in the (D) normal,
(E) diabetic and (F) intervention groups. (G–I) Positive expression
of p-JNK1 protein for myocardial tissue in the (G) normal, (H)
diabetic and (I) intervention groups. (J–L) Positive expression of
IRS1 protein for myocardial tissue in the (J) normal, (K) diabetic
and (L) intervention groups. PKCII, protein kinase C II; JNK1,
c-Jun N-terminal kinase 1; p-JNK1, phosphorylated JNK1; IRS1,
insulin receptor substrate 1. |
 | Table IIIComparison of the average optical
density value for PKC, JNK1, p-JNK and IRS-1 in rat myocardium
among the treatment groups. |
Table III
Comparison of the average optical
density value for PKC, JNK1, p-JNK and IRS-1 in rat myocardium
among the treatment groups.
| Group | Cases (n) | PKC | JNK1 | p-JNK | IRS1 |
|---|
| Control | 10 | 0.283±0.051 | 0.132±0.015 | 0.149±0.005 | 0.193±0.033 |
| Diabetic | 10 | 0.408±0.013a,b | 0.387±0.064a,b | 0.392±0.030a,b | 0.380±0.071a,b |
| Intervention | 10 | 0.352±0.009 | 0.243±0.111 | 0.201±0.021 | 0.238±0.014 |
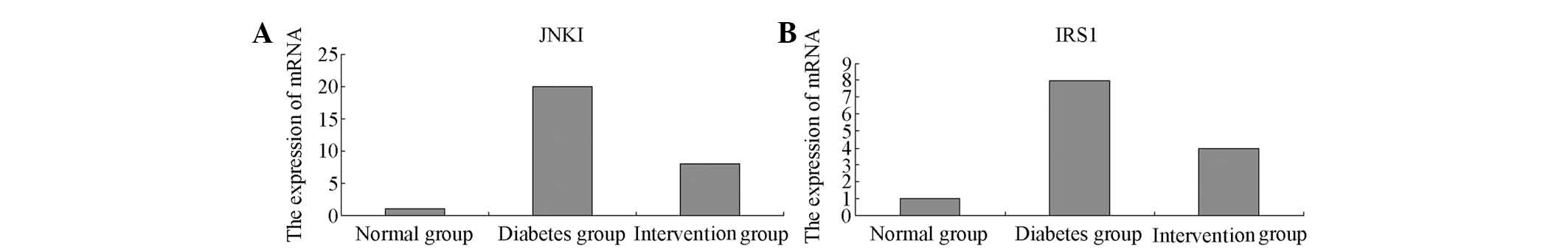
qPCR
The levels of JNK1 and IRS-1 mRNA expressed in the
myocardial cells of the normal group were low, while significantly
increasing in the diabetes model group (30-fold or 20-fold the
level of the control group) (P<0.01). Following treatment with
breviscapine, the expression levels of the JNK1 mRNA and IRS-1 mRNA
were markedly downregulated (Fig.
4).
Discussion
Previous studies on the pathogenesis of DCM mainly
focused on myocardial cell metabolism, calcium ion transport and
oxygen radical abnormalities (6,7). The
study of intracellular signal transduction and cell function
regulation in DCM has drawn increasing focus, and excessive
activation of the DAG-PKC pathway is suggested to have a pivotal
role in the pathogenesis. The activation of PKC was shown to be an
important mechanism in the early cardiac dysfunction caused by
diabetes (8). PKC regulates the
cascaded downstream signals in the DAG-PKC pathway by the
diacylglycerol kinase subtype DGKζ controlling the DAG levels. The
cardiomyocytes of mice with Type I DM induced by STZ were analyzed
(9), and a causal connection
between the translocation from the endochylema to the cytomembrane
of the PKC-β and -δ subtypes and a reduction in the heart’s
blood-pumping function or an improvement in myocardium interstitial
fibrosis were found. However, in DGKζ transgenic DM mice, the
translocation from the endochylema to the cytomembrane of the PKC-β
and -δ subtypes was significantly reduced, and there was no
significant left ventricular systolic dysfunction or myocardial
fibrosis (9). In the present
study, the expression of PKC-β2 in the myocardium of the diabetic
group was significantly higher than that in the control and
intervention groups. The preliminary applications of the highly
selective PKC-β2 inhibitor, LY333531, have further defined the role
of PKC-β2 in diabetic microangiopathy (10). The large number of PKC isozymes may
have various roles in the remodeling of the myocardium, including
the possibility of PKC-β2 affecting the downstream signaling by
activating JNK1 (11–14). Increasing the expression of
downstream signaling agents, including PKC-β2, JNK1, p-JNK and
IRS1, has been shown to accelerate the progression of diabetic
myocardial interstitial fibrosis (15).
This study revealed that, compared with the control
group, the expression of JNK1, p-JNK1 and IRS1 in the diabetic
group was significantly increased (P<0.01), while their
expression was reduced following the inhibition of the DAG-PKC
pathway. The activated JNK1 not only had a role in the
mitogen-activated protein kinase (MAPK) signaling pathway, but also
phosphorylated IRS1, which was stimulated by insulin or
insulin-like growth factor (IGF-1). Accordingly, JNK1 may be able
to regulate IRS1 signaling in the insulin/IGF1 pathway comprising
phosphatidylinositol 3-kinase (PI3K) activation by IRS1, resulting
in cardiac hypertrophy and heart failure (16)through further activation of the
serine/threonine protein kinase/protein kinase B (Akt/PKB) pathway
by PI3K. The latest study revealed a role for mammalian target of
rapamycin (mTOR) through phosphorylation at mTORser2448
in the Akt/PKB pathway; however, this complex pathway has yet to be
fully elucidated (17). In this
study, only one of the effects was revealed, which is that
mTORser2448 phosphorylation activates ribosomal protein
S6 kinase 1 (p70S6K1), promotes the generation of the
hypoxia-inducible factor 1 (HIF1) and vascular endothelial growth
factor (VEGF), and accelerates cardiac hypertrophy (18–20).
In conclusion, the DAG-PKC pathway may affect
downstream signaling through JNK1 (the common signal point of the
G-protein receptor pathway and the insulin receptor pathway at the
cell membrane), which results in the occurrence and development of
DCM. The series of signal points
DAG-PKC-JNK1-IRS1-Akt/PKB-mTOR-p70S6K1 may be a potential pathway
for inducing DCM via the DAG-PKC signal transduction pathway.
Current challenges include controlling high-risk factors, such as
the DAG-PKC signal transduction systems and significant increases
in the expression of JNK1, p-JNK1 and IRS1, in DCM. If the general
mechanism of the signal transduction during the formation and
progression of DCM is elucidated and targeted interventions are
performed, the incidence of DCM may be reduced and the patients’
quality of life may be improved.
Acknowledgements
This study was supported by the Chinese
Pharmaceutical Science Development Prize (No. L2012057) and science
fund of the First Hospital of China Medical University (No.
FSFH1207).
References
|
1
|
Dhalla NS, Rangi S, Zieroth S and Xu YJ:
Alterations in sarcoplasmic reticulum and mitochondrial functions
in diabetic cardiomyopathy. Exp Clin Cardiol. 17:115–120.
2012.PubMed/NCBI
|
|
2
|
Hamblin M, Friedman DB, Hill S, Caprioli
RM, Smith HM and Hill MF: Alterations in the diabetic myocardial
proteome coupled with increased myocardial oxidative stress
underlies diabetic cardiomyopathy. J Mol Cell Cardiol. 42:884–895.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Retnakaran R and Zinman B: Type 1
diabetes, hyperglycaemia, and the heart. Lancet. 371:1790–1799.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chavali V, Tyagi SC and Mishra PK:
Predictors and prevention of diabetic cardiomyopathy. Diabetes
Metab Syndr Obes. 6:151–160. 2013.PubMed/NCBI
|
|
5
|
Otsui K, Inoue N, Tamagawa A and Onishi K:
A case of mitochondrial cardiomyopathy with restrictive transmitral
filling pattern. Int Med Case Rep J. 5:19–22. 2012.PubMed/NCBI
|
|
6
|
Shi FH, Cheng YS, Dai DZ, Peng HJ, Cong XD
and Dai Y: Depressed calcium-handling proteins due to endoplasmic
reticulum stress and apoptosis in the diabetic heart are attenuated
by argirein. Naunyn Schmiedebergs Arch Pharmacol. 386:521–531.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yildirim SS, Akman D, Catalucci D and
Turan B: Relationship between downregulation of miRNAs and increase
of oxidative stress in the development of diabetic cardiac
dysfunction: junctin as a target protein of miR-1. Cell Biochem
Biophys. 13–May;2013.(Epub ahead of print). View Article : Google Scholar
|
|
8
|
Durgan DJ, Smith JK, Hotze MA, et al:
Distinct transcriptional regulation of long-chain acyl-CoA
synthetase isoforms and cytosolic thioesterase 1 in the rodent
heart by fatty acids and insulin. Am J Physiol Heart Circ Physi.
290:H2480–H2497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bilim O, Takeishi Y, Kitahara T, et al:
Diacylglycerol kinase zeta inhibits myocardial atrophy and restores
cardiac dysfunction in streptozotocin-induced diabetes mellitus.
Cardiovasc Diabetol. 7:22008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arikawa E, Ma RC, Isshiki K, et al:
Effects of insulin replacements, inhibitors of angiotensin, and
PKCbeta’s actions to normalize cardiac gene expression and fuel
metabolism in diabetic rats. Diabetes. 56:1410–1420.
2007.PubMed/NCBI
|
|
11
|
Asghar O, Al-Sunni A, Khavandi K, et al:
Diabetic cardiomyopathy. Clin Sci (Lond). 116:741–760. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Wang J, Takeda N, Binaglia L,
Panagia V and Dhalla NS: Changes in cardiac protein kinase C
activities and isozymes in streptozotocin-induced diabetes. Am J
Physiol. 277:E798–E804. 1999.PubMed/NCBI
|
|
13
|
Ishii H, Koya D and King GL: Protein
kinase C activation and its role in the development of vascular
complications in diabetes mellitus. J Mol Med (Berl). 76:21–31.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mebazaa A, Gheorghiade M, Zannad FM and
Parrillo JE: Acute Heart Failure. Springer-Verlag; London: 2008,
View Article : Google Scholar
|
|
15
|
Lei S, Li H, Xu J, et al:
Hyperglycemia-induced protein kinase C β2 activation induces
diastolic cardiac dysfunction in diabetic rats by impairing
caveolin-3 expression and Akt/eNOS signaling. Diabetes.
62:2318–2328. 2013.
|
|
16
|
Liang Q and Molkentin JD: Redefining the
roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy
between cultured myocytes and animal models. J Mol Cell Cardiol.
35:1385–1394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gordon BS, Kazi AA, Coleman CS, Dennis MD,
Chau V, Jefferson LS and Kimball SR: RhoA modulates signaling
through the mechanistic target of rapamycin complex 1 (mTORC1) in
mammalian cells. Cell Signal. Dec 2–2013.(E-pub ahead of
print).
|
|
18
|
Spangenburg EE: Changes in muscle mass
with mechanical load: possible cellular mechanisms. Appl Physiol
Nutr Metab. 34:328–335. 2009.PubMed/NCBI
|
|
19
|
Vary TC, Deiter GG and Lantry R: Chronic
alcohol feeding impairs mTOR(Ser2448) phosphorylation in rat
hearts. Alcohol Clin Exp Res. 32:43–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanchez Canedo C, Demeulder B, Ginion A,
et al: Activation of the cardiac mTOR/p70(S6K) pathway by leucine
requires PDK1 and correlates with PRAS40 phosphorylation. Am J
Physiol Endocrinol Metab. 298:E761–E769. 2010.PubMed/NCBI
|