Introduction
Cancer therapy is often based on surgery,
chemotherapy and radiation therapy. Chemotherapy is one of the main
therapies for the majority of cancers, particularly for those with
a proneness to invade adjacent tissues and to metastasize to other
organs. The effectiveness of chemotherapy is often limited by
toxicity to other normal tissues in the body and multidrug
resistance (MDR). Malignant tumors exhibit MDR and toxicity, which
are major therapy-limiting factors that lead to a poor patient
prognosis. As a consequence, the requirement to understand these
resistance mechanisms on a molecular level is urgent. The
development of resistance to chemotherapeutic agents is a serious
problem in the treatment of numerous human cancers. A tolerance to
one agent is often accompanied by cross-resistance to a variety of
other compounds. This MDR is caused by several mechanisms,
including increased drug efflux (1–8),
enhanced drug detoxification, alterations of targets, and
modification of DNA damage repair systems (9–11)
and apoptosis pathways (12). The
drug efflux effect is mediated by the enhanced expression of one or
more ATP-binding cassette (ABC) transporters (2,13–15).
One of these ABC transporters is breast cancer resistant protein
(BCRP), which is encoded by the ABCG2 gene (16). A number of chemotherapeutic agents,
including adriamycin (ADM), mitoxantrone (MIT) and daunorubicin
(DNR), are substrates for the ABCG2 drug efflux pump (2). The exposure of the Eca109 cell line,
an esophageal squamous cell carcinoma cell line, to increasing
concentrations of ADM leads to the development of the MDR
phenotype, the resulting subline being designated Eca109/ADM. The
objective of the present study was to investigate the correlation
between ABCG2 expression and the MDR of esophageal cancer.
Materials and methods
Cell line and cell culture
The Eca109 esophageal squamous cell carcinoma cell
line was obtained from the Tumor Institute of The Fourth Hospital
of Hebei Medical University (Shijiazhuang, China). The Eca109 cells
were maintained in RPMI-1640 medium supplemented with 10% fetal
bovine serum and 5% penicillin (100 U/ml) and streptomycin (100
mg/ml) in a humidified atmosphere of 95% air and 5% CO2
at 37°C. The medium was changed three times a week.
Establishment of the ADM-resistant cell
line
An ADM-resistant cell line was established from the
Eca109 cells by continuous exposure to increasing concentrations of
ADM, from 0.002 ng/μl to 0.02 ng/μl, for eight months. One of the
surviving clones was isolated and designated as Eca109/ADM.
Cytotoxicity assay
The sensitivity of the Eca109 and Eca109/ADM cells
to the anticancer drugs ADM, DNR and MIT was determined using the
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT)
assay, which is based on the capacity of viable cells to metabolize
(via mitochondrial succinate dehydrogenase) a yellow tetrazolium
salt MTT into purple formazan crystals dissolved in acidified
propan-2-ol. The resulting purple solution is
spectrophotometrically measured at 490 nm. An increase or decrease
in cell number results in a concomitant change in the quantity of
formazan formed, indicating the degree of cytotoxicity caused by
the test material. The absorbance at 490 nm was read using a
microplate reader (BioTek, Winooski, VT, USA). The cells were
seeded into 96-well culture plates at a density of 5×104
cells/ml. A serial concentration of ADM, DNR or MIT was added in a
final volume of 200 μl per well. Following drug treatment for 24 h,
the medium was replaced with an equal volume of fresh medium
containing 0.5 mg/ml MTT (Sigma, St. Loius, MO, USA) and incubated
for 4 h. The medium was then removed and 180 μl dimethylsulfoxide
was added and incubated for 10 min at room temperature. The
cytotoxic effects of the drugs were determined according to the
optical density values using a microplate reader at an absorption
wavelength of 490 nm. Cell viability was expressed as the relative
formazan formation in the treated samples compared with the control
cells [(A490-treated cells/A490 control cells) × 100]. The
IC50 (i.e., the drug concentration causing 50% growth
inhibition), was determined using the MTT assay. The resistance
index was determined as the IC50 of the resistant
cells/the IC50 of the parental cells.
ABCG2 mRNA levels determined by reverse
transcription polymerase chain reaction (RT-PCR)
The total RNA was isolated from the Eca109 and
Eca109/ADM cells using TRI reagent (Sigma) according to the
manufacturer’s instructions, and then treated with avian
myeloblastosis virus reverse transcriptase (Promega, Madison, WI,
USA) to form cDNA. The cDNA was amplified by PCR using Taq DNA
polymerase (Promega), which was performed by denaturation at 94°C
for 30 sec, annealing at 57°C for 30 sec and extension at 72°C for
30 sec. This was repeated for 35 cycles. The PCR-amplified products
were then run on a 1.5% agarose gel and visualized by ethidium
bromide staining. The expression intensities of the optimized bands
were quantified with Quantity One software (Bio-Rad, Mississauga,
ON, Canada), and expressed as a ratio (ABCG2 versus
GAPDH). The PCR primers were as follows: Forward: 5′-GGT CAG
AGT GTG GTT TCT GTA GCA-3′ and reverse: 5′-GTG AGA GAT CGA TGC CCT
GCT TTA-3′ for ABCG2 (product of 280 bp); and forward:
5′-ACC ACA GTC CAT GCC ATC AC-3′ and reverse: 5′-TCC ACC ACC CTG
TTG CTG TA-3′ for GAPDH (product of 452 bp).
ABCG2 protein levels determined by flow
cytometry
The flow cytometric analysis was performed based on
the standard staining procedure. The Eca109 and Eca109/ADM cells
were grown to 80% confluence in cell culture bottles, gently
dislodged with pancreatic enzymes solution (Gibco, Carlsbad, CA,
USA), centrifuged for 5 min at 1,200 × g (Bai Yang, Beijing, China)
and resuspended in phosphate-buffered saline (PBS). Samples of
unfixed Eca109 and Eca109/ADM cells (106 cells/100 μl)
in flow cytometry tubes were incubated subsequently with mouse
anti-ABCG2 labeled with fluorescein isothiocyanate (BioLegend, San
Diego, CA, USA) for 30 min in the dark. Fluorescence was measured
on a Epics-XL II flow cytometer (Beckman Coulter, Miami, FL,
USA).
ABCG2 protein level detection by western
blotting
The ABCG2 protein expression level was detected by
western blotting. Each cell line was grown to 80% confluence,
trypsinized, transferred to eppendorf tubes and rinsed with
ice-cold PBS. The contents of each tube were suspended in 200 μl
lysis buffer in the presence of protease inhibitors (Sigma). The
cell suspension was incubated for 20 min (4°C) and centrifuged (10
min, 32,000 × g) to provide a clear supernatant. The protein
concentration was measured by the Bio-Rad protein assay, with
bovine serum albumin used as a standard (Bio-Rad Laboratories,
Mississauga, ON, USA). For the western blotting analysis, the
protein extract was analyzed by SDS gel electrophoresis on
appropriate polyacrylamide gels (5%), with 50 μg protein loaded on
each lane. The proteins on the gels were then transferred to
polyvinylidene difluoride membranes by a semi-dry transfer method.
Following blocking with Tris-buffered saline containing 5% skimmed
milk and 0.1% Tween-20, the membranes were probed with a primary
antibody against ABCG2 (mouse monoclonal antibody, clone no
sc-18841; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The
antibody was used at a dilution of 1:1,000. The membrane blots were
then reacted with secondary antibody (horseradish peroxidase
anti-mouse IgG), washed extensively with Tris-buffered saline (0.1%
Tween-20) and submerged in DAB, then developed to visualize the
antibody-antigen complexes.
ADM accumulation and efflux by flow
cytometry
The cellular accumulation and efflux of ADM were
analyzed by flow cytometry. In total, 4×105 cells
(Eca109 and Eca109/ADM) were incubated with 0.02 μg/ml ADM at 37°C
for 2 h and then washed twice with ice-cold PBS. The cells were
resuspended in ADM-free RPMI-1640 for 1 h. The cells were washed
with ice-cold PBS and the ADM retained in the cells was detected by
flow cytometry. The ADM generated red fluorescence (fluorescent
peak at 620 nm) after being stimulated by a 488-nm laser (Beckman
Coulter).
Apoptosis rate of cells
The apoptosis rate of the Eca109 and Eca109/ADM
cells following treatment with 0.02 μg/ml ADM for 24 h was analyzed
by flow cytometry. The cells were harvested with trypsin-EDTA
(1:20), washed with PBS and then centrifuged (5 min, 1,200 × g).
The cells were dyed in 1 ml flourescence liquor containing 50 μg/ml
propidium iodide following incubation for 30 min in the dark at
4°C, then kept at 4°C until used. The cells were analyzed by an
Epics-XL II type cytometer (Beckman Coulter) equipped with a 488 nm
argon ion laser. For each sample, 10,000 events selected in the
living cell gate were measured. Forward scatter and side scatter
data were used to establish a gate excluding dead cells and
debris.
Statistical analysis
The statistical analysis was performed with SPSS
11.5 software (SPSS, Inc., Chicago, IL, USA). The data are
displayed as the mean ± standard deviation, and three individual
experiments were conducted in triplicate. Student’s t-test was used
to compare data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Cell morphology of Eca109/ADM
The Eca109/ADM cell line was successfully
established from the Eca109 cells by continuous exposure to
increasing concentrations of ADM, from 0.002 ng/μl to 0.02 ng/μl,
for eight months. One of the surviving clones was isolated and
designated as Eca109/ADM. The cell morphology of the Eca109/ADM
cells was more irregular compared with that of the Eca109 cells
(Fig. 1).
Drug-resistant phenotype
An ADM-resistant subline derived from the parental
sensitive cell line, Eca109, was established by a stepwise
selection in ADM. The subline was designated as Eca109/ADM. The
IC50 value of ADM, DNR and MIT in the Eca109/ADM cells
was 15.45±1.15, 7.27±0.30 and 3.91±0.53 μg/ml, respectively,
compared with 4.69±0.88, 1.94±0.21 and 1.24±0.30 μg/ml,
respectively, in the Eca109 cells. The Eca109/ADM cells exhibited
3.29-, 3.75- and 3.15-fold resistance, respectively (Fig. 2).
 | Figure 2IC50 value of cells against
ADM, DNR and MIT detected by MTT. The IC50 value of
Eca109/ADM against ADM, DNR and MIT was 15.45±1.15, 7.27±0.30 and
3.91±0.53 μg/ml, respectively, compared with 4.69±0.88, 1.94±0.21
and 1.24±0.30 μg/ml, respectively, for Eca109. The Eca109/ADM cells
exhibited 3.29-, 3.75- and 3.15-fold resistance. ADM, adriamycin;
DNR, daunorubicin; MIT, mitoxantrone; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. |
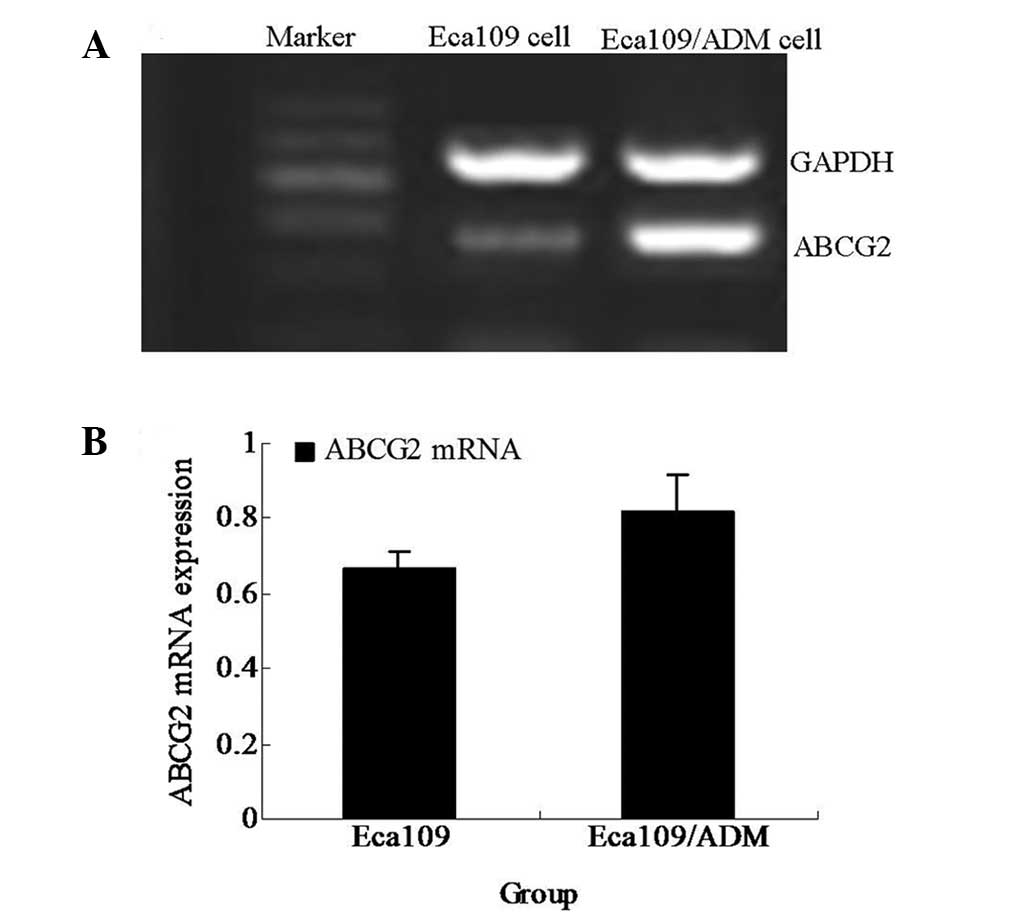
ABCG2 gene expression level
The ABCG2 gene expression level was detected
by RT-PCR and compared with that of the parental cell line, Eca109.
The ABCG2 mRNA expression level of the Eca109/ADM cells was
significantly higher than that of the Eca109 cells (P<0.05;
Fig. 3).
ABCG2 protein expression level
The ABCG2 protein expression level was examined by
flow cytometry and western blotting. The result of the flow
cytometry revealed that the ABCG2 protein expression level of the
Eca109/ADM cells was significantly higher than that of the parental
cell line, Eca109 (P<0.05; Fig.
4). The western blotting result was consistent with the flow
cytometry result (Fig. 5).
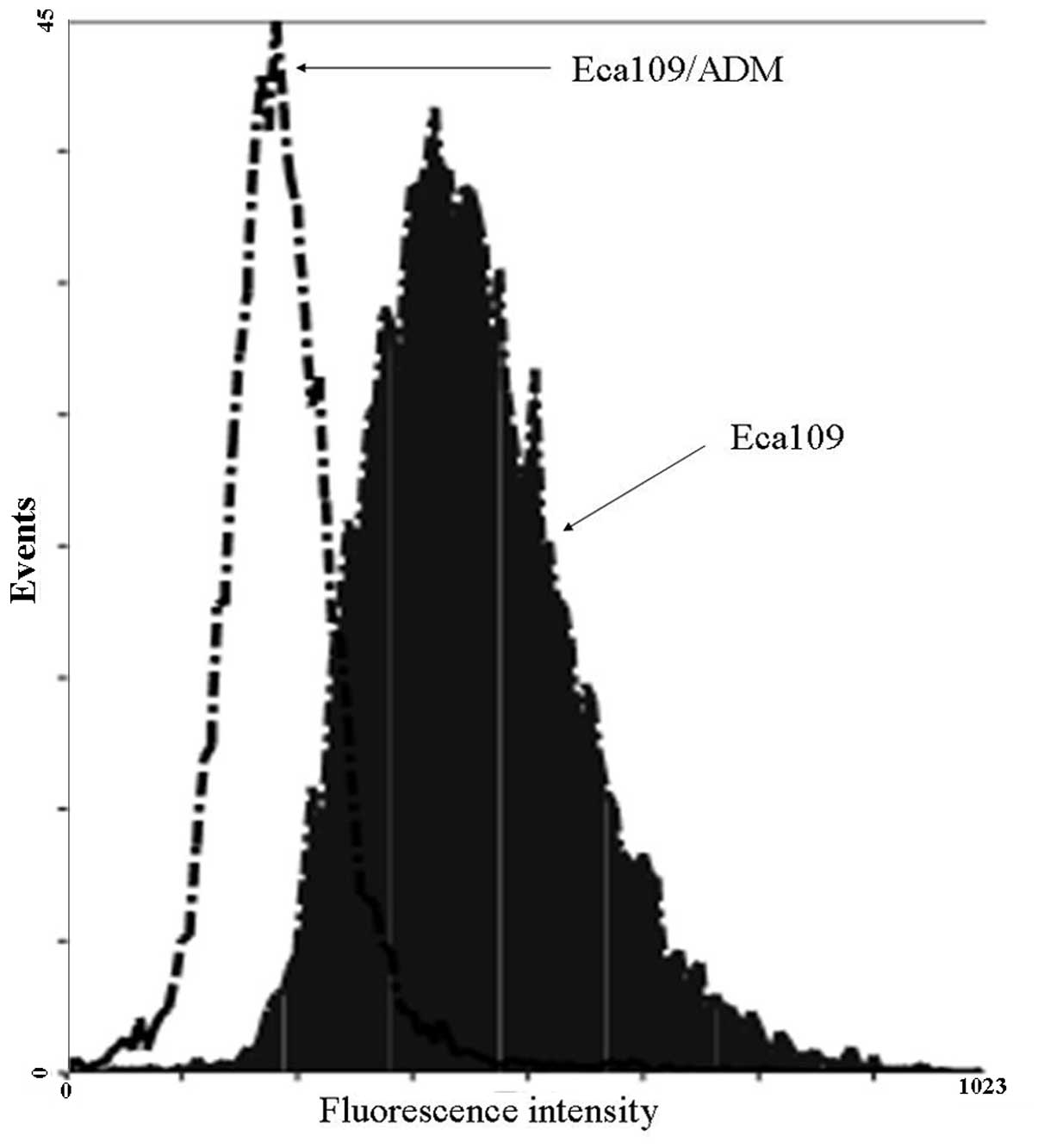
Drug efflux effect of Eca109/ADM
cells
To investigate the resistance of the Eca109/ADM
cells to the anticancer agent, ADM, the ADM efflux effect was
detected using flow cytometry (Fig.
6). When the cells were incubated at 37°C with 0.02 μg/ml ADM
for 2 h and then without ADM for 1 h, the level of ADM in the
Eca109/ADM cells was decreased more than that in the Eca109 cells.
The ADM efflux effect of the Eca109/ADM cells was more than that of
the Eca109 cells.
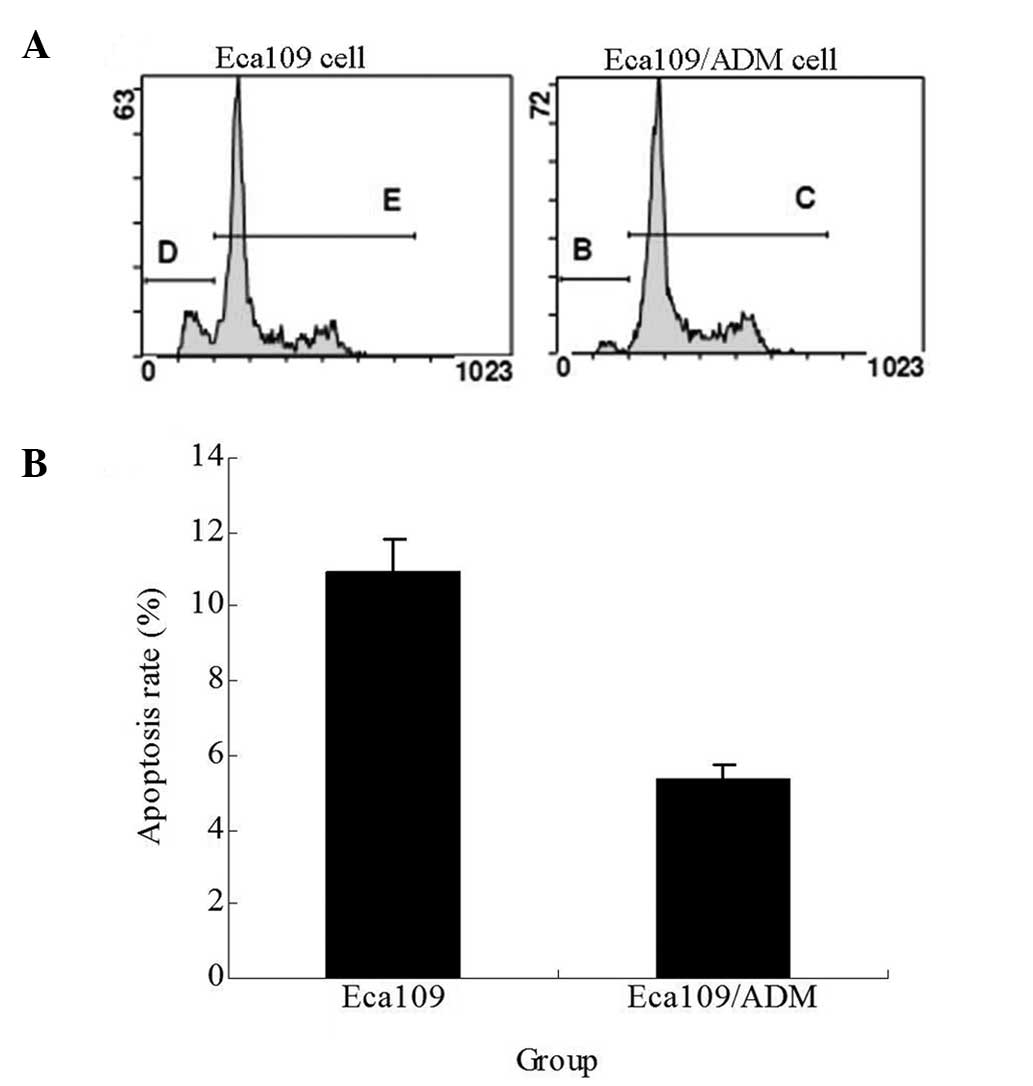
Apoptosis rate of cells
The apoptosis rate of the Eca109/ADM cells following
the treatment with 0.02 μg/ml ADM for 24 h was significantly lower
than that of the Eca109 cells (P<0.01; Fig. 7).
Discussion
At present, the high mortality rate of cancer is a
serious threat to an individual’s life and health. Chemotherapy is
a major form of treatment for various cancers. However, cancer
cells often become resistant to chemotherapy via MDR, thereby
resulting in chemotherapy failure. This resistance may be due to
the drug extrusion activity of MDR ABC transporters, particularly
ABCG2. MDR, by which cells resist numerous structurally and
functionally unrelated drugs, is a major obstacle for the effective
chemotherapy of cancer. As for the mechanisms of MDR, the most
significant is the decreased accumulation of drug within cells by
an increased drug efflux, including overexpression of the cell
membrane transporters, the majority of which are ABC transporters.
ABC transporters are transmembrane proteins capable of expelling a
large variety of structurally and mechanistically diverse
anticancer drugs, which consequently reduces the concentration of
anticancer drugs in the cells. ABCG2 is also known as BCRP and is a
member of the ABC protein family. A large number of hematological
malignancies and solid tumors have been detected as exhibiting BCRP
(17). This indicates that this
transporter may be significant in the clinical drug resistance of
cancers, however there have been few studies in esophageal
cancer.
The multidrug transporters (ABC transporters) are
present in a number of normal tissues with barrier functions
(18–23), and may modulate the absorption of
orally administered cytotoxic compounds. For example, ABCG2 is
expressed mainly in the placenta, and it is indicated that ABCG2
may control the penetration of drugs from the maternal plasma into
the fetus, thus protecting the fetus against the potential toxicity
of the drugs (24).
Based on the ability of ABCG2 to extrude drug agents
(25,26), it is possible that multidrug
transporters affect the absorption, distribution, cellular levels
and effectiveness of these agents (27), resulting in drug resistance. There
is a significant correlation between ABCG2 expression and the drug
resistance of tumors.
In order to investigate the correlation between
ABCG2 expression level and the drug resistance of esophageal
cancer, the ADM-resistant subline, Eca109/ADM, was generated from
the Eca109 esophageal cancer cell line by continuous exposure to
ADM, an anticancer drug agent. The Eca109/ADM cells exhibited
3.29-, 3.75-and 3.15-fold resistance against ADM, DNR and MIT,
respectively, and the MDR phenotype against various anticancer
drugs. Analysis of the gene and protein expression profile using
RT-PCR, flow cytometry and western-blotting demonstrated the highly
elevated expression level of ABCG2 in the Eca109/ADM cells,
compared with its parental cell line, Eca109. The MDR phenotype of
the Eca109/ADM cells may be associated with the level of ABCG2
overexpression.
ABCG2, a member of one of the ABC family, may
extrude the drug agents from cells. For further research in the
present study, the drug efflux effect of the Eca109/ADM cells was
detected using flow cytometry. The flow cytometry results
demonstrated that the drug efflux effect of the Eca109/ADM cells
was stronger than that of its parental cell line, Eca109.
The MTT and cell apoptosis rates as detected by flow
cytometry were compared with the Eca109 and Eca109/ADM cells
sensitivity to ADM. The MTT results demonstrated that the
IC50 of the Eca109/ADM cells following the treatment of
various concentrations of ADM for 24h was significantly higher than
that of the Eca109 cells. The Eca109/ADM cells exhibited 3.29-fold
resistance against ADM compared with its parental cell line,
Eca109. The apoptosis rate results demonstrated that the apoptosis
rate of the Eca109/ADM cells following the treatment of 0.02 μg/ml
ADM for 24 h was significantly lower than that of the Eca109 cells.
These results indicated that the Eca109/ADM cell line was resistant
to the ADM drug compared with its parental Eca109 cell line.
Overall, the Eca109/ADM cell line was a multidrug
cell line with an ABCG2 MDR phenotype. High expression levels of
ABCG2 in Eca109/ADM cells may extrude ADM from cells, which
decreases the ADM concentration in the cells and results in MDR.
Therefore, ABCG2 amplification and expression in esophageal cancer
cells may acquire resistance.
Acknowledgements
This study was supported by grants from The Natural
Science Foundation of Hebei, China (grant no. H2012206107) and the
Hebei Province Medical Scientific Research Key Project (grant no.
20110131).
References
|
1
|
Ding R, Shi J, Pabon K and Scotto KW:
Xanthines down-regulate the drug transporter ABCG2 and reverse
multidrug resistance. Mol Pharmacol. 81:328–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ishikawa T, Saito H, Hirano H, Inoue Y and
Ikegami Y: Human ABC transporter ABCG2 in cancer chemotherapy: drug
molecular design to circumvent multidrug resistance. Methods Mol
Biol. 910:267–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalalinia F, Elahian F, Hassani M,
Kasaeeian J and Behravan J: Phorbol ester TPA modulates
chemoresistance in the drug sensitive breast cancer cell line MCF-7
by inducing expression of drug efflux transporter ABCG2. Asian Pac
J Cancer Prev. 13:2979–2984. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mo W and Zhang JT: Human ABCG2: structure,
function, and its role in multidrug resistance. Int J Biochem Mol
Biol. 3:1–27. 2012.PubMed/NCBI
|
|
5
|
Natarajan K, Xie Y, Baer MR and Ross DD:
Role of breast cancer resistance protein (BCRP/ABCG2) in cancer
drug resistance. Biochem Pharmacol. 83:1084–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sodani K, Tiwari AK, Singh S, et al:
GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse
ABCG2- and ABCB1-mediated drug resistance. Biochem Pharmacol.
83:1613–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu CP, Hsieh CH and Wu YS: The emergence
of drug transporter-mediated multidrug resistance to cancer
chemotherapy. Mol Pharm. 8:1996–2011. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xia CQ and Smith PG: Drug efflux
transporters and multidrug resistance in acute leukemia:
therapeutic impact and novel approaches to mediation. Mol
Pharmacol. 82:1008–1021. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oguri T, Ozasa H, Uemura T, et al:
Preclinical rationale for synergistic interaction of pemetrexed and
cytotoxic nucleoside analogues. Oncol Lett. 4:571–575.
2012.PubMed/NCBI
|
|
10
|
Oplustilova L, Wolanin K, Mistrik M, et
al: Evaluation of candidate biomarkers to predict cancer cell
sensitivity or resistance to PARP-1 inhibitor treatment. Cell
Cycle. 11:3837–3850. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spanswick VJ, Lowe HL, Newton C, et al:
Evidence for different mechanisms of ‘unhooking’ for melphalan and
cisplatin-induced DNA interstrand cross-links in vitro and in
clinical acquired resistant tumour samples. BMC Cancer.
12:4362012.
|
|
12
|
Yang YI, Lee KT, Park HJ, et al:
Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer
cells through downregulation of the Akt and NFκB pathway.
Carcinogenesis. 12:2488–2498. 2012.PubMed/NCBI
|
|
13
|
Hagiya Y, Endo Y, Yonemura Y, et al:
Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette
(ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based
photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis
Photodyn Ther. 9:204–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Flores-Martín J, Rena V, Márquez S,
Panzetta-Dutari GM and Genti-Raimondi S: StarD7 knockdown modulates
ABCG2 expression, cell migration, proliferation, and
differentiation of human choriocarcinoma JEG-3 cells. PLoS One.
7:e441522012.PubMed/NCBI
|
|
15
|
Shen B, Dong P, Li D and Gao S: Expression
and function of ABCG2 in head and neck squamous cell carcinoma and
cell lines. Exp Ther Med. 2:1151–1157. 2011.PubMed/NCBI
|
|
16
|
Doyle L and Ross DD: Multidrug resistance
mediated by the breast cancer resistance protein BCRP (ABCG2).
Oncogene. 22:7340–7358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Omran OM: The prognostic value of breast
cancer resistance protein (BCRP/ABCG2) expression in breast
carcinomas. J Environ Pathol Toxicol Oncol. 31:367–376. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Campos CR, Schröter C, Wang X and Miller
DS: ABC transporter function and regulation at the blood-spinal
cord barrier. J Cereb Blood Flow Metab. 32:1559–1566. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Decleves X, Jacob A, Yousif S, Shawahna R,
Potin S and Scherrmann JM: Interplay of drug metabolizing CYP450
enzymes and ABC transporters in the blood-brain barrier. Curr Drug
Metab. 12:732–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kania KD, Wijesuriya HC, Hladky SB and
Barrand MA: Beta amyloid effects on expression of multidrug efflux
transporters in brain endothelial cells. Brain Res. 1418:1–11.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masereeuw R and Russel FG: Regulatory
pathways for ATP-binding cassette transport proteins in kidney
proximal tubules. AAPS J. 14:883–894. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller DW, Hinton M and Chen F: Evaluation
of drug efflux transporter liabilities of darifenacin in cell
culture models of the blood-brain and blood-ocular barriers.
Neurourol Urodyn. 30:1633–1638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pinzón-Daza M, Garzón R, Couraud P, et al:
The association of statins plus LDL receptor-targeted
liposome-encapsulated doxorubicin increases in vitro drug delivery
across blood-brain barrier cells. Br J Pharmacol. 167:1431–1447.
2012.PubMed/NCBI
|
|
24
|
Kobayashi D, Ieiri I, Hirota T, et al:
Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein
expression in human placenta. Drug Metab Dispos. 33:94–101. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krishnamurthy P and Schuetz JD: Role of
ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol.
46:381–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pan G, Giri N and Elmquist WF: Abcg2/Bcrp1
mediates the polarized transport of antiretroviral nucleosides
abacavir and zidovudine. Drug Metab Dispos. 35:1165–1173. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mao Q and Unadkat JD: Role of the breast
cancer resistance protein (ABCG2) in drug transport. AAPS J.
7:E118–E133. 2005. View Article : Google Scholar : PubMed/NCBI
|