Introduction
Cisplatin is a chemotherapeutic agent. It was
approved by the Food and Drug Administration (FDA) in 1978 and has
been widely used in the treatment of tumors, such as testicular,
ovarian, head and neck, bladder, and small cell lung cancer. Two
disadvantages limit its clinical application: severe adverse drug
reactions and drug resistance (1,2).
Severe adverse drug reactions limit the clinical dose applied to
patients, which in turn attenuates its effects. Drug resistance,
including natural and acquired drug resistance, leads to failure of
the chemotherapeutic treatment. It is thus important to improve the
sensitivity of malignant tumors to chemotherapeutic agents, in
order to reduce drug dosage and improve the efficacy of
treatment.
Gap junction (GJ), a special channel composed of
proteins known as connexins (Cx), connects adjacent cells, allowing
for direct exchange of small hydrophilic molecules and ions less
than 1–2 kDa in size, including metabolites and messengers such as
sodium, potassium, calcium, cAMP/cGMP, ADP/ATP, and thereby
resulting in the metabolic and electric coupling of cells (3). GJ can promote apoptosis induced by
several chemical agents in tumor cells (4,5). Our
previous study showed that the enhancement of cisplatin and
etoposide cytotoxicity is dependent on the GJ intercellular
communication (GJIC) (6). It was
hypothesized that GJs may transmit ‘death signals’, i.e., the
induced apoptotic or necrotic processes from one cell to
neighboring cells, which is known as the ‘bystander effect’. In
light of this, intercellular amplification of the death signals by
enhancement of GJ formation or function would increase the
cytotoxic action of cisplatin, and thus, cisplatin
sensitization.
There are a few approaches to increase GJIC in tumor
cells, including the use of certain plant-derived flavonoids
(7,8). Baicalein is a flavonoid known to
display antitumor effects (9–13).
However, it remains unknown whether baicalein can improve GJ
function and cisplatin cytotoxicity. This study aimed to
investigate the effect of baicalein on the cytotoxicity of
cisplatin and the relationship between this effect and the
modulation of GJ function by baicalein in HeLa cells expressing
Cx26. We found that baicalein significantly enhances cell coupling
and cisplatin cytotoxicity only in the presence of functional GJs
composed of Cx26. These results indicated that baicalein can
increase the cytotoxicity of cisplatin through enhancement of GJ
function.
Materials and methods
Drugs, antibodies and reagents
Baicalein was purchased from the China National
Institutes for Food and Drug Control, (Beijing, China). Cisplatin,
anti-haemagglutinin (HA) mouse IgG, sulforhodamine B,
trichloroacetic acid, acetic acid, Tris, dimethylsulfoxide (DMSO)
and oleamide were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Cell culture reagents were purchased from Gibco-BRL (Grand
Island, NY, USA) and calcein acetoxymethyl ester (AM) was purchased
from Invitrogen Life Technologies (Calrsbad, CA, USA). G418,
hygromycin, and doxycycline were purchased from Calbiochem (San
Diego, CA, USA). Secondary antibodies for western blotting were
purchased from Amersham Biosciences (Piscataway, NJ, USA). All
other reagents were purchased from Sigma-Aldrich unless otherwise
stated.
Cell lines and culture
The HeLa cell line expressing Cx26 was previously
described and characterized (14).
In this cell line, obtained via transfection, Cx26 expression is
under the control of a single bidirectional tetracycline-inducible
promoter. Cx26 has a thrombin-cleavable C-terminal epitope tag (3.2
kDa) that includes an HA epitope. Cx26-expressing HeLa cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM), 10% newborn
bovine serum, 100 μg/ml G418 sulfate, and 200 μg/ml hygromycin B at
37°C in a 5% CO2 humidified incubator. Connexin
expression was induced by incubation with 1 μg/ml doxycycline for
48 h prior to all experiments.
Sulforhodamine B (SRB) assay
The SRB colorimetric assay was used to measure the
toxic effect of baicalein on cell viability (15). Cells were seeded onto 96-well
plates for 24 h, followed by incubation with different
concentrations of baicalein for 24 h, and were cultured for an
additional 12 h. The culture medium was then removed and the cells
were fixed with 50 μl of ice-cold 50% trichloroacetic acid solution
at 4°C for 60 min, rinsed five times with tap water and dried at
room temperature overnight. Next, 100 μl of 0.4% SRB solution was
added to each well and incubated at room temperature for 30 min.
Unbound dye was removed by washing five times with 1% acetic acid
solution and drying at room temperature. A total of 10 mM Tris base
solution (pH 10.5) was used to dissolve the protein-bound dye, and
the plate was placed on a plate shaker for 15 min. The optical
density (OD)570 nm was measured using a 96-well MRX
plate reader (Dynex Technologies, Chantilly, VA, USA).
Gap junction dye-coupling (parachute)
assay
Functional GJs were examined as described by
Goldberg et al (16). Cells
were grown to confluence in 12-well plates. Donor cells from one
well were incubated with growth medium supplemented with a freshly
made solution of 5 μmol/l calcein AM for 30 min at 37°C. Calcein AM
is intracellularly converted into the GJ-permeable dye calcein.
After three consecutive washes with phosphate-buffered saline (PBS)
to remove excess dye, the donor cells were trypsinized and seeded
onto the receiver cells at a 1:150 donor/receiver ratio. They were
allowed to form GJs for 4 h at 37°C and then examined under a
fluorescence microscope (IX71; Olympus, Tokyo, Japan). Images were
captured of ~20 cells per well (magnification, ×400) to count
receiver cells containing calcein per donor cell. The average
number of receiver cells containing calcein per donor cell was used
as a measure of the GJIC.
Western blotting
Following three washes with ice-cold PBS, cells were
lysed using lysis buffer (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM
EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM
Na3VO4, 1 mM β-glycerophosphate, 1:1,000
protease inhibitors). The cell lysate was sonicated and centrifuged
at 14,167 × g for 30 min at 4°C. The DC protein assay kit was used
to determine the protein concentration (Bio-Rad, Hercules, CA,
USA). A total of 20 μg of protein from each sample was analyzed by
sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis
(PAGE) and transferred to nitrocellulose blotting membranes,
followed by immunoblotting. Monoclonal antibodies against HA IgG
(1:1,000) or β-actin (1:10,000) were used. Immunopositive bands
were visualized using the Amersham ECL™ Plus Western Blotting
Detection kit (GE Healthcare).
Colony-forming assay
All experiments of cell exposure to cisplatin were
performed in the dark at 37°C. Baicalein was dissolved in DMSO.
When combined treatment with cisplatin and baicalein was performed,
baicalein was added to the cells 3 h prior to cisplatin addition.
The GJ inhibitor oleamide was added to the cell culture medium at a
50 μM concentration 3 h prior to the exposure to cisplatin.
Cisplatin toxicity was assessed by a standard colony
forming assay as described by Jenser et al (5) with a few modifications. Briefly,
cells were cultured at low (100 cells/cm2) or high
density (30,000 cells/cm2), corresponding to conditions
permissive of gap junction formation or not, respectively. For the
high-density condition, cells were grown to confluence prior to
cisplatin exposure. Following treatment with cisplatin for 1 h,
cells were washed with PBS, harvested by trypsinization, counted,
and seeded onto 6-well dishes at a 500 cells/well density. The
cells were incubated for an additional 7-day period, and next fixed
and stained with 1% crystal violet in ethanol. Colonies containing
≥50 cells were counted. Colony formation rates were normalized to
the efficiency of colony formation of cells not treated with drugs
(control). For the low-density condition, cells were seeded onto
6-well plates at a 100 cells/ml density. After 4 h adherence, cells
were exposed to cisplatin and then replenished with fresh medium.
They were rinsed and assessed for colony formation as described
above. Cells in this condition were unable to form GJs, since there
is no possibility to contact each other at such low density.
To avoid discrepancy in results caused by the fact
that cells are at different cell cycle phases, we incubated the
cells in serum-free medium for 24 h prior to the addition of
cisplatin, to ensure cell synchronicity at the G1 phase.
Statistical analysis
Data from the experiments of different treatments
were analyzed by unpaired Student’s t-tests using the SigmaPlot
10.0 software (Systat Software Inc., San Jose, CA, USA). Data are
presented as mean ± SEM. P<0.05 was considered to indicate
statistically significant differences.
Results
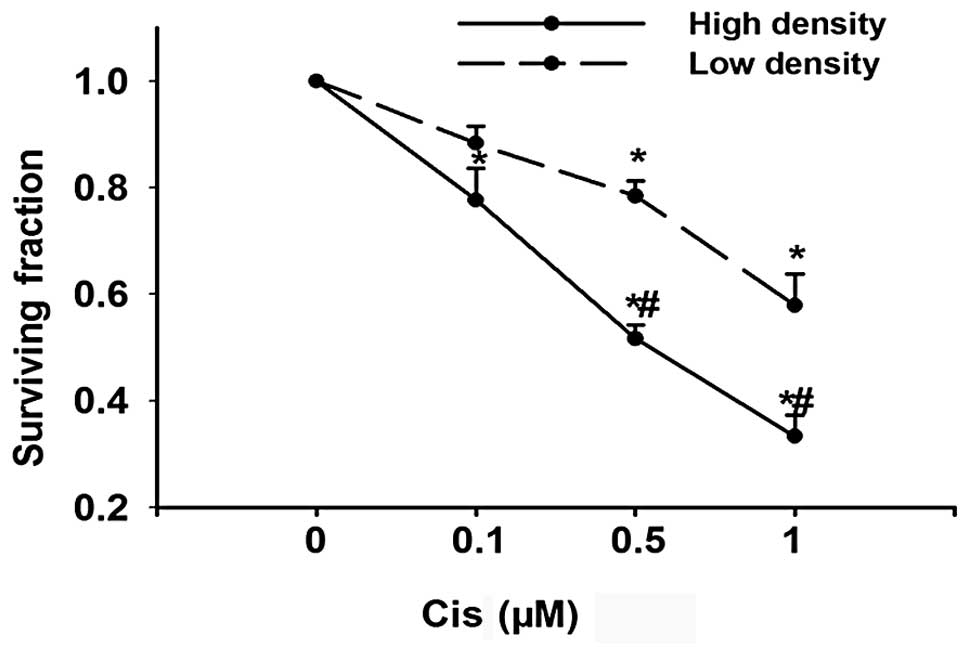
Cisplatin cytotoxicity is cell
density-dependent
GJ channels are formed by the end-to-end docking of
two hemichannels in adjacent cells; GJIC occurs only when cells
contact each other. As an initial experiment to determine the
effect of GJIC on cisplatin toxicity, Cx26-expressing HeLa cells
were cultured under conditions where GJ formation was possible
(high density; 30,000 cells/cm2) or not (low density;
100 cells/cm2). In both conditions, cisplatin caused
cell death in a concentration-dependent manner. However, the toxic
effect of cisplatin was substantially greater at high-density
compared to low-density cultures (Fig.
1). This indicates that cell culture density might have an
effect on cisplatin cytotoxicity. The concentration of cisplatin
used in the experiment was within the therapeutic range used during
chemotherapy (17).
Effects of cell density are due to
GJIC
The formation of GJs is only one of the numerous
differences between cells cultured at low and high density. To
determine whether the effects of high cell density were due to
GJIC, GJ coupling was examined in the cultures under different
conditions of connexin expression and chemical inhibition.
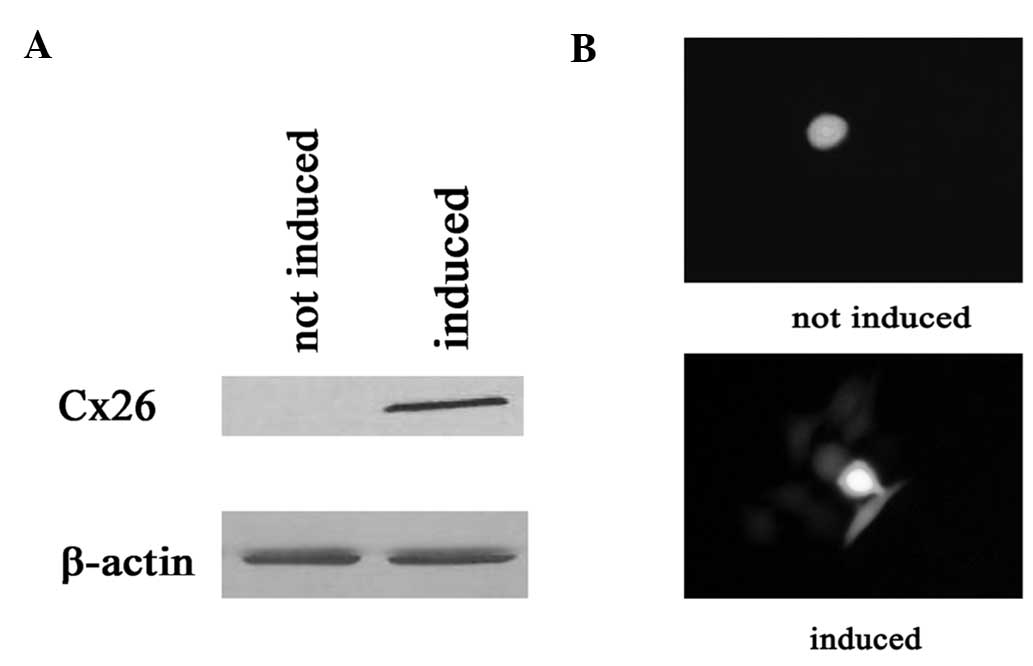
First, connexin expression was studied in
Cx26-expressing HeLa cells. Connexin expression was induced by
incubation with 1 μg/ml doxycycline for 48 h (see Materials and
methods). Fig. 2A and B show the
expression of connexin and GJ dye coupling 48 h following exposure
to doxycycline.
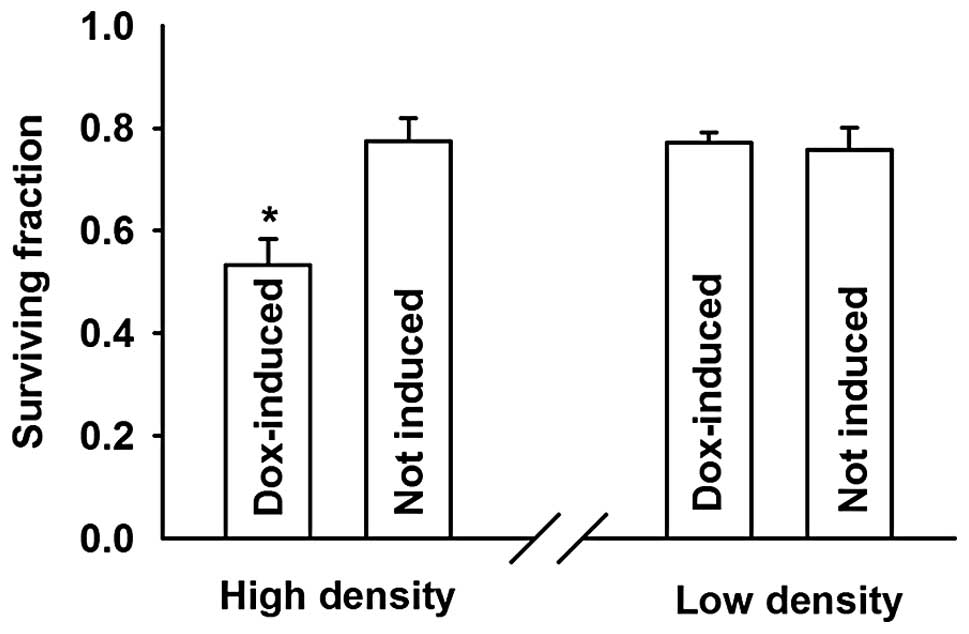
The cells treated with doxycycline (expressing
connexin, GJs formed) showed a markedly higher sensitivity to
cisplatin at high compared to low density growth conditions. The
surviving fraction decreased from 0.78±0.04 to 0.53±0.05,
respectively. Notably, the inhibition of cell survival by cisplatin
was not affected by addition of doxycycline in low density cultures
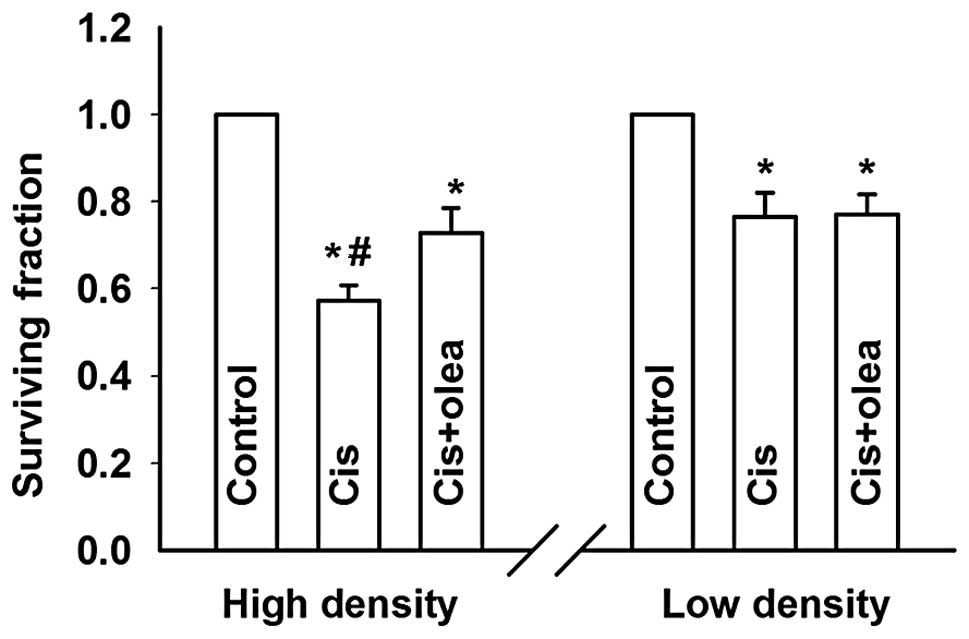
(Fig. 3).
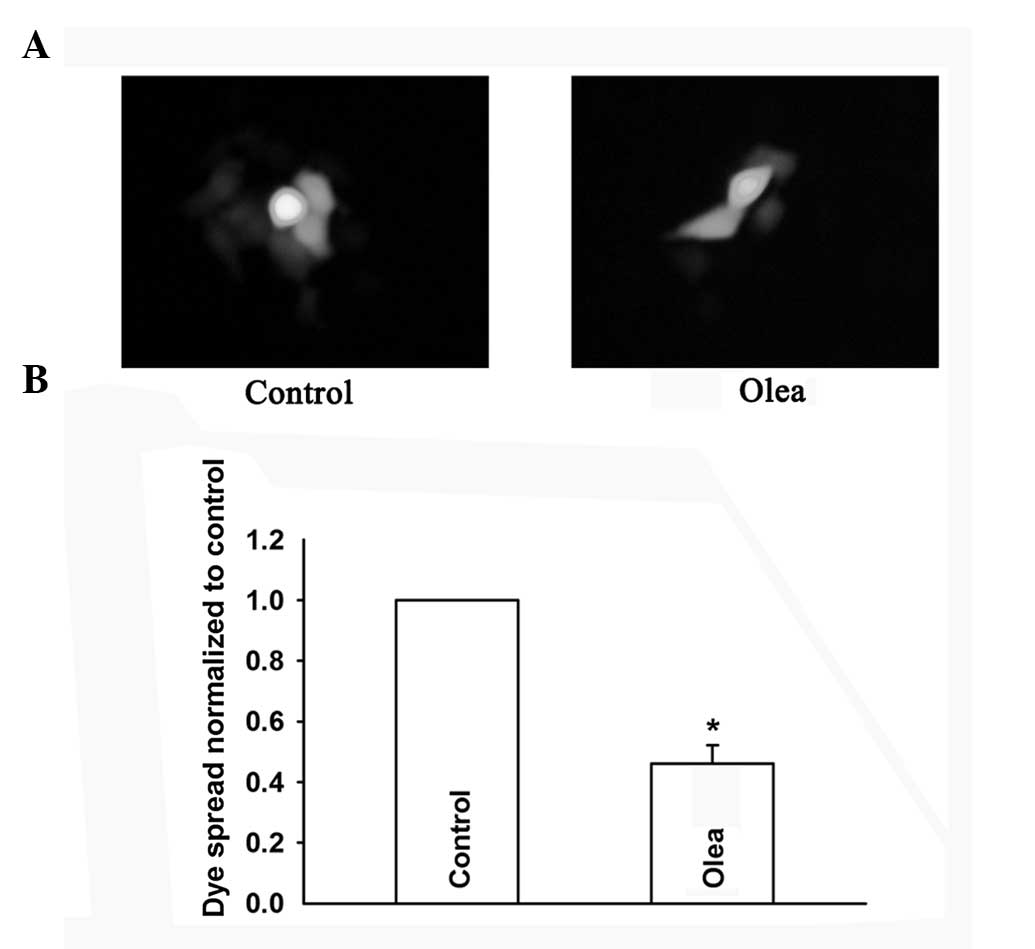
To further assess the role of GJIC on cell
density-dependent cisplatin sensitivity, oleamide, a GJ channel
inhibitor (18), was used to
inhibit GJIC in HeLa cells, and its effect was assessed by the GJ
dye-coupling assay (Fig. 4A and
B). In low-density cultures (without GJIC), treatment with
oleamide did not affect cisplatin toxicity. However, in
high-density cultures (with GJIC), cisplatin toxicity was
attenuated upon inhibition of GJIC by oleamide, manifested as a
significant increase of the cell surviving fraction from 0.57±0.04
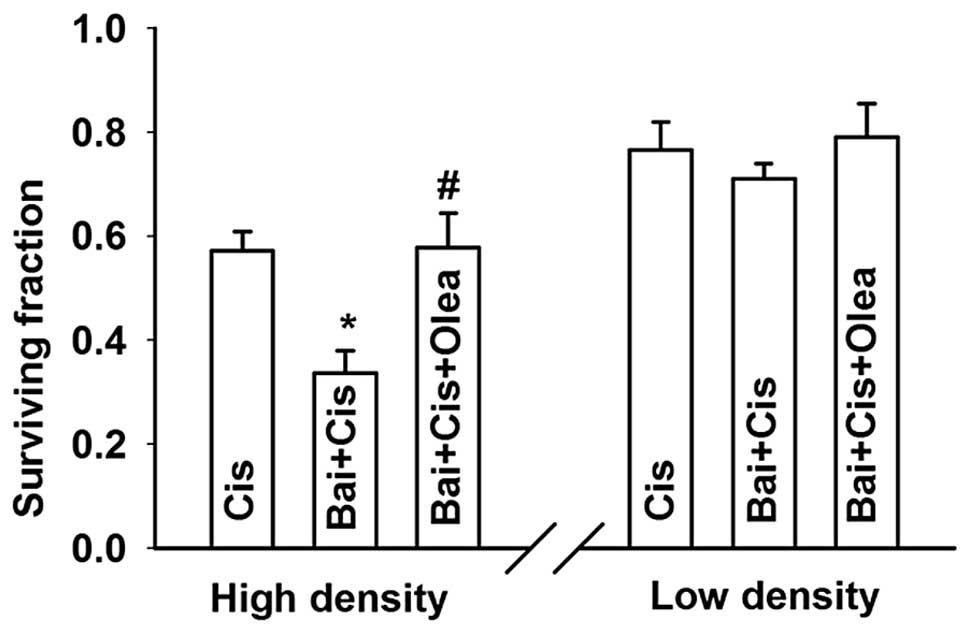
to 0.73±0.06 (Fig. 5). Thus,
inhibition of GJIC during the 1 h of exposure to cisplatin reversed
the effect of high density cell culture on cisplatin toxicity.
These results indicated that GJIC enhances cisplatin
toxicity and fully accounts for the observed density-dependent
effect of cisplatin toxicity.
Effects of baicalein on cell
viability
The formation of GJs composed of Cx26 affected the
cytotoxicity of cisplatin. This observation was consistent with our
hypothesis that enhancement of the GJ function would increase the
cytotoxic action of cisplatin, and thus increase cisplatin
sensitization. Would baicalein affect the GJ function and cisplatin
cytotoxicity? To determine this, we first examined the cytotoxic
effect of baicalein using the SRB assay.
As shown in Fig. 6,
baicalein had no effect on cell viability until the concentration
reached 100 μM. Thus, the concentrations of baicalein used in the
following experiments (≤0.1 μM) had no cytotoxic effect on HeLa
cells.
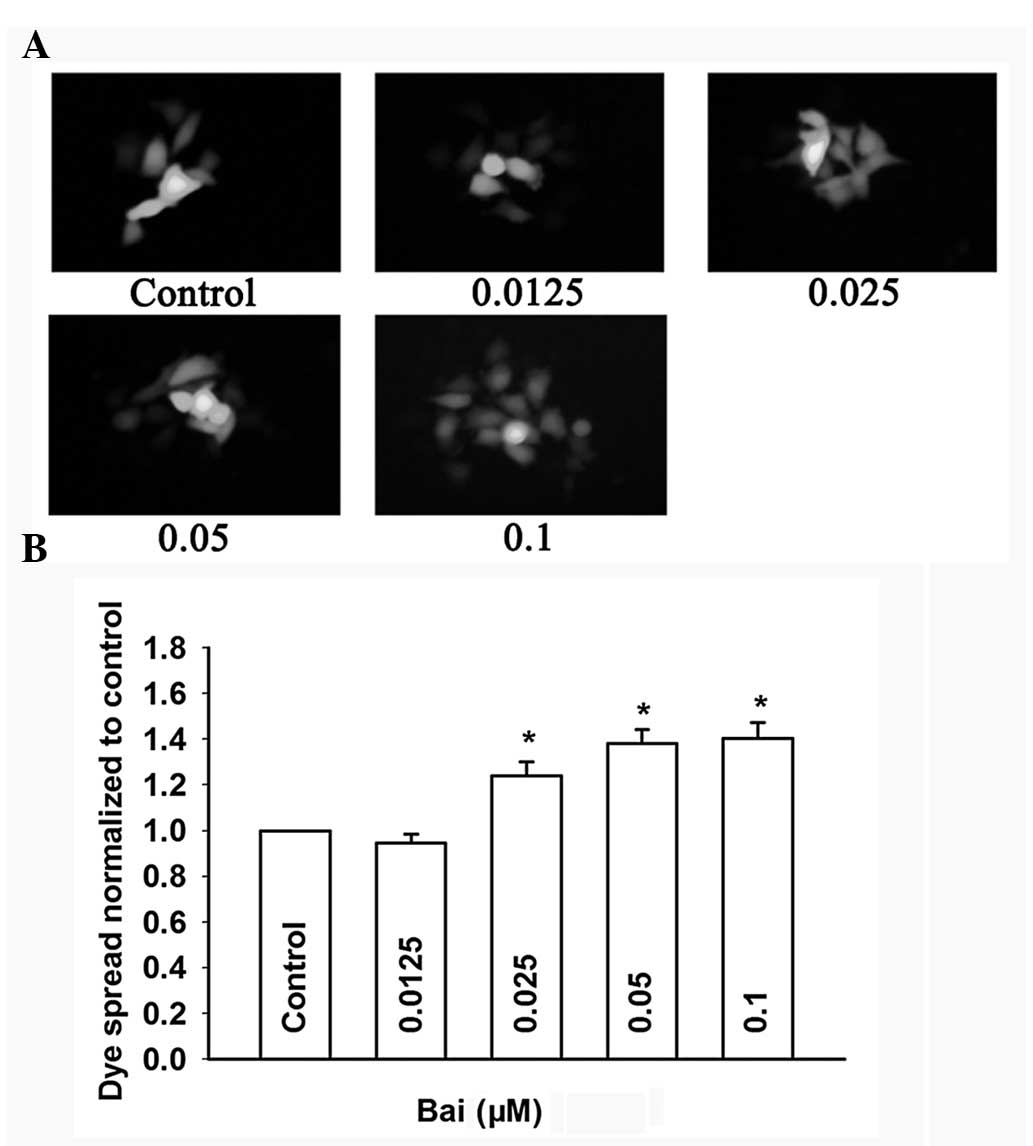
Effects of baicalein on GJ function
The effect of baicalein on dye coupling among
cultured cells was assayed by the parachute assay. Donor cells were
labeled with the junction-permeable dye calcein and then seeded
onto unlabeled receiver cells with different concentrations of
baicalein for 4 h. GJ intercellular communication was expressed as
the number of receiver cells receiving calcein from a labeled cell,
normalized to that of control cells (non-treated). As shown in
Fig. 7A and B, baicalein markedly
increased the dye spread from donor cells to receiver cells in a
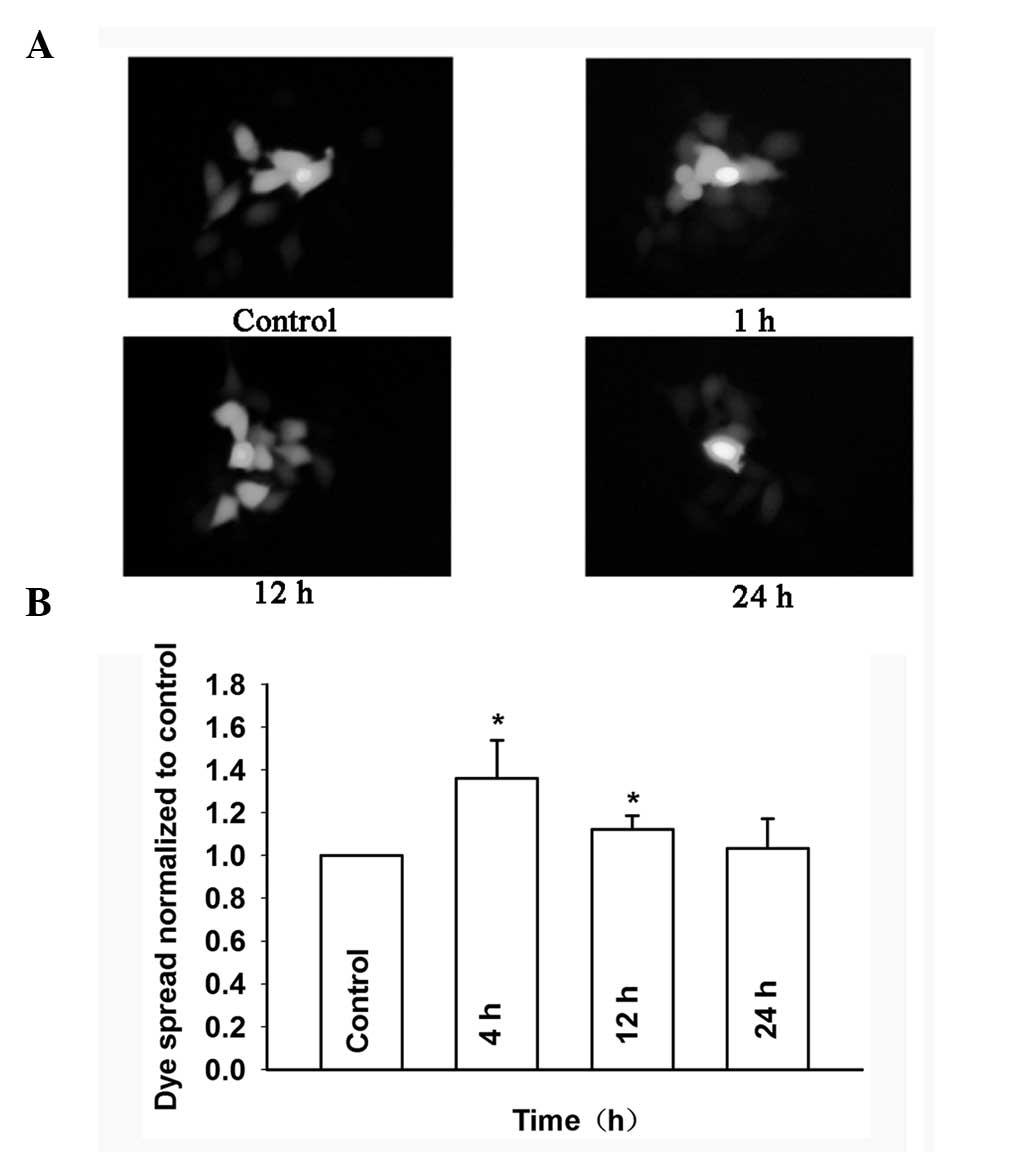
dose-dependent manner. Fig. 8A and
B show that the dye spread through GJs treated with 0.1 μM
baicalein was markedly decreased from 4 to 24 h in Cx26-transfected
HeLa cells. These results indicated that baicalein can enhance GJIC
in Cx26-expressing HeLa cells.
Effects of baicalein on cisplatin
cytotoxicity and GJ
The effects of baicalein on cisplatin-induced
cytotoxicity were examined in HeLa cells expressing Cx26. Cells
seeded at high or low cell density were treated with baicalein (0.1
μM) for 3 h, followed by exposure to 0.5 μM cisplatin + baicalein
for 1 h. The clonogenic survival of HeLa cells was assessed 7 days
following exposure to cisplatin and baicalein. Baicalein enhanced
cisplatin cytotoxicity in high-density cultures but had no effect
in low-density cultures; the surviving fraction substantially
decreased from 0.57±0.04 in low-density cultures to 0.34±0.04 in
high-density cultures. This effect of baicalein was only observed
in conditions permissive of GJ formation.
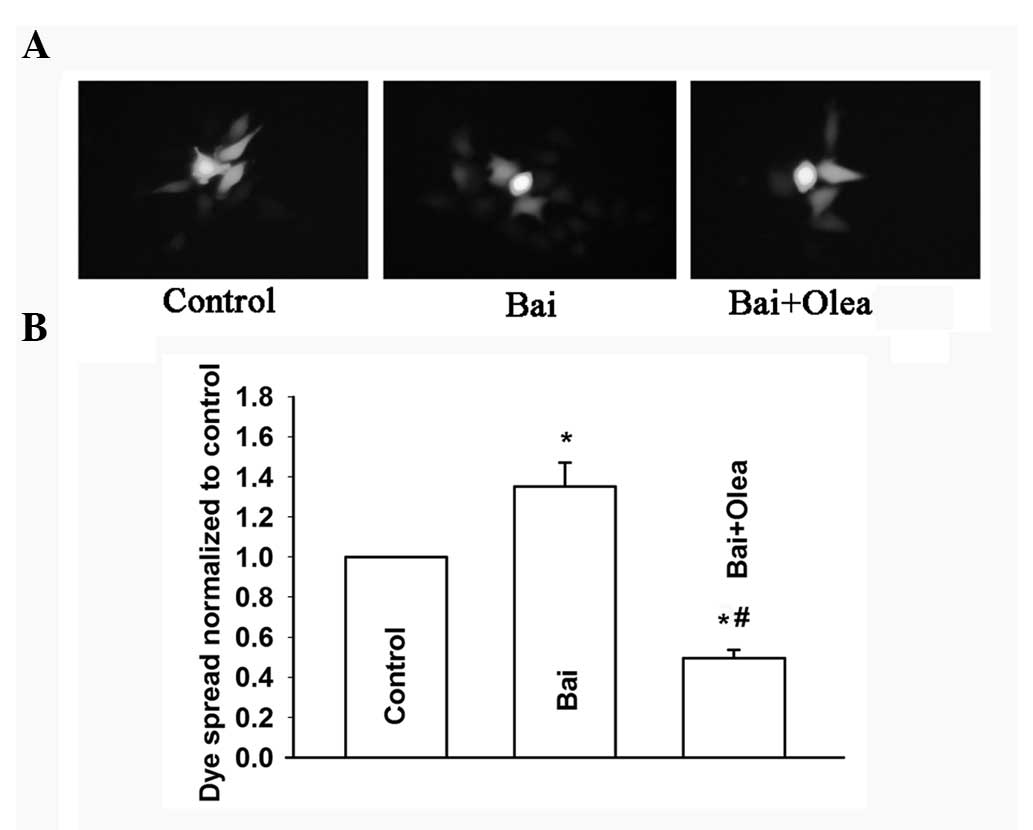
To further assess the role of GJIC in the
improvement of cisplatin sensitivity induced by baicalein, oleamide
was used to inhibit GJIC in HeLa cells (Fig. 9A and B). Consistent with our
previous results, treatment with oleamide and baicalein did not
affect cisplatin toxicity in low-density cell cultures (without
GJIC). However, the improvement of cisplatin toxicity induced by
baicalein was attenuated upon inhibition of GJIC by oleamide in
high-density cultures (with GJIC). An increase of the surviving
fraction, from 0.34±0.04 to 0.58±0.07, was observed (Fig. 10). These results showed that
oleamide can inhibit the improvement of the dye spread through GJs
induced by baicalein, and thus reduce cisplatin cytotoxicity
improved by baicalein at high cell density. Therefore, baicalein
improves cisplatin cytotoxicity by enhancing GJIC in
Cx26-expressing HeLa cells.
Effects of baicalein on connexin
expression
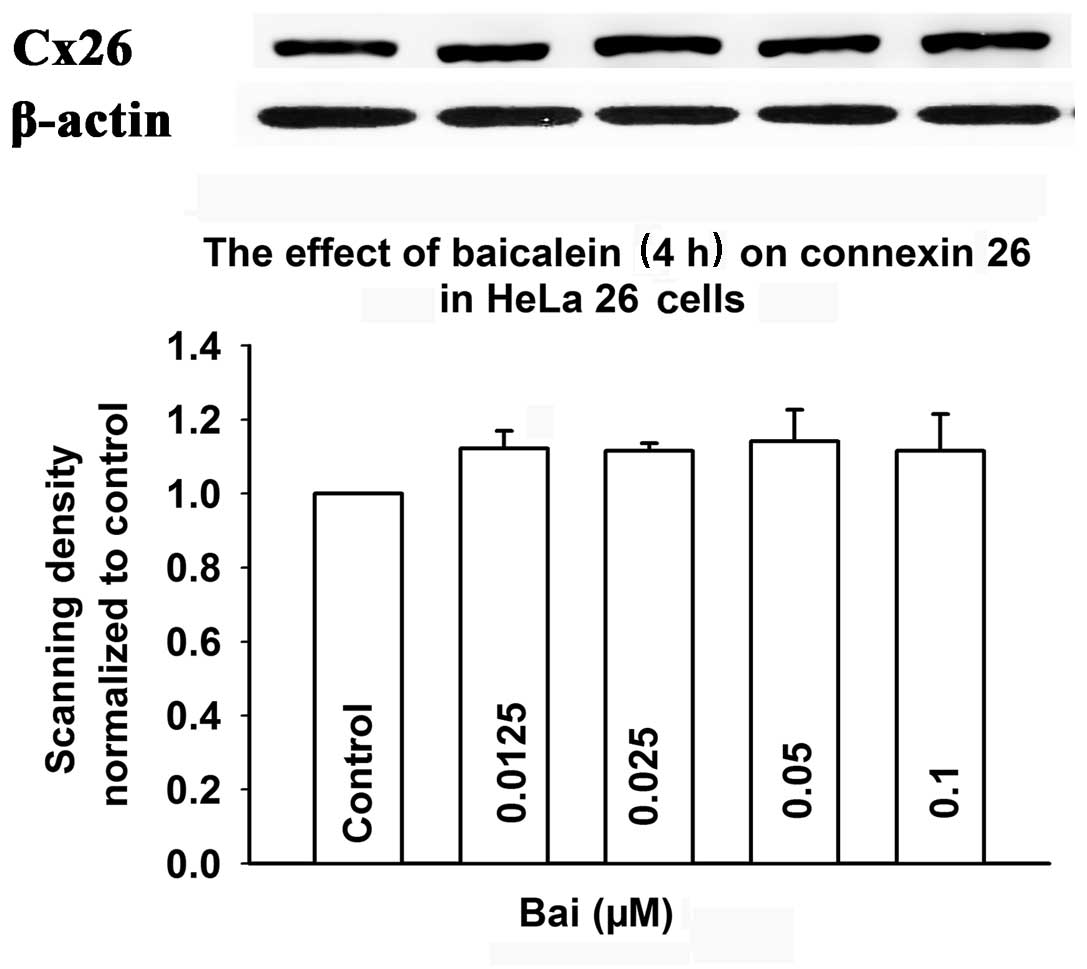
To determine whether baicalein affects the
expression of connexin, the level of Cx26 was assessed by western
blotting in cells induced by doxycycline and exposed to baicalein.
Fig. 11 shows that treatment with
baicalein (0.0125–0.1 μM) for 4 h did not affect Cx26 expression.
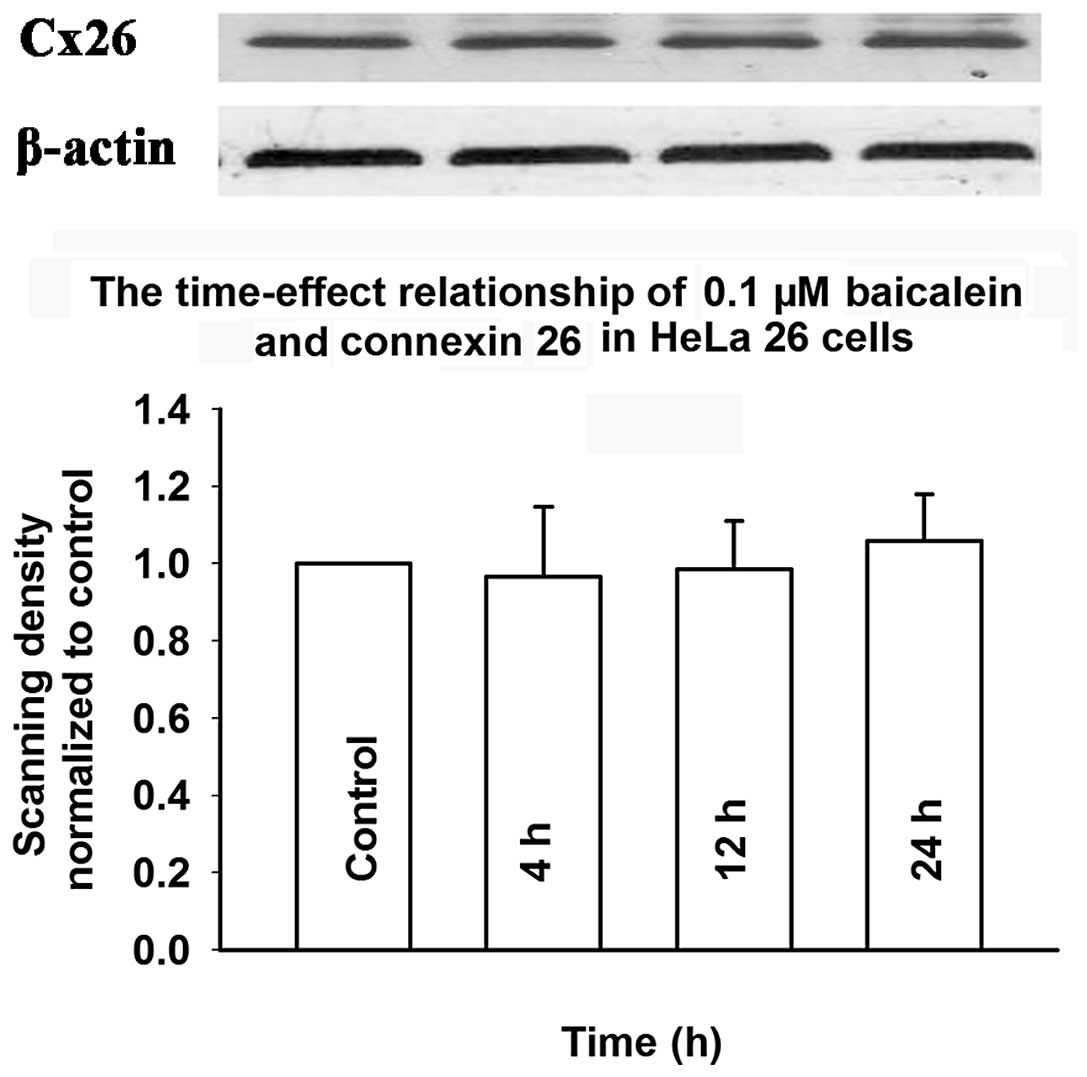
Fig. 12 shows that treatment with
baicalein (0.1 μM) for 4 to 24 h did not change Cx26 expression.
These results suggested that the mechanism of baicalein-induced
enhancement of GJIC does not involve an increase in the expression
of connexin.
Discussion
Cx26 is widely and highly expressed in numerous
tissues such as liver, mammary gland, skin and appendages, mucous
membranes, pancreas, intestine, endometrium, lung and brain
(19). GJs composed by Cx26 play
an important role in the ‘bystander effect’. HeLa cells transfected
with the herpes simplex virus thymidine kinase gene (HSV-tk)
were more susceptible to death when expressing Cx26 and forming
GJs. The bystander effect disappeared when GJIC was inhibited
(20). This phenomenon was also
observed in a bladder cancer cell line transfected with
HSV-tk. Cx26 expression and the induction of functional GJs
facilitated HSV-tk/ganciclovir (GCV) gene therapy through
the bystander effect (21).
Gemcitabine administration in mice bearing tumors that overexpress
Cx26 resulted in marked regression of the tumor (22). These findings have prompted us to
study the effect of Cx26 channels on cisplatin cytotoxicity.
Consistent with previous reports (4–6), the
results of the present study showed that cisplatin toxicity is
GJ-dependent. Cisplatin toxicity was enhanced in high-density
cultures where GJIC occurred, but not in low-density cultures,
which lacked junction contacts. Moreover, cisplatin cytotoxicity
was attenuated upon inhibition of GJIC by oleamide. Our results
thus demonstrated that GJs composed of Cx26 play an important role
in cisplatin cytotoxicity. We further argue that cisplatin
cytotoxicity can be increased by enhancing GJIC.
The antitumor effects of baicalein have been
extensively studied (9–13). Few studies however investigated the
effect of baicalein on GJ function and cisplatin-induced
cytotoxicity. Our results showed that treatment with baicalein
(0.0125–0.1 μM) for 4 h increased the cell coupling mediated by the
Cx26 channels. Importantly, we found that in high-density cultures,
where there was substantial intercellular contact, baicalein
enhanced the function of GJ and the cytotoxicity of cisplatin, with
the cell surviving fraction decreasing from 0.57±0.04 to 0.34±0.04.
This decrease was not observed in low-density cultures, which
lacked intercellular contacts. The results of the experiments with
the GJ inhibitor oleamide further supported the link between GJIC
and the enhancement of cisplatin cytotoxicity. In high-density
cultures, inhibition of GJIC via oleamide attenuated the
improvement of cisplatin cytotoxicity induced by baicalein, with
the surviving fraction significantly increasing from 0.34±0.04 to
0.58±0.07. By contrast, in low-density cultures, oleamide did not
significantly alter the effects of baicalein. Our study provided
the first evidence that the enhancement of cisplatin cytotoxicity
by baicalein may be achieved through the enhancement of the GJ
function in HeLa cells.
It is very common in clinical tumor therapy to
combine chemotherapeutic agents to obtain additive or synergistic
effects. Cisplatin is one of the most widely used cancer
chemotherapeutic agents. Baicalein was also shown in recent studies
to exert beneficial effects in clinic tumor therapy (9–11).
While the underlying mechanism was not clarified, it was
hypothesized that baicalein inhibits multi-drug resistance gene
expression and decreases the level of the permeability glycoprotein
(P-gp), thus increasing the intracellular concentration of
chemotherapeutic agents (23). Our
study found that the Cx26 channel may also mediate the improvement
of cisplatin cytotoxicity induced by baicalein. We also found that
in the presence of GJ, baicalein (0.1 μM) is not toxic to HeLa
cells, but increases the cytotoxicity of cisplatin by ~23%,
suggesting that when baicalein is combined with cisplatin, the
dosage of the latter can be reduced without compromising the
efficiency of its tumoricidal effect.
As for the mechanism of enhancement of GJIC induced
by baicalein, our results showed that baicalein did not affect the
expression of Cx26 at any tested concentration (0.0125–0.1 μM) or
duration of treatment (4–24 h). Therefore, baicalein improved GJIC
without changing the expression of Cx26. There are numerous factors
modulating the activity of GJ channels. Cx26 is a connexin, and
connexins have long been reported to be regulated by
phosphorylation at serine and threonine residues (24,25).
However, Cx26 is not a phosphoprotein (26,27).
It is thus unlikely that baicalein can modulate the activity of
Cx26-composed GJs by phosphorylation. A number of studies showed
that flavonoids mostly influence GJ function by changing the
expression of connexin and its phosphorylation status (7,8,28,29).
It was reported that two flavonoids, apigenin and tangeretin, can
counteract tumor promoter-induced inhibition of intercellular
communication of rat liver epithelial cells without changing
connexin 43 and in its phosphorylation state (7). Our results are similar to results of
this study, but the mechanism by which baicalein enhances GJ
function in HeLa cells was not elucidated. Nevertheless, our
results demonstrated that baicalein enhances GJ function without
changing connexin expression. Moreover, the present results also
suggest that baicalein may be developed as a non-toxic
chemo-adjuvant and could be used to increase the efficacy of
existing anticancer chemotherapies by enhancing the GJ
functionality.
Acknowledgements
This study was supported by grants from the
Department of Science and Technology of Xinjiang Uygur Autonomous
Regions (no. 201233150) and the National Natural Science Foundation
of China (nos. 30973434 and 30901807).
References
|
1
|
Giaccone G: Clinical perspectives on
platinum resistance. Drugs. 59(Suppl 4): 9–17. 2000. View Article : Google Scholar
|
|
2
|
Fuertes MA, Alonso C and Pérez JM:
Biochemical modulation of cisplatin mechanisms of action:
enhancement of antitumor activity and circumvention of drug
resistance. Chem Rev. 103:645–662. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
King TJ and Bertram JS: Connexins as
targets for cancer chemoprevention and chemotherapy. Biochim
Biophys Acta. 1719:146–160. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalvelyte A, Imbrasaite A, Bukauskiene A,
Verselis VK and Bukauskas FF: Connexins and apoptotic
transformation. Biochem Pharmacol. 66:1661–1672. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jensen R and Glazer PM:
Cell-interdependent cisplatin killing by Ku/DNA-dependent protein
kinase signaling transduced through gap junctions. Proc Natl Acad
Sci USA. 101:6134–6139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Q, You T, Yuan D, Han X, Hong X, He
B, Wang L, Tong X, Tao L and Harris AL: Cisplatin and oxaliplatin
inhibit gap junctional communication by direct action and by
reduction of connexin expression, thereby counteracting cytotoxic
efficacy. J Pharmacol Exp Ther. 333:903–911. 2010. View Article : Google Scholar
|
|
7
|
Chaumontet C, Bex V, Gaillard-Sanchez I,
Seillan-Heberden C, Suschetet M and Martel P: Apigenin and
tangeretin enhance gap junctional intercellular communication in
rat liver epithelial cells. Carcinogenesis. 15:2325–2330. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conklin CM, Bechberger JF, MacFabe D,
Guthrie N, Kurowska EM and Naus CC: Genistein and quercetin
increase connexin43 and suppress growth of breast cancer cells.
Carcinogenesis. 28:93–100. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng YH, Li LA, Lin P, Cheng LC, Hung CH,
Chang NW and Lin C: Baicalein induces G1 arrest in oral cancer
cells by enhancing the degradation of cyclin D1 and activating AhR
to decrease Rb phosphorylation. Toxicol Appl Pharmacol.
263:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, Hines
OJ, Gukovskaya AS, Go VL and Eibl G: Baicalein, a component of
Scutellaria baicalensis, induces apoptosis by Mcl-1
down-regulation in human pancreatic cancer cells. Biochim Biophys
Acta. 1813:1465–1474. 2011.
|
|
12
|
Chen CH, Huang TS, Wong CH, Hong CL, Tsai
YH, Liang CC, Lu FJ and Chang WH: Synergistic anti-cancer effect of
baicalein and silymarin on human hepatoma HepG2 cells. Food Chem
Toxicol. 47:638–644. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Song L, Cai L, Wei R, Hu H and
Jin W: Effects of baicalein on apoptosis, cell cycle arrest,
migration and invasion of osteosarcoma cells. Food Chem Toxicol.
53:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koreen IV, Elsayed WA, Liu YJ and Harris
AL: Tetracycline-regulated expression enables purification and
functional analysis of recombinant connexin channels from mammalian
cells. Biochem J. 383:111–119. 2004. View Article : Google Scholar
|
|
15
|
Papazisis KT, Geromichalos GD, Dimitriadis
KA and Kortsaris AH: Optimization of the sulforhodamine B
colorimetric assay. J Immunol Methods. 208:151–158. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldberg GS, Bechberger JF and Naus CC: A
pre-loading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
17
|
Erdlenbruch B, Nier M, Kern W, Hiddemann
W, Pekrun A and Lakomek M: Pharmacokinetics of cisplatin and
relation to nephrotoxicity in paediatric patients. Eur J Clin
Pharmacol. 57:393–402. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan X, Cravatt BF, Ehring GR, Hall JE,
Boger DL, Lerner RA and Gilula NB: The sleep-inducing lipid
oleamide deconvolutes gap junction communication and calcium wave
transmission in glial cells. J Cell Biol. 139:1785–1792. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krysko DV, Leybaert L, Vandenabeele P and
D’Herde K: Gap junctions and the propagation of cell survival and
cell death signals. Apoptosis. 10:459–469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mesnil M, Piccoli C and Yamasaki H: A
tumor suppressor gene, Cx26, also mediates the bystander effect in
HeLa cells. Cancer Res. 57:2929–2932. 1997.PubMed/NCBI
|
|
21
|
Tanaka M, Fraizer GC, De La Cerda J,
Cristiano RJ, Liebert M and Grossman HB: Connexin 26 enhances the
bystander effect in HSVtk/GCV gene therapy for human bladder cancer
by adenovirus/PLL/DNA gene delivery. Gene Ther. 8:139–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garcia-Rodríguez L, Pérez-Torras S, Carrió
M, Cascante A, García-Ribas I, Mazo A and Fillat C: Connexin-26 is
a key factor mediating gemcitabine bystander effect. Mol Cancer
Ther. 10:505–517. 2011.PubMed/NCBI
|
|
23
|
Li DR, Zhang W, Tang DP, Tu WS and Qin J:
A study of the reverse effect of scutellarein on
multidrug-resistant human ovarian carcinoma cell line A2780/ADM.
Tumor. 24:111–113. 2004.
|
|
24
|
Lampe PD and Lau AF: The effects of
connexin phosphorylation on gap junctional communication. Int J
Biochem Cell Biol. 36:1171–1186. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Warn-Cramer BJ and Lau AF: Regulation of
gap junctions by tyrosine protein kinases. Biochim Biophys Acta.
1662:81–95. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Traub O, Look J, Dermietzel R, Brümmer F,
Hülser D and Willecke K: Comparative characterization of the 21-kD
and 26-kD gap junction proteins in murine liver and cultured
hepatocytes. J Cell Biol. 108:1039–1051. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sáez JC, Nairn AC, Czernik AJ, Spray DC,
Hertzberg EL, Greengard P and Bennett MV: Phosphorylation of
connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent
protein kinase, protein kinase C and
Ca2+/calmodulin-dependent protein kinase II. Eur J
Biochem. 192:263–273. 1990.PubMed/NCBI
|
|
28
|
Chaumontet C, Droumaguet C, Bex V,
Heberden C, Gaillard-Sanchez I and Martel P: Flavonoids (apigenin,
tangeretin) counteract tumor promoter-induced inhibition of
intercellular communication of rat liver epithelial cells. Cancer
Lett. 114:207–210. 1997. View Article : Google Scholar
|
|
29
|
Huard C, Druesne N, Guyonnet D, Thomas M,
Pagniez A, Le Bon AM, Martel P and Chaumontet C: Diallyl disulfide
(DADS) enhances gap-junctional intercellular communication by both
direct and indirect mechanisms in rat liver cells. Carcinogenesis.
25:91–98. 2004. View Article : Google Scholar : PubMed/NCBI
|