1. Introduction
Glaucoma is a serious and irreversible disease that
causes blindness and is commonly termed the ‘silent thief of
sight’, because loss of vision often occurs gradually. In the
majority of cases, glaucoma is associated with intraocular pressure
and low blood perfusion, and is characterized by progressive death
of retinal ganglion cells (RGCs). As glaucoma progresses, it may
lead to optic nerve degeneration and loss of retinal nerve fibers,
and ultimately result in the loss of vision. So, facilitating the
survival of RGCs may be an effective therapeutic target in the
treatment of glaucoma. Within the retina, RGCs are the only
projecting neurons that transmit visual information to the brain
(1), representing the third-order
neurons of the visual pathway (2).
Following optic nerve injury, RGCs are unable to regenerate their
axons and cell apoptosis is rapidly initiated (3).
Numerous studies have indicated that apoptosis may
be the final common pathway for RGC death in glaucoma (4–7).
However, it has become increasingly evident that apoptosis and
necrosis alone do not adequately encompass cell death. Studies have
revealed that although apoptosis is actively executed by
cysteine-aspartic acid proteases (caspase), RGC death occurs
independently of caspase-mediated pathways (8,9),
which are a family of cysteine proteases that are also essential in
necrosis and inflammation (10).
These data imply that RGC death involves apoptosis as well as
non-apoptotic programmed cell death (PCD). Autophagy is a type of
cell death that is morphologically distinct from apoptosis and
which usually mediates cytoprotection, avoiding the apoptotic or
necrotic demise of cells (11,12).
Activation of autophagy reduces or inhibits the apoptosis of the
cells. Several studies have provided evidence that the survival of
RGCs may be improved by autophagy following optic nerve axotomy in
mice (13,14). These investigations demonstrated
that besides from apoptosis and necrosis, other pathways may also
be responsible for RGC death. The accumulating literature providing
evidence of the mechanisms of non-apoptotic RGC death suggests that
other possible pathways are involved.
Cell death is categorized into PCD and passive
(necrotic) cell death (15,16).
While apoptosis is the best characterized form of PCD, other
non-apoptotic modes also exist, including autophagy, paraptosis and
mitotic catastrophe (16).
Autophagy, as a form of PCD, is a self-degradative process that
involves the breakdown of intracellular proteins and organelles
(17). Paraptosis is a novel type
of PCD, that is caspase independent and is characterized by
cytoplasmic vacuolization derived from the endoplasmic reticulum
and/or mitochondria swelling. Paraptosis is distinguished from
apoptosis by its non-response to caspase inhibitors (that block
apoptosis) and lack of apoptotic morphology. This type of cell
death is also insensitive to autophagic inhibitors and lacks DNA
fragment and poly (ADP-ribose) polymerase (PARP) cleavage (15,18).
Several studies have identified AIP-1/Alix and
phosphatidylethanolamine binding protein 1 (PEBP-1) as inhibitors
of paraptosis (15,19).
Paraptosis is fundamentally different from other
forms of PCD, such as apoptosis, autophagy and oncosis. Apoptosis
typically involves the activation of caspases, responds to caspase
inhibitors and exhibits morphological features including chromatin
condensation, nuclear fragmentation and the formation of apoptotic
bodies. Autophagy exhibits a contrasting morphology during
cytoplasmic vacuolization in comparison with paraptosis. The
process of autophagy is the formation of autophagosomes, which are
double-membrane vesicles responsible for delivering long-lived
proteins and excess or damaged organelles, into the lysosome for
degradation (20). In addition,
paraptosis is not inhibited by autophagy inhibitors, including
3-mathyladenine (3-MA). Oncosis is another form of non-apoptotic
PCD, which is distinguished from paraptosis and occurs in the
destroyed cell membrane.
2. Hypothesis
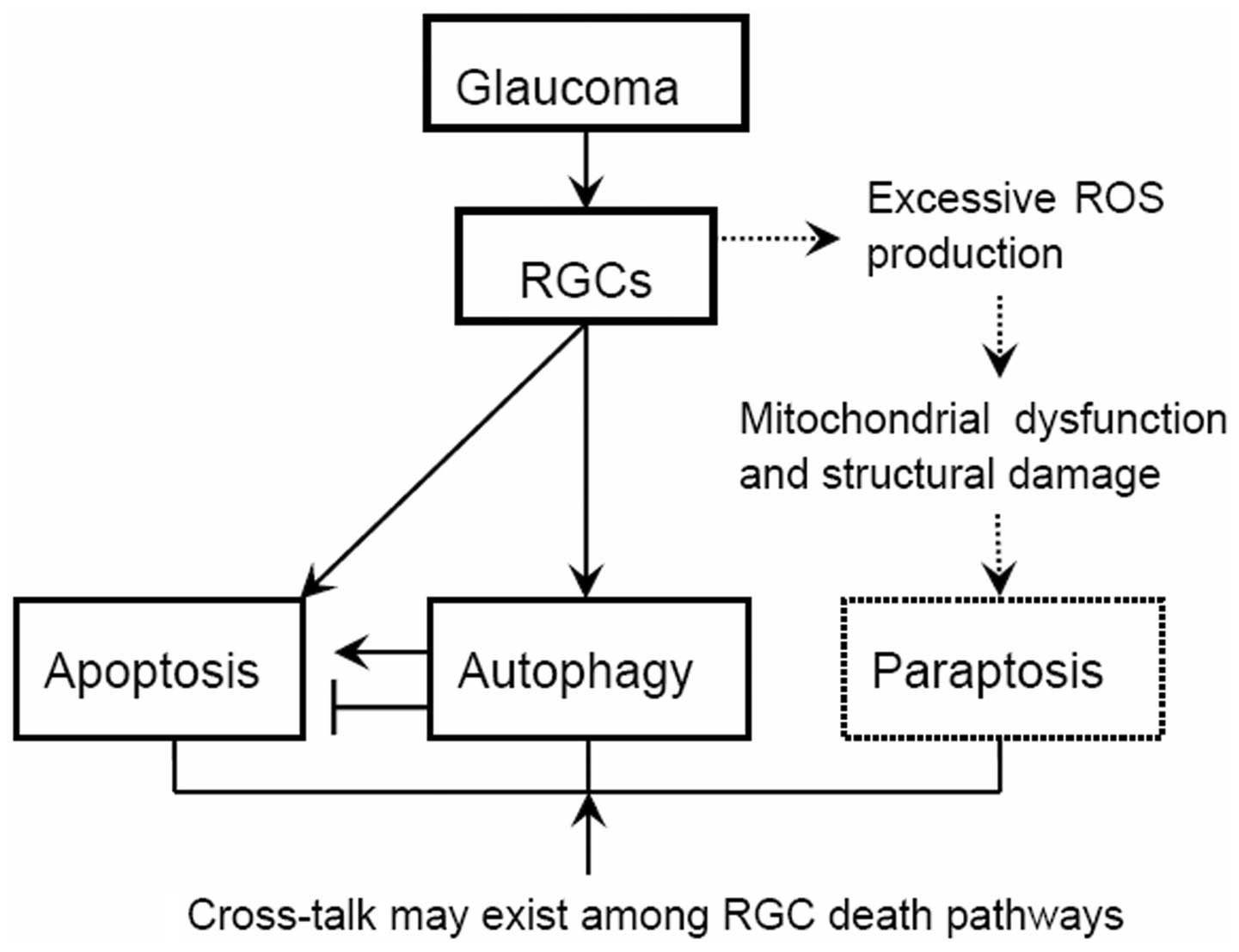
In the present review, it was hypothesized that the
progressive death of the RGCs in glaucoma is associated with a
distinguished form of cell death, aside from the well characterized
apoptosis, necrosis and autophagy, termed paraptosis (Fig. 1). Paraptosis may be triggered in
the early stages of glaucoma (such as the appearance of optic nerve
abnormalities, consistent with glaucoma, but with no visual field
abnormalities). Furthermore, it was hypothesized that paraptosis
and apoptosis may, together with autophagy, occur alone or
simultaneously in RGCs, depending not only on the clinical severity
but also on the duration of glaucoma development.
The second hypothesis is that paraptosis in
glaucomatous RGCs may be derived from damage to the mitochondria.
Mitochondria destruction plays a key role in inducing paraptosis in
glaucomatous RGCs, and this may be associated with
mitochondria-derived reactive oxygen species (ROS) reactions.
3. Evidence of hypothesis
Paraptosis occurs in retinal pigment
epithelial cells (RPEs) and retinal Müller glial cells (RMGs)
Triamcinolone acetonide (TA), a synthetic
corticosteroid, has been demonstrated to induce retinal toxicity
via mechanisms predominantly associated with paraptosis in rat
RPEs, RMGs and in ARPE-19 cells (human RPE cells) in vitro
(21). However, whether TA induces
paraptosis in RGCs has not been investigated. Nevertheless, these
observations suggest that paraptosis may be triggered in retinal
cells in certain conditions.
Glaucomatous RGCs are associated with the
occurrence of paraptosis
Mechanisms of RGC death in glaucoma include
neurotrophic factor deprivation, hypoperfusion/ischemia, glial cell
activation, glutamate excitotoxicity and abnormal immune response
(4). Paraptosis may be the
mechanism of cell death in a number of pathological conditions,
including excitotoxicity, ischemia and neurodegeneration (19), which suggests that the mechanisms
of pathological damage in RGCs may be associated with the
occurrence of paraptosis.
In addition, numerous studies have proved the
importance of paraptosis in the nervous system. In one study,
polymorphonuclear leukocytes and macrophages induced evident
paraptosis in T9 glioma cells (22). Similarly, in human U251MG glioma
cells expressing the membrane form of macrophage colony-stimulating
factor (mM-CSF), cell death was induced by human monocytes in
vitro and these cells were rejected within immunodeficient mice
via the paraptosis pathway (23).
Furthermore, intracellular acidification, by inhibition of the
Na+/H+-exchanger, triggered
caspase-independent cell death that resembles paraptosis in
cerebellar granule neurons (24).
As is well established, RGCs are a specialized type of neuron that
are located near to the inner surface of the retina, which suggests
that paraptosis may also have a role in the pathophysiological
processes that lead to RGC damage.
Paraptosis may be underestimated
The widespread occurrence of apoptosis and autophagy
is readily recognized by numerous specific markers that are coupled
to these processes. However, due to a lack of specific markers
(19), paraptosis is often
concealed by the effects of apoptosis or autophagy (25,26)
and thus its occurrence and distribution may be underestimated.
Evidence demonstrates that paraptosis,
apoptosis and/or autophagy may occur simultaneously
Several studies have revealed that paraptosis,
accompanied by autophagy and apoptosis, was induced by
celastrol, a natural compound (25). Our previous investigations
demonstrated that the cell death pathway was concentration- and
time-dependent and associated with cell type. We identified that
honokiol, a constituent of Magnolia officinalis, induced
paraptosis at low concentrations. By contrast, at higher
concentrations, honokiol induced caspase-dependent apoptosis in
leukemic cells, and these two cellular death processes were in
sequence and parallel with the increase in honokiol concentration
(26,27). These results imply that, in the
same cell, more than one death program may be activated
simultaneously. Therefore, based on the hypothesis that
glaucomatous RGC death involves the process of paraptosis, we also
propose that paraptosis and apoptosis and/or autophagy, may occur
alone or simultaneously in RGCs.
High levels of ROS are important in RGC
death
ROS, including oxygen ions and peroxides, are
synthesized during the production of mitochondrial energy, and are
highly reactive in signaling pathways for regulating cell growth,
differentiation, survival and death. High levels of ROS are
important in the signaling of cell death in several neuronal
systems, including RGCs (28).
Mitochondria may produce enhanced ROS production, that may modulate
the autophagy process (29,30).
Evidence has revealed that optic nerve crush caused RGCs to produce
superoxide anions in response to external oxidative stress, as an
early step in signaling apoptosis (31). Furthermore, mitochondrial
superoxide was a critical signal in curcumin (a major active
component of turmeric)-induced paraptosis in malignant breast
cancer cells (32). From the above
analysis, we further hypothesize that mitochondria may generate
enhanced ROS production, that subsequently contributes to the
induction of paraptosis in glaucomatous RGCs.
4. Preliminary findings of hypothesis
Our results indicated that swelling mitochondria and
vacuoles were detected within 5 and 7 days, following optic nerve
crush injury by clamping, in RGCs of adult rats and that these
vacuoles originated from the mitochondria. In this investigation,
the optic nerve of adult wistar rats underwent crushing as
described previously (31). Male
wistar rats weighing between 200 and 250 g were purchased from the
Laboratory Animal Center, School of Medicine of Xi’an Jiaotong
University (Xi’an, Shaanxi, China). All animals received human care
in compliance with the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research. The present study was approved by
the ethics committee of Shaanxi Institute of Ophthalmology (Xi’an,
China). Briefly, the rats were anesthetized and the optic nerves
were gently crushed with blunt forceps for 50 sec, sparing the
retinal and optic disc circulations. The rats were then returned to
their cages and sacrificed at the time points of day 3, 5 or 7 and
retinal ultrastructure impairment was analyzed using a transmission
electron microscope (H-7650; Hitachi, Tokyo, Japan). All procedures
were performed aseptically and on the left eye, with the right eye
serving as a sham-operated control. The optic nerve crush injury by
clamping induced ultrastructure changes in the RGCs, as
demonstrated in Fig. 2.
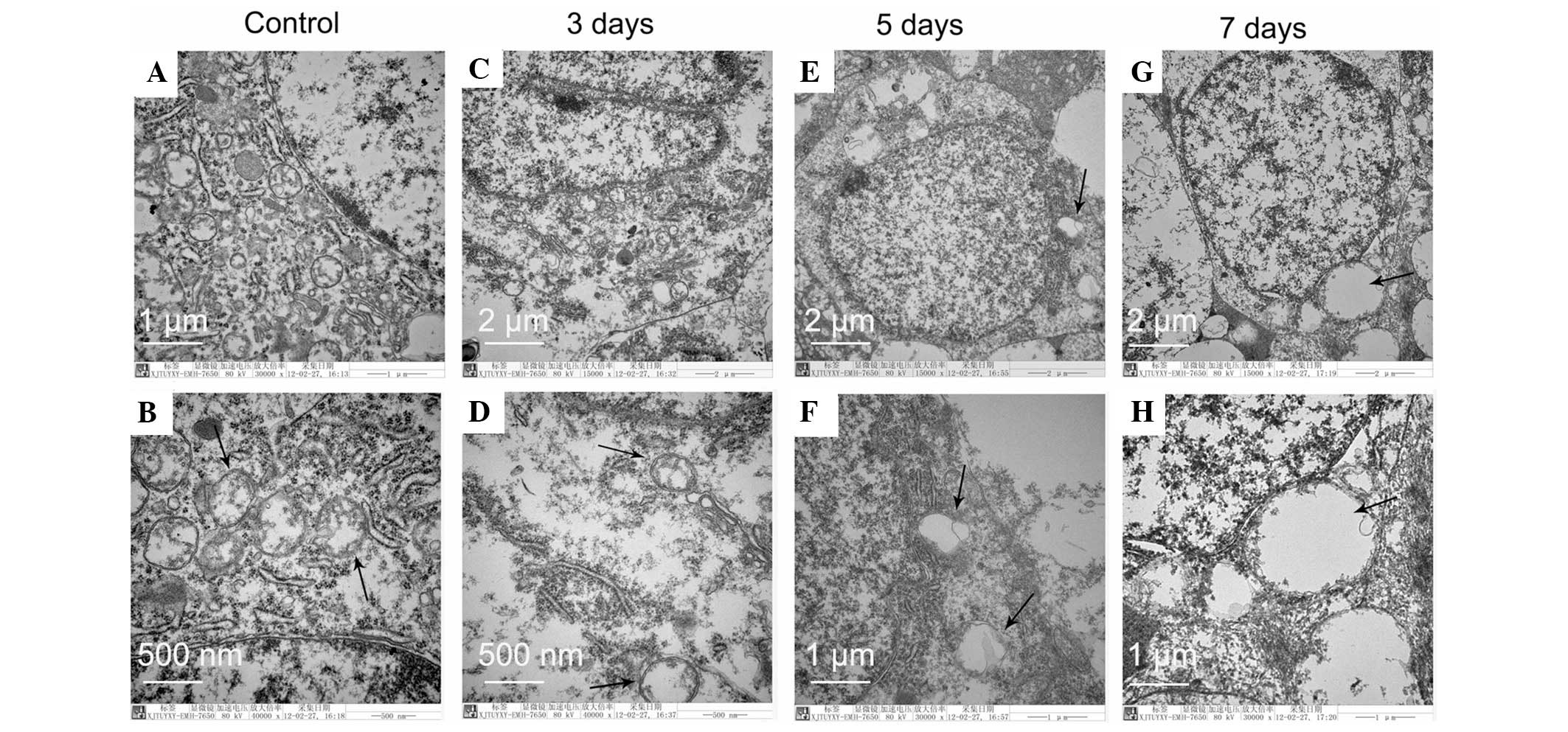 | Figure 2Electron microscopy analysis of RGCs,
following optic nerve crush injury by clamping, indicates vacuoles
have originated from the mitochondria. (A and B) Control cells with
normal mitochondria, endoplasmic reticulum and uncondensed
chromatin. (C and D) Swollen mitochondria with disintegrated
cristae and decreased matrix density. (E–H) Images demonstrate that
mega mitochondria are derived from swelling and fusion among
mitochondria. Condensed chromatin and nuclear fragmentation are not
detected. These morphologic features are consistent with the
characteristics of the type of cell death termed paraptosis. (D, F
and H) Enlarged images in the black squares of images (C, E and G),
respectively.. The representative sections from three rats in each
group are presented. The black arrows indicate the mitochondria.
Original magnifications: (C), (E) and (G): ×15,000; (A), (F) and
(H): ×30,000; (B) and (G): ×40,000. RGCs, retinal ganglion
cells. |
All the images of RGCs had well-defined plasma
membranes and uniformly distributed chromatin in the nucleus,
suggesting apoptosis had not evolved in the RGCs within 5 and 7
days following optic nerve crush injury. However, the images in
Fig. 2C and 2D demonstrate swollen
mitochondria with disintegrated cristae and decreased matrix
density. At day 5 and 7, the rate of mitochondrial fusion increased
until the cells were almost fully occupied by several large mega
mitochondria. However, autophagosomes containing cytoplasmic
organelles were not detected, demonstrating that the cell death was
not due to autophagy. All the ultrastructural analysis confirmed
that the mitochondria were targeted in the RGCs within 5 and 7 days
following optic nerve crush injury and the type of RGC death was
neither apoptosis nor autophagy, but instead fitted the criteria of
paraptosis (15,18).
Glaucoma is a multi-factorial group of eye diseases
that result in optic nerve damage, particularly axonal damage,
which triggers RGC cell death and the subsequent loss of vision.
The optic nerve crush injury rat model is an important experimental
disease model for glaucoma and in our study, this model was
utilized to examine the injury and morphological changes of
RGCs.
Taken together, the above study confirmed that optic
nerve crush injury triggered paraptosis in RGCs, suggesting that
paraptosis may be involved in glaucomatous RGC death. Further
investigations are required to determine whether the excessive
production of ROS is triggered in glaucomatous RGCs. However,
ultrastructural analysis of swollen mitochondria with disintegrated
cristae and intact endoplasmic reticulum further confirmed the
hypothesis that mitochondria are important in triggering paraptosis
in glaucomatous RGCs.
5. Conclusion
In the present review, the evidence suggesting that
progressive death of RGCs in glaucoma involves another novel
non-apoptotic PCD termed ‘paraptosis’ in the early stages of
glaucoma has been summarized. We conceive this to be a two step
process: (i) an excessive production of ROS impairs the
mitochondria in glaucomatous RGCs and (ii) the damage to the
mitochondria leads to RGC paraptosis. In addition, paraptosis and
apoptosis, possibly even together with autophagy, may occur
simultaneously in RGCs in the moderate and/or severe stages of
glaucoma. Further studies are under way to confirm this theory.
These hypotheses stand to enhance the understanding of the
mechanisms of glaucomatous RGC damage and provide a novel strategy
for protecting RGC by inhibiting paraptosis. Furthermore, we expect
this will facilitate the identification of a number of chemical
compounds/proteins that directly delay or prevent
paraprosis-induced RGC death and thus improve visual damage caused
by glaucoma.
Acknowledgements
This study was supported by the International
Cooperation Project of Science and Technology Department of Shaanxi
Province (No. 2011 KW-39), Xiamen Science and Technology Project
(No. 3502Z20116011, 3502Z20134040) and National Natural Science
Foundation of China (No. 81170841).
References
|
1
|
Garcia-Frigola C, Carreres MI, Vegar C and
Herrera E: Gene delivery into mouse retinal ganglion cells by in
utero electroporation. BMC Dev Biol. 7:1032007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ebneter A, Casson RJ, Wood JP and Chidlow
G: Microglial activation in the visual pathway in experimental
glaucoma: spatiotemporal characterization and correlation with
axonal injury. Invest Ophthalmol Vis Sci. 51:6448–6460. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leon S, Yin Y, Nguyen J, Irwin N and
Benowitz LI: Lens injury stimulates axon regeneration in the mature
rat optic nerve. J Neurosci. 20:4615–4626. 2000.PubMed/NCBI
|
|
4
|
Kuehn MH, Fingert JH and Kwon YH: Retinal
ganglion cell death in glaucoma: mechanisms and neuroprotective
strategies. Ophthalmol Clin North Am. 18:383–395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo L, Moss SE, Alexander RA, Ali RR,
Fitzke FW and Cordeiro MF: Retinal ganglion cell apoptosis in
glaucoma is related to intraocular pressure and IOP-induced effects
on extracellular matrix. Invest Ophthalmol Vis Sci. 46:175–182.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo L, Salt TE, Maass A, Luong V, Moss SE,
Fitzke FW and Cordeiro MF: Assessment of neuroprotective effects of
glutamate modulation on glaucoma-related retinal ganglion cell
apoptosis in vivo. Invest Ophthalmol Vis Sci. 47:626–633. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen J, Miao Y, Wang XH and Wang Z:
Elevation of p-NR2A(S1232) by Cdk5/p35 contributes to retinal
ganglion cell apoptosis in a rat experimental glaucoma model.
Neurobiol Dis. 43:455–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tezel G and Yang X: Caspase-independent
component of retinal ganglion cell death, in vitro. Invest
Ophthalmol Vis Sci. 45:4049–4059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Spalding KL, Dharmarajan AM and Harvey AR:
Caspase-independent retinal ganglion cell death after target
ablation in the neonatal rat. Eur J Neurosci. 21:33–45. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ravindran J, Prasad S and Aggarwal BB:
Curcumin and cancer cells: how many ways can curry kill tumor cells
selectively? AAPS J. 11:495–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madeo F, Tavernarakis N and Kroemer G: Can
autophagy promote longevity? Nat Cell Biol. 12:842–846. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morselli E, Galluzzi L, Kepp O, Criollo A,
Maiuri MC, Tavernarakis N, Madeo F and Kroemer G: Autophagy
mediates pharmacological lifespan extension by spermidine and
resveratrol. Aging (Albany NY). 1:961–970. 2009.PubMed/NCBI
|
|
13
|
Rodríguez-Muela N, Germain F, Mariño G,
Fitze PS and Boya P: Autophagy promotes survival of retinal
ganglion cells after optic nerve axotomy in mice. Cell Death
Differ. 19:162–169. 2012.PubMed/NCBI
|
|
14
|
Kim SH, Munemasa Y, Kwong JM, Ahn JH,
Mareninov S, Gordon LK, Caprioli J and Piri N: Activation of
autophagy in retinal ganglion cells. J Neurosci Res. 86:2943–2951.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sperandio S, Poksay K, de Belle I,
Lafuente MJ, Liu B, Nasir J and Bredesen DE: Paraptosis: mediation
by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ.
11:1066–1075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bröker LE, Kruyt FA and Giaccone G: Cell
death independent of caspases: a review. Clin Cancer Res.
11:3155–3162. 2005.
|
|
17
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sperandio S, de Belle I and Bredesen DE:
An alternative, nonapoptotic form of programmed cell death. Proc
Natl Acad Sci USA. 97:14376–14381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sperandio S, Poksay KS, Schilling B,
Crippen D, Gibson BW and Bredesen DE: Identification of new
modulators and protein alterations in non-apoptotic programmed cell
death. J Cell Biochem. 111:1401–1412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z and Klionsky DJ: Mammalian
autophagy: core molecular machinery and signaling regulation. Curr
Opin Cell Biol. 22:124–131. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valamanesh F, Torriglia A, Savoldelli M,
Gandolphe C, Jeanny JC, BenEzra D and Behar-Cohen F:
Glucocorticoids induce retinal toxicity through mechanisms mainly
associated with paraptosis. Mol Vis. 13:1746–1757. 2007.PubMed/NCBI
|
|
22
|
Chen Y, Douglass T, Jeffes EW, Xu Q,
Williams CC, Arpajirakul N, Delgado C, Kleinman M, Sanchez R, Dan
Q, et al: Living T9 glioma cells expressing membrane macrophage
colony-stimulating factor produce immediate tumor destruction by
polymorphonuclear leukocytes and macrophages via a
‘paraptosis’-induced pathway that promotes systemic immunity
against intracranial T9 gliomas. Blood. 100:1373–1380.
2002.PubMed/NCBI
|
|
23
|
Jadus MR, Chen Y, Boldaji MT, Delgado C,
Sanchez R, Douglass T, Al-Atar U, Schulz W, Lloyd C and Wepsic HT:
Human U251MG glioma cells expressing the membrane form of
macrophage colony-stimulating factor (mM-CSF) are killed by human
monocytes in vitro and are rejected within immunodeficient mice via
paraptosis that is associated with increased expression of three
different heat shock proteins. Cancer Gene Ther. 10:411–420.
2003.
|
|
24
|
Schneider D, Gerhardt E, Bock J, Müller
MM, Wolburg H, Lang F and Schulz JB: Intracellular acidification by
inhibition of the Na+/H+-exchanger leads to caspase-independent
death of cerebellar granule neurons resembling paraptosis. Cell
Death Differ. 11:760–770. 2004. View Article : Google Scholar
|
|
25
|
Wang WB, Feng LX, Yue QX, Wu WY, Guan SH,
Jiang BH, Yang M, Liu X and Guo DA: Paraptosis accompanied by
autophagy and apoptosis was induced by celastrol, a natural
compound with influence on proteasome, ER stress and Hsp90. J Cell
Physiol. 227:2196–2206. 2010. View Article : Google Scholar
|
|
26
|
Wang Y, Yang Z and Zhao X: Honokiol
induces paraptosis and apoptosis and exhibits schedule-dependent
synergy in combination with imatinib in human leukemia cells.
Toxicol Mech Methods. 20:234–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Zhu X, Yang Z and Zhao X: Honokiol
induces caspase-independent paraptosis via reactive oxygen species
production that is accompanied by apoptosis in leukemia cells.
Biochem Biophys Res Commun. 430:876–882. 2013. View Article : Google Scholar
|
|
28
|
Geiger LK, Kortuem KR, Alexejun C and
Levin LA: Reduced redox state allows prolonged survival of
axotomized neonatal retinal ganglion cells. Neuroscience.
109:635–642. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ravikumar B, Sarkar S, Davies JE, Futter
M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M,
Korolchuk VI, Lichtenberg M, Luo S, et al: Regulation of mammalian
autophagy in physiology and pathophysiology. Physiol Rev.
90:1383–1435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li ZY, Yang Y, Ming M and Liu B:
Mitochondrial ROS generation for regulation of autophagic pathways
in cancer. Biochem Biophys Res Commun. 414:5–8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lieven CJ, Hoegger MJ, Schlieve CR and
Levin LA: Retinal ganglion cell axotomy induces an increase in
intracellular superoxide anion. Invest Ophthalmol Vis Sci.
47:1477–1485. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoon MJ, Kim EH, Lim JH, Kwon TK and Choi
KS: Superoxide anion and proteasomal dysfunction contribute to
curcumin-induced paraptosis of malignant breast cancer cells. Free
Radic Biol Med. 48:713–726. 2010. View Article : Google Scholar : PubMed/NCBI
|