Introduction
Ischemic brain injury is a common cause of permanent
disability and is associated with dementia and cognitive decline in
the elderly (1). Certain surgical
procedures that involve the reduction or interruption of the blood
supply to the brain and events such as strokes, are often
accompanied by memory loss that persists for several months during
recovery. These changes are believed to be associated with focal
cerebral ischemia. The middle cerebral artery occlusion model
(MCAO) that was originally developed in rats is considered to be a
reliable and reproducible model. The model induces deficits in
cognitive function in the rats, which appear to remain fairly
stable (2,3).
Neurotrophic factors are known to be critical in
neurite outgrowth and cell survival. Nerve growth factor (NGF) and
brain-derived neutrophic factor (BDNF) may bind to acceptors,
stimulate the downstream signaling pathways and protect rat
hippocampal neurons against ischemic cell damage (4). Mitogen-activated protein kinase
(MAPK) family members, including extracellular signal-regulated
kinases (ERK)1/2, p38 MAPK and c-Jun N-terminal kinase (JNK),
respond to various extracellular stimuli, thereby transmitting
extracellular signals into the nucleus. The JNK signaling pathway
is a potential cascade mediating neuronal apoptosis triggered by
focal ischemia (5). While, the
MEK/ERK pathway is important in cell growth and differentiation
following ischemia, activation of the Akt kinase pathway by growth
factors also has neuroprotective effects (6).
Astragalus is a traditional Chinese medicine, also
known as legumes Mongolia astragalus or the dried root of
Astragalus membranaceus (Fisch.) Bge. Astragalosides (ASTs)
are the main component of Astragalus and function as antioxidants,
in immune regulation and to promote intelligence. ASTs have been
commonly used in the prevention and treatment of cardiovascular and
cerebrovascular diseases, aging, immune function disorders and
other diseases. Our previous studies revealed that ASTs have
protective effects against ischemic damage (7,8) and
improve behavioral disorders, including problems with spatial
learning and memory in AD rats (9); therefore, we hypothesize that AST may
improve the learning and memory abilities of rats following
ischemia reperfusion (I/R) injury. On the basis of the association
between cognitive decline and AST, the current study was performed
to observe whether AST regulates the behavioral disorder in rats
following ischemia, and its possible mechanism.
Materials and methods
Reagents
AST brown powder (content, >95% AST) was provided
by the Institute of Anhui Hengxing Medicine (Hefei, China). Ginaton
(Gin) was provided by Willmar Schwabe Pharmaceuticals (Karlsruhe,
Germany). Hochest 33258 was provided by Sigma (St. Louis, MO, USA).
ERK, phospho (p)-ERK, JNK, p-JNK, Akt and p-Akt monoclonal
antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA).
Animals
Sprague-Dawley rats (weight, 250–325 g) were
supplied by The Experimental Animal Center of Anhui Medical
University (Hefei, China). Animals were housed four per cage,
allowed access to water and food ad libitum and maintained
in a constant temperature of 22±2°C, with a humidity of 50±10%
under a 12 h light/dark cycle. Animal treatment and maintenance was
conducted in accordance with the guidelines for the humane
treatment of animals set by the association of Laboratory Animal
Sciences and The Center for Laboratory Animal Sciences at Anhui
Medical University.
Rat I/R model and treatment
The MCAO surgery was conducted according to
previously described methods (10). The rats were anesthetized by 10%
chloral hydrate (300 mg/kg, intraperitonealy) and placed in dorsal
recumbency. A longitudinal incision of 1.5 cm in length was made in
the midline of the ventral cervical skin. The right common carotid
artery, internal carotid artery (ICA) and external carotid artery
(ECA) were exposed and carefully isolated. A nylon monofilament (40
mm in length and 0.24 mm in diameter), its tip rounded by
flame-heating, was inserted from the lumen of the ECA to that of
the right ICA to occlude the origin of the right middle cerebral
artery (MCA). The right MCA was occluded for 120 min, following
which, the cerebral blood flow was restored by withdrawal of the
nylon thread.
As a means of assessing the adequacy of the
occlusion, a neurological score was assigned to each animal 5 min
prior to removing the occlusion and at 22 h after reperfusion: 0,
no deficit; 1, forelimb weakness; 2, circling to the affected side,
3, partial paralysis on the affected side; and 4, no spontaneous
motor activity. Following 120 min of occlusion, all the animals
were randomly assigned according to the neurological function
deficit score (11) to one of the
following groups: I/R, AST (20, 40 and 80 mg/kg) or Gin (40 mg/kg).
The rats were administered treatment by gavage at 0, 8 and 24 h
reperfusion, then once a day for 7 days. Sham-control animals were
prepared in the same way, with the exception of the insertion of a
nylon surgical thread into the right ICA. The animal body
temperature was maintained at 37±1°C during and following the
surgery. The animals were sacrificed by decapitation following a
Morris water maze (MWM) test at reperfusion day 7, and the brain
was used for immunohistochemistry.
Another batch of rats was randomly divided into the
following five groups: Sham, I/R, Gin (40 mg/kg) and AST (40 and 80
mg/kg). These rats were administrated treatment by gavage once a
day for 7 days. After the last intragastric administration, the
rats were underwent MCAO for 120 min, then the thread was withdrawn
and the blood flow for was restored for 22 h, before the rats were
sacrificed. A neurological score was assigned to each animal 5 min
prior to removing the occlusion and at 22 h post-reperfusion. At 22
h after reperfusion, the rats were sacrificed and the brains were
dissected, frozen in powdered dry ice and stored at −80°C until
further use for western-blotting.
MWM test
An MWM was used to test spatial learning and memory,
and was performed on days 4, 5, 6 and 7 post-ischemia. The maze
consisted of a black circular pool 2.14 m in diameter and 80 cm in
height, filled with water at 21–22°C to a height of 50 cm. A black
circular platform that was 9 cm in diameter was placed 2.0 cm below
the water line in the center of one quadrant and remained in the
same position. Several, constant, large visual cues surrounded the
tank at a height of 120–150 cm to facilitate orientation. The rat
was placed in the water facing the wall at one of four random start
locations (north, south, east and west, locating at equal distances
from each other on the pool rim). Each rat was allowed to locate
the submerged platform within 90 sec and rest on it for 20 sec. If
the rat failed to locate the hidden platform within 90 sec, it was
placed on it for 20 sec. The procedure was repeated for all the
four starting locations. The latency to reach the platform and the
swimming speed were recorded, each representing the average of four
trials. The escape latency, i.e. the time to reach the platform,
and the length of the path the animal swam to find the platform
were used to assess the acquisition of the water-maze task. The
shorter the latency to locate the platform, the better the rats
memory for its location was considered to be.
Histological examination
For the histological examination, the rats were
perfused transcardially with normal saline, followed by fixation
with 4% paraformaldehyde. The brains were removed and stored in the
same paraformaldehyde solution. Serial paraffin sections were cut
coronally on a Leica microtome (Mannheim, Germany). The sections
were stained with hematoxylin and eosin (H&E) and examined
under a light microscope (Olympus, Tokyo, Japan).
Hoeschst 33258 staining
For nuclear staining, the paraffin sections were
deparaffinized with xylene (Beyotime, Shanghai, China) twice for 15
min at 37°C, washed with phosphate-buffered saline (PBS), mounted
onto slides using antifade mounting medium (Beyotime,) and then
examined by fluorescence microscopy (Olympus). Morphologically,
cells undergoing apoptosis should appear smaller than normal, with
chromatin that appears condensed and deeply stained. Cellular
fragmentation into apoptotic bodies also occurs.
Western blotting
The rats were sacrificed by decapitation at a
specified time under anesthesia. The infarcted brains were
separated, frozen quickly in liquid nitrogen and stored at −80°C.
The tissues were homogenized in ice-cold homogenization buffer (HB;
Beyotime). Following the protein concentration measurement using
the Lowry method, with bovine serum used as a standard, each sample
was diluted to equal protein concentrations with HB. Next, 4X
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) sample buffer was added into the sample, which was then
boiled in a 100°C waterbath for 10 min. Protein (50 μg) was loaded
onto each lane, separated by 15% SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
5% skimmed milk for 2 h and then probed with p-ERK1/2 (1:1,000;
9101; Cell Signaling, Beverly, MA, USA) or ERK1/2 (1:1,000; 9102;
Cell Signaling, Beverly, MA, USA) at 4°C overnight. The detection
was performed using horseradish peroxidase-conjugated goat
anti-rabbit IgG and developed using 0.05% 3,3′-diaminobenzidine in
PBS containing 0.01% H2O2. The bands on the
membrane were scanned and analyzed by Eaglesight software
(Stratagene, La Jolla, CA, USA). The proteins were visualized by an
enhanced chemiluminescence system (Bioshine, Shanghai, China).
Statistical analysis
The data were expressed as the mean ± standard
deviation. Significant differences between groups were determined
by a one-way analysis of variance and the t-test. P<0.05 was
used to indicate a statistically significant difference.
Results
Effects of AST on learning and memory
impairment in I/R rats
To measure the correlation between the brain I/R
injury and cognitive deficits and the protective effects of AST in
rats, learning and memory was assessed by the MWM test. At each day
of observation of the MWM test, the ischemic rats spent more time
locating the submerged platform when compared with the sham rats.
At days 4, 5 and 6, the AST (20 mg/kg)-treated ischemic rats showed
a trend towards locating the platform in a shorter time relative to
the ischemic group. The AST (40 mg/kg)-treated group exhibited a
shortened latency at day 6, while AST (80 mg/kg) also had the same
effect at days 5 and 6. At the last testing day (day 7), AST (20,
40, 80 mg/kg) markedly shortened the time it took to locate the
hidden platform compared with the ischemic rats (P<0.05 or
P<0.01) (Table I).
 | Table IEffects of AST on the escape latency
during the training session in the MWM test in I/R rats (mean ±
standard deviation, n=8). |
Table I
Effects of AST on the escape latency
during the training session in the MWM test in I/R rats (mean ±
standard deviation, n=8).
| | Latency, sec |
|---|
| |
|
|---|
| Group | Dose, mg/kg | 4 days | 5 days | 6 days | 7 days |
|---|
| Sham | - | 70.92±17.31 | 60.70±15.42 | 47.55±11.99 | 33.57±9.86 |
| Model | - | 94.19±18.47a | 85.29±15.02b | 79.84±18.40b | 67.81±15.60b |
| Gin | 40 | 78.42±22.21 | 73.88±15.25 | 60.22±16.17c | 48.90±7.71d |
| AST | 20 | 79.87±20.61 | 70.48±19.50 | 64.64±12.62 | 51.85±9.04c |
| 40 | 78.01±22.48 | 75.63±12.80 | 61.34±15.73c | 49.05±6.73d |
| 80 | 75.59±20.86 | 69.59±13.19c | 55.78±18.34c | 46.01±13.65d |
The length of the path the rats swam to locate the
platform was also recorded to assess the acquisition of the
water-maze task. Compared with the sham group, the I/R group rats
swam a longer distance to locate the platform (P<0.01). AST (40
mg/kg) shortened the distance at day 6, and AST (40 mg/kg) had the
same action at days 5 and 6. On the last testing day, AST (20, 40,
80 mg/kg) markedly shortened the distance swam when compared with
the I/R group (P<0.01) (Table
II).
 | Table IIEffects of AST on the swim distance
during the training session in the MWM in I/R rats (mean ± standard
deviation, n=8). |
Table II
Effects of AST on the swim distance
during the training session in the MWM in I/R rats (mean ± standard
deviation, n=8).
| | Distance, cm |
|---|
| |
|
|---|
| Group | Dose, mg/kg | 4 days | 5 days | 6 days | 7 days |
|---|
| Sham | - | 1808.19±661.94 | 1502.32±503.84 | 1221.18±307.44 | 833.62±173.04 |
| Model | - |
2982.97±746.86a |
2648.16±473.43a |
2312.10±425.62a |
1763.64±524.13a |
| Gin | 40 | 2312.00±589.51 |
2065.43±445.45b |
1551.98±404.00c |
1113.76±224.62c |
| AST | 20 | 2558.83±552.58 | 2254.27±565.87 |
1862.86±411.14b |
1217.34±290.95b |
| 40 | 2303.95±693.06 |
2060.13±451.50b |
1528.46±431.47c |
1083.47±260.98c |
| 80 | 2258.92±640.11 |
1875.59±481.58c |
1423.50±377.50c | 967.20±270.72c |
Effect of AST on neuronal degenerative
changes and apoptosis in the hippocampus of I/R rats
To investigate the correlation between memory
impairment and neuronal degeneration and apoptosis, the
neuropathology and levels of apoptosis were measured (Fig. 1). The model group demonstrated
neuron degeneration in the hippocampus (CA1), whereas no evident
neuronal abnormalities were present in the control group. The
neuronal cell bodies became small and deeply stained with dye. AST
may improve the pathomorphological changes of the hippocampal
neurons and reduce the neuronal chromatin, which is condensed.
Hippocampal neuronal apoptosis was explored further
by staining with Hoechst 33258, which binds to chromatin and allows
fluorescent visualization of normal and condensed chromatin.
Morphologically, the cells undergoing apoptosis became small and
deeply stained and cellular fragmentation into apoptotic bodies
occured (Fig. 2A). Compared with
the control group, the percentage of condensed cells in the
hippocampus increased in the MCAO rats, whereas AST significantly
decreased the percentage in the hippocampus (CA1) (Fig. 2B).
Effect of AST on the neurological
function evaluation of MCAO rats
A neurological deficit score may indicate the
neurological function impairment of rats following ischemia
reperfusion injury. AST (40, 80 mg/kg) significantly reduced the
neurological deficit score when compared with the I/R group,
indicating that pretreatment with AST may improve the neurological
disorder induced by cerebral I/R (Table III).
 | Table IIIEffect of AST on the neurological
function evaluation of rats following I/R injury (mean±standard
deviation, n=8). |
Table III
Effect of AST on the neurological
function evaluation of rats following I/R injury (mean±standard
deviation, n=8).
| Group | Dose, mg/kg | Neurological deficit
score |
|---|
|
|---|
| 2 h | 22 h |
|---|
| Sham | - | 0.00±0.00 | 0.00±0.00 |
| Model | - | 2.38±0.52 | 2.63±0.52 |
| Gin | 40 | 2.25±0.71 | 1.88±0.64a |
| AST | 40 | 2.50±0.53 | 2.00±0.53a |
| 80 | 2.13±0.64 | 1.88±0.35b |
Effects of AST on the activity of p-ERK,
p-JNK and p-Akt in the brains of I/R rats
To further analyze the cell signaling pathways
responsible for the AST neuroprotective effects, western blotting
was performed with tissue samples obtained from the brain tissue of
the ischemic rats. As shown in Fig.
3, the bands for p-ERK, p-JNK and p-Akt were observed in each
group. The level of p-ERK, p-JNK and p-Akt was quantified by
densitometry. There was increased phosphorylation of ERK and Akt,
and decreased phosphorylation of JNK in the I/R group; pretreatment
with AST (40 or 80 mg/kg) may activate ERK and Akt phosphorylation
while depressing JNK phosphorylation further.
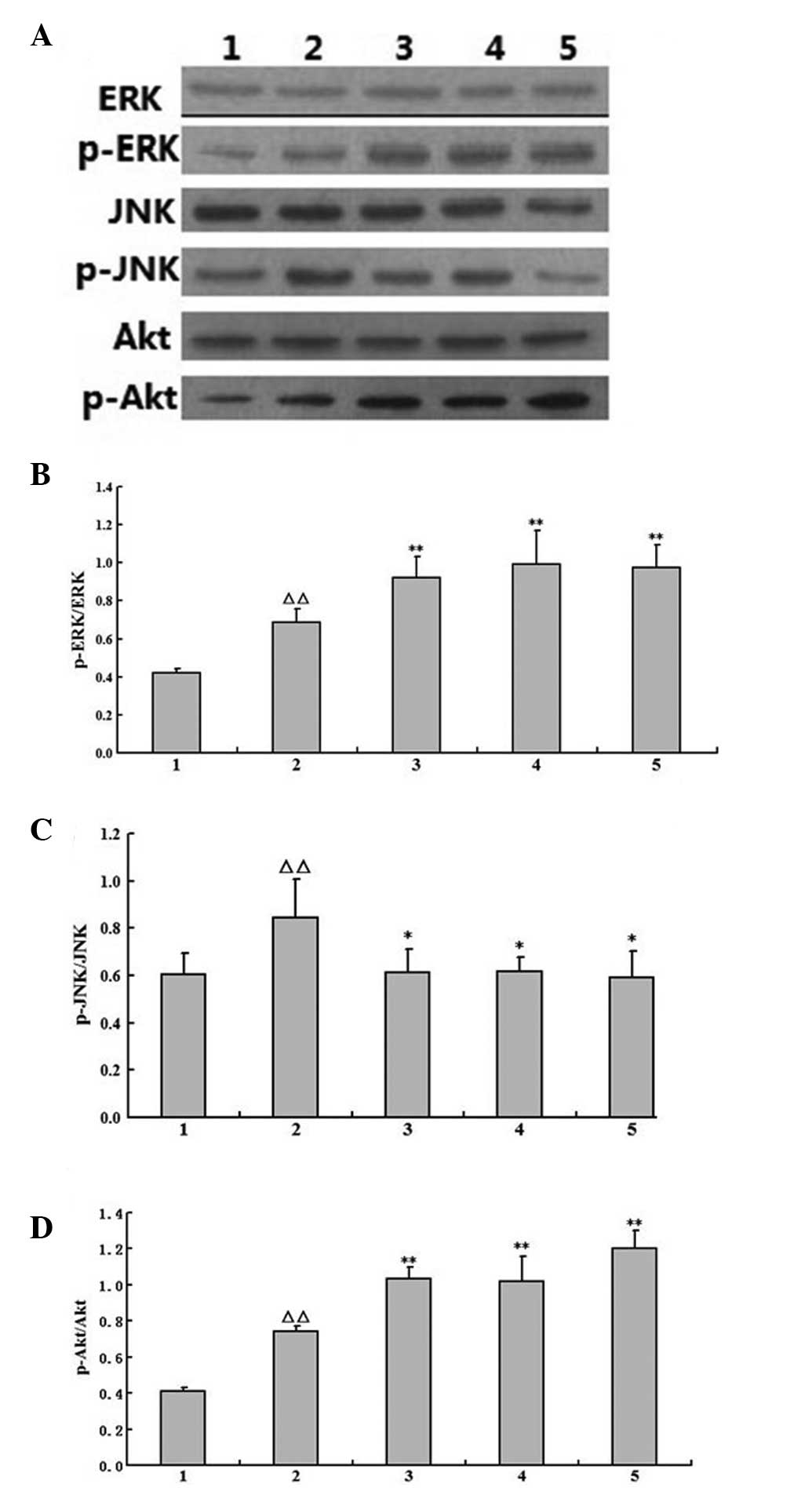 | Figure 3Effects of AST on the activity of
p-ERK, p-Akt and p-JNK in the I/R rats (mean ± standard deviation,
n=5). (A) Representative western blotting of ERK, p-ERK, JNK,
p-JNK, Akt and p-Akt in cerebral ischemia tissue from the different
groups of rats at 22 h post-reperfusion. (B, C and D). The data of
the (B) p-ERK/ERK, (C) p-JNK/JNK and (D) p-Akt/Akt. 1, sham; 2,
model; 3, Gin (40 mg/kg); 4, AST (40 mg/kg) and 5, AST (80 mg/kg).
The presented data are based on at least three independent
experiments (ΔΔP<0.01 vs. sham group;
*P<0.05 and **P<0.01 vs. model group by
Fisher’s LSD post-hoc after one-way analysis of variance). AST,
astragalosides; ERK, extracellular signal-regulated kinases; I/R,
ischemia/reperfusion; Gin, ginaton; p-, phospho. |
Discussion
The development of mazes to investigate spatial
learning and memory has provided a method to determine the
protective effects of drugs on the behavioral consequences and the
neurological damage to the hippocampus and other areas involved in
the neurotoxic effects of ischemia (12). The deficits on the learning and
memory of rats following I/R injury was therefore measured by MWM
in the present study. MCAO induced a failure in the spatial memory
function of the rats when they were tested on days 4 to 7 following
the surgery. This result demonstrated that AST shortened the
latency and distance of swimming compared with the I/R group, which
indicated that AST was able to diminish the abnormal behavioral
performance following I/R. Gin is extracted from the leaves of
Ginkgo biloba and is a famous Chinese traditional medicine
used for treating cerebrovascular diseases and Alzheimer’s disease,
therefore it was selected as a positive control. The data revealed
that Gin may shorten the latency and distance of swimming, similar
to the effects of AST.
The I/R injury induced neuronal apoptosis and
learning and memory impairments, therefore, the protective effects
of AST on neuronal apoptosis in the hippocampus were investigated
further. Apoptosis is a subtype of cell death that is involved in
diverse physiological and pathological processes (13). In the present study, a histological
examination revealed that the neuronal cell bodies became short and
deeply stained with dye following I/R injury, and nuclear staining
with Hoechst 33258 displayed nuclear condensation and fragmentation
in the cells undergoing apoptosis. AST may ameliorate these
pathological changes and inhibit the apoptosis in the hippocampus
in MCAO rats. This may be the protective mechanism of AST on I/R
injury.
Our previous data demonstrated that AST may increase
the expression of NGF and BDNF and the mRNA expression of TrkA and
TrkB, while decreasing the p75 NTR mRNA level (7,8).
Therefore we hypothesize that AST may produce a marked effect
through growth factors and downstream signaling pathways. The ERK,
JNK and Akt signaling pathways may regulate neuronal survival and
apoptosis following ischemia, but the majority of studies agree
that the levels of p-ERK, p-JNK and p-Akt decrease after 24 h or
more following ischemia (14,15),
therefore the level of p-ERK, p-JNK and p-Akt was detected 22 h
after reperfusion in the present study. The p-ERK levels were
enhanced following use of a variety of protective agents, such as
BDNF and erythropoietin (16–18).
The phosphorylation of ERK is an essential component in inducing
neuroprotection. The serine/threonine kinase, Akt, also known as
protein kinase B, enhances survival with cerebral ischemia through
a PI3-kinase-dependent signaling pathway. PI3K/Akt signaling is
also important in neurogenesis. In addition, Akt is a critical
mediator of cellular responses to growth factors, whereas JNK may
be a key mediator for the transmission of apoptotic signals to
mitochondrial apoptosis-related proteins (19,20).
The JNK signaling pathway has been demonstrated to be activated
following focal cerebral ischemia. Furthermore, ischemia-induced
JNK activity may promote neuronal apoptosis (21). In the present study, the changes in
ERK, JNK and Akt phosphorylation were measured in the rats treated
with AST, and the results revealed that AST was able to increase
ERK and Akt phosphorylation and decrease the expression of p-JNK
following I/R.
In conclusion, the present study indicated that AST
may attenuate learning and memory impairments in rats following
ischemia-reperfusion, and that this may be associated with the
regulation of ERK, JNK and Akt phosphorylation and their expression
levels.
Acknowledgements
This study was supported by grants from The Nature
Science Foundation of Anhui Province Education (grant nos.
KJ2008B301 and KJ2009A81), Young Teachers in Colleges and
Universities Provincial Research Projects of Anhui Province (grant
no. 2008jq1059zd).
References
|
1
|
Hattori K, Lee H, Hurn PD, Crain BJ,
Traystman RJ and DeVries AC: Cognitive deficits after focal
cerebral ischemia in mice. Stroke. 31:1939–1944. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouët V, Freret T, Toutain J, Divoux D,
Boulouard M and Schumann-Bard P: Sensorimotor and cognitive
deficits after transient middle cerebral artery occlusion in the
mouse. Exp Neurol. 203:555–567. 2007.PubMed/NCBI
|
|
3
|
Shioda N, Han F, Morioka M and Fukunaga K:
Bis(1-oxy-2-pyridinethiolato)oxovanadium(IV) enhances neurogenesis
via phosphatidylinositol 3-kinase/Akt and extracellular signal
regulated kinase activation in the hippocampal subgranular zone
after mouse focal cerebral ischemia. Neuroscience. 155:876–887.
2008. View Article : Google Scholar
|
|
4
|
Lee TH, Kato H, Chen ST, Kogure K and
Itoyama Y: Expression of nerve growth factor and trkA after
transient focal cerebral ischemia in rats. Stroke. 29:1687–1697.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Irving EA and Bamford M: Role of mitogen-
and stress-activated kinases in ischemic injury. J Cereb Blood Flow
Metab. 22:631–647. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, Shimohata T, Wang JQ, Sun G,
Schaal DW, Sapolsky RM and Steinberg GK: Akt contributes to
neuroprotection by hypothermia against cerebral ischemia in rats. J
Neurosci. 25:9794–9806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin YY, Li WP, Gong HL, Zhu FF, Li WZ and
Wu GC: Protective effect of astragaloside on focal cerebral
ischemia/reperfusion injury in rats. Am J Chin Med. 38:517–527.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin YY, Li WP, Li WZ, Gong HL, Zhu FF and
Wu GC: Effects of astragalosides on the expression of BDNF, TrkB
and p75NTR mRNA against focal cerebral ischemia-reperfusion injury.
Chinese Pharmacological Bulletin. 25:672–676. 2009.
|
|
9
|
Li W, Li W, Yin Y, Gong H, Wu G and Zhu F:
Protective effects of AST and ASI on memory impairment and its
mechanism in senescent rats treated by GC. Zhongguo Zhong Yao Za
Zhi. 34:199–203. 2009.(In Chinese).
|
|
10
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wiard RP, Dickerson MC, Beek O, Norton R
and Cooper BR: Neuroprotective properties of the novel
antiepileptic lamotrigine in a gerbil model of global cerebral
ischemia. Stroke. 26:466–472. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li WZ, Li WP, Zhang W, Yin YY, Sun XX,
Zhou SS, Xu XQ and Tao CR: Protective effect of extract of
Astragalus on learning and memory impairments and neurons’
apoptosis induced by glucocorticoids in 12-month-old male mice.
Anat Rec (Hoboken). 294:1003–1014. 2011.PubMed/NCBI
|
|
14
|
Kim SJ, Yoo KY, Jeong CW, Kim WM, Lee HK,
Bae HB, Kwak SH, Li M and Lee J: Urinary trypsin inhibitors afford
cardioprotective effects through activation of PI3K-Akt and ERK
signal transduction and inhibition of p38 MAPK and JNK. Cardiology.
114:264–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang PR, Wang JS, Zhang C, Song XF, Tian N
and Kong LY: Huang-Lian-Jie-Du-Decotion induced protective
autophagy against the injury of cerebral ischemia/reperfusion via
MAPK-mTOR signaling pathway. J Ethnopharmacol. 149:270–280. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang F, Wang S, Signore AP and Chen J:
Neuroprotective effects of leptin against ischemic injury induced
by oxygen-glucose deprivation and transient cerebral ischemia.
Stroke. 38:2329–2336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kilic E, Kilic U, Soliz J, Bassetti CL,
Gassmann M and Hermann DM: Brain-derived erythropoietin protects
from focal cerebral ischemia by dual activation of ERK-1/-2 and Akt
pathways. FASEB J. 19:2026–2028. 2005.PubMed/NCBI
|
|
18
|
Lange-Asschenfeldt C, Raval AP, Dave KR,
Mochly-Rosen D, Sick TJ and Pérez-Pinzón MA: Epsilon protein kinase
C mediated ischemic tolerance requires activation of the
extracellular regulated kinase pathway in the organotypic
hippocampal slice. J Cereb Blood Flow Metab. 24:636–645. 2004.
View Article : Google Scholar
|
|
19
|
Tournier C, Hess P, Yang DD, Xu J, Turner
TK, Nimnual A, Bar-Sagi D, Jones SN, Flavell RA and Davis RJ:
Requirement of JNK for stress-induced activation of the cytochrome
c-mediated death pathway. Science. 288:870–874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lei K, Nimnual A, Zong WX, Kennedy NJ,
Flavell RA, Thompson CB, Bar-Sagi D and Davis RJ: The Bax subfamily
of Bcl2-related proteins is essential for apoptotic signal
transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol.
22:4929–4942. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuno S, Saito A, Hayashi T and Chan PH:
The c-Jun N-terminal protein kinase signaling pathway mediates Bax
activation and subsequent neuronal apoptosis through interaction
with Bim after transient focal cerebral ischemia. J Neurosci.
24:7879–7887. 2004. View Article : Google Scholar
|

















