Introduction
Mesangial proliferative glomerulonephritis (MsPGN)
is the most common form of primary glomerular disease, and it is
characterized by mesangial cell proliferation and activation and
extracellular matrix (ECM) expansion in the mesangial area
(1). However, despite the severity
and incidence of MsPGN, there is a lack of effective therapies.
Polycystin-1 (PC-1) is encoded by the polycystic
kidney disease gene 1 (PKD1) (2).
Structural and functional abnormalities in PC-1 account for 85–90%
of all cases of autosomal dominant polycystic kidney disease
(ADPKD) (3). In previous studies
we developed a novel PC-1 N-terminal fragment (PC-1 NF) fusion
protein, which encodes part of the leucine-rich repeat (LRR) and
the cell wall integrity and stress response component (CWI&SRC)
domains of the PC-1 extracellular region, and we found that this
fusion protein may inhibit the proliferation of ADPKD cyst-lining
epithelial cells (4,5). A previous study by Zheng et al
(6) demonstrated that, when
transfected with PC-1, the cancer cell lines HepG2, A549 and SW480
exhibited a significant increase in the levels of apoptosis
compared with the respective control cell lines. This increase in
apoptosis was accompanied by cell cycle arrest at the
G0/G1 phase (6). An excessive proliferation of ADPKD
cyst-lining epithelial, cancer and mesangial cells is
characteristic of ADPKD, cancer and MsPGN, respectively. It was
found that the PC-1 NF fusion protein inhibits the progression of
ADPKD cyst-lining epithelial cells and cancer cells; however,
little is known about the role of the PC-1 NF fusion protein in
mesangial cells in MsPGN. Therefore, in this study the role of the
PC-1 NF fusion protein in MsPGN was investigated.
Materials and methods
Materials
Horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G (IgG) was obtained from Santa Cruz Biotechnology,
Inc. (Santa Cruz, CA, USA). Dulbecco’s modified Eagle’s medium
(DMEM)/F12 medium, TRIzol®, SuperScript® II
Reverse Transcriptase and RNAsin® were obtained from
Gibco®-BRL (Carlsbad, CA, USA). Fetal bovine serum was
obtained from Hangzhou Sijiqing Biological Engineering Materials
Co., Ltd. (Hangzhou, China). The BrdU-ELISA kit was obtained from
Roche Diagnostics (Indianapolis, IN, USA) and the fluorescence
quantitative polymerase chain reaction (qPCR) detection kit was
obtained from Takara Bio, Inc. (Shiga, Japan). The ELISA regimen of
collagen IV was obtained from the Cell Bank at the Chinese Academy
of Science (Shanghai, China). Monoclonal rat anti-GAPDH antibody
was obtained from Ambion® (Carlsbad, CA, USA). The
secondary antibody was purchased from Beijing Zhongshan Corp.
(Beijing, China). Polyclonal rabbit anti-protein kinase Cα (PKCα),
-Bcl-2-associated X protein (Bax) and -B-cell lymphoma-2 (Bcl-2)
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Heidelberg, Germany). Enhanced chemiluminescence detection
reagents and [γ-32P]ATP were obtained from Amersham
Pharmacia Biotech (Braunschweig, Germany). Diethylpyrocarbonate and
other reagents were purchased from Sigma-Aldrich Chemie GmbH
(Deisenhofen, Germany).
Immunohistochemistry
To determine the role of the PC-1 NF fusion protein
in MsPGN, the distribution of PC-1 in normal and IgA nephropathy
(Haas II and III class) renal tissues was investigated. The study
was approved by the Ethics Committee of Zhongshan Hospital, Xiamen
University, Xiamen, China and consent was obtained from patients.
Tissue sections were dewaxed and the endogenous peroxidases were
inactivated. The sections were then immersed in 0.01 M citrate
buffer (pH 6.0), heated in a microwave oven to retrieve
antigenicity and blocked for non-specific binding using 5% bovine
serum albumin in phosphate-buffered saline (PBS) for 20 min.
Blocked sections were incubated with mouse anti-PC-1 monoclonal
antibody at 4°C overnight. Following washing with PBS, the sections
were treated with horseradish peroxidase-conjugated goat anti-mouse
IgG at 37°C for 20 min. Secondary antibody incubation was followed
by color development using 3,3′-diaminobenzidine and
counterstaining, as described above. Incubation with an irrelevant
non-immune mouse IgG primary antibody served as the negative
control.
Cell culture
The rat mesangial cell (RMC) line was established in
the Division of Nephrology, Center of Kidney Disease, Changzheng
Hospital, Second Military Medical University, Shanghai, China
(7). The cells were cultured in
DMEM supplemented with 10% fetal bovine serum/F12 medium at 37°C
with 5% CO2. The cells were treated with PC-1 NF fusion
protein at various concentrations (0, 0.5, 1, 2 and 4 μg/ml) in
media for 24, 48, 72 and 96 h.
Cell cycle analysis
The cell cycle phase was examined using
fluorescence-activated cell sorting (FACS) analysis. The cells were
trypsinized, washed with PBS and fixed in 70% pre-cooled ethanol
overnight at −20°C. Cells were then washed twice with PBS and
stained with propidium iodide for FACS analysis in the dark. The
FACS data were analyzed using Multicycle AV DNA Content and Cell
Cycle Analysis software (De Novo Software, San Diego, CA, USA). The
proliferation index (PI) was determined using the following
equation: PI (%) = (S+G2/M)/(G0/G1+S+G2/M)x100.
qPCR
The effect of the PC-1 NF fusion protein on the
proliferation, cell cycle and apoptosis of RMCs, as well as changes
in the mRNA levels of proliferating cell nuclear antigen (PCNA),
cyclin D1, p21Waf1, Bax, Bcl-2, matrix metalloproteinase
2 (MMP2) and tissue inhibitor of metalloproteinase 1 (TIMP1) were
analyzed using qPCR. Total RNA was extracted from cells in the
logarithmic growth phase, and the cells were treated with 4 μg/ml
PC-1 NF fusion protein for 48 h using TRIzol. Genomic DNA
contaminants were removed by digestion with DNase (Takara Bio,
Inc.). Briefly, cDNA was synthesized from a total of 2 μg RNA in a
20-μl reaction mixture that included 20 units RNAsin, 100 units
SuperScript II Reverse Transcriptase, 4 μl 5X 1st strand buffer,
0.5 μmol/l primers and 1 μl 10 mmol/l deoxynucleotide triphosphate.
The mixture was incubated at 25°C for 10 min, 42°C for 1 h and 52°C
for 15 min. The reverse transcriptase was inactivated by incubation
at 70°C for 15 min. The primer sequences used for the qPCR are
shown in Table I. qPCR was
performed in a 25-μl reaction mixture [1 μl cDNA, 2.5 μl
10−3 buffer, 0.3 μl 250 mM Mg2+, 0.3 μl 25 mM
dAGCU, 1 μl 10 μmol/primers, 1 μl 10−3X calibration
buffer, 0.8 μl 25X SYBR Green (Bio-Rad, Hercules, CA, USA), 1.25
units Taq polymerase and 0.4 units uracil-N-glycosylase]. qPCR was
performed under the following cycle conditions: Predenaturation at
95°C for 90 sec; five cycles of denaturation at 95°C for 5 sec,
annealing at 55°C for 15 sec, extension at 72°C for 20 sec; 35
additional denaturing cycles at 95°C for 5 sec; annealing at 60°C
for 30 sec to reach the fluorescent signal detection point; another
40 cycles of denaturing at 95°C for 1 min and finally annealing at
55°C for 1 min (increasing 0.5°C/cycle every 10 sec). The target
gene expression was calculated from the respective standard curves,
and quantitative expression was normalized to GAPDH using an
iCycler Thermal Cycler (Bio-Rad).
 | Table IQuantitative polymerase chain reaction
primers. |
Table I
Quantitative polymerase chain reaction
primers.
| Name | Sequence | Length (bp) |
|---|
| PCNA | Forward: 5′-CAT GGG
CGT GAA CCT CAC-3′
Reverse: 5′-CAC AGC TGT ACT CCT GTT CTG G-3′ | 206 |
| Cyclin D1 | Forward: 5′-GCT GGC
CAT GAA CTA CCT G-3′
Reverse: 5′-GCC TCT GGC ATT TTG GAG-3′ | 275 |
|
P21Waf1 | Forward: 5′-CCG ATC
CTG GTG ATG TCC G-3′
Reverse: 5′-TCC GAA CAC GCT CCC AGA C-3′ | 201 |
| Type IV collagen | Forward: 5′-GCC CTA
CGT TAG CAG ATG TAC C-3′
Reverse: 5′-TAT AAA TGG ACT GGC TCG GAA T-3′ | 217 |
| MMP2 | Forward: 5′-TTC AAC
GGT CGG GAA TAC A-3′
Reverse: 5′-GGA AGC GGA ACG GAA ACT-3′ | 188 |
| TIMP1 | Forward: 5′-AAG TCC
CAG AAC CGC AGC-3′
Reverse: 5′-TCC AGT TTG CAR GGG ATG G-3′ | 197 |
| Bax | Forward: 5′-GCT GAT
GGC AAC TTC AAC TG-3′
Reverse: 5′-CAG CCA CAA AGA TGG TCA CT-3′ | 235 |
| Bcl-2 | Forward: 5′-GTG GAG
GAA CTC TTC AGG GAT-3′
Reverse: 5′-CAG CCA GGA GAA ATC AAA CAG-3′ | 246 |
| GAPDH | Forward: 5′-TGC TGA
GTA TGT CGT GGA GTC-3′
Reverse: 5′-TGC TGA CAA TCT TGA GGG AG-3′ | 173 |
Analysis of apoptosis
Following treatment with PC-1 NF fusion protein for
48 h, the cells were harvested by trypsin digestion, fixed in 4%
paraformaldehyde and 2% glutaraldehyde for 4 h and then washed in
PBS. Cells were clotted by the addition of plasma and 1% osmium
acid for 2–3 h, rinsed with PBS for 30 min and embedded in Epon 812
at 37°C overnight. Ultrathin sections were stained with 4% uranyl
acetate for 30 min. The sections were then washed and subjected to
lead citrate staining for 5–10 min, prior to further washing and
drying. Ultrastructural changes in the epithelia were observed
using a transmission electron microscope (Hitachi H800, Hitachi,
Tokyo, Japan). Cell preparation and analysis methods for the FACS
apoptosis index (AI) analysis were the same as those employed for
the cell cycle analysis with the exception that the samples
included suspension cells in the media.
Western blot analysis
Following individual incubations, as described in
the cell culture section, the medium was removed and the cells were
scraped into ice-cold PBS supplemented with 1 mM sodium vanadate, 1
mM sodium fluoride and 1 mM phenylmethylsulfonyl fluoride. The
cells were centrifuged at 700 × g for 10 min at 4°C and resuspended
in 200 ml lysis buffer (50 mM Tris, 5 mM EDTA, 150 mM NaCl, 0.5%
Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium
vanadate and 1 mM sodium fluoride; pH 8.0). Cell debris was
sonicated using a Branson sonifier® (10 sec; duty cycle,
100%; output control, 1) and centrifuged (14,000 × g, 15 min), and
the protein content was measured. To ensure equal protein loading,
150 mg protein was mixed with the same volume of SDS sample buffer
(125 mM Tris HCl, 2% SDS, 10% glycerin, 1 mM dithiothreitol and
0.002% bromophenol blue; pH 6.9) and boiled for 5 min. Proteins
were resolved on 10% SDS-polyacrylamide gels and were blotted onto
nitrocellulose membranes. The membranes were blocked using 10 mM
Tris (pH 7.5), 100 mM NaCl and 0.1% Tween-20 (TBST) containing 5%
non-fat dry milk for 1 h at room temperature. The membranes were
then incubated with polyclonal rabbit anti-PKCα, -Bax and -Bcl-2
antibodies and monoclonal rat anti-GAPDH antibody (1:2,000) at 4°C
overnight. The membranes were subjected to three 15-min TBST washes
prior to being incubated at room temperature with horseradish
peroxidase-conjugated goat anti-rabbit IgG (1:2,500). Following
further washes with TBST, signals were detected using enhanced
chemiluminescence (Amersham Pharmacia Biotech, Amersham, UK).
ELISA
The concentration of type IV collagen in the medium
was measured using an ELISA kit in accordance with the
manufacturer’s instructions. The absorbance at 492 nm was
determined using a microplate reader (Wellscan MK3, Labsystems
Dragon, Helsinki, Finland).
Statistical analysis
The data are presented as the mean ± standard
deviation. Comparisons between results from different groups were
performed using a Student’s t-test or a one-way analysis of
variance, as appropriate. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using the SPSS 18.0 software package (SPSS, Inc.,
Chicago, IL, USA).
Results
Distribution and expression level of PC-1
in renal tissues
PC-1 protein was expressed predominantly in the
medullary and cortical collecting ducts and the distal convoluted
tubules in the normal adult and IgA nephropathy kidney tissues,
which is consistent with the expression pattern observed in rats in
the preliminary experiment. Although only weak expression of PC-1
was observed in the glomerular area, the difference was great among
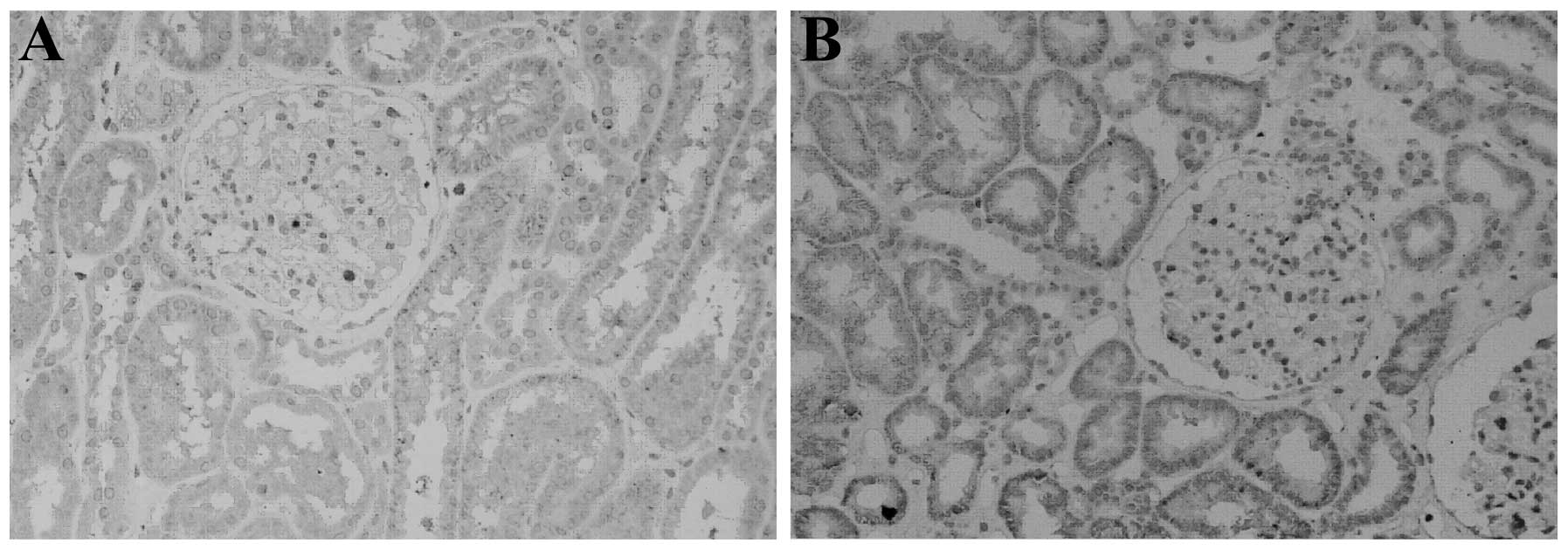
the individual subjects. As shown in Fig. 1, positive staining appeared as
brown granules throughout the cytoplasm. The amount of positive
staining observed in normal renal tissue was greater than that in
the IgA nephropathy (Haas III) renal tissue.
PC-1 NF fusion protein inhibits the
proliferation of RMCs and affects the proliferation-related mRNA
expression levels of PCNA
The results indicated that proliferation was
progressively inhibited by treatment with increasing concentrations
of PC-1 NF fusion protein. Treatment with 4 μg/ml PC-1 NF fusion
protein for 24 h effectively inhibited the proliferation of RMCs
(P<0.01). This inhibitory effect was time-dependent and reached
a peak at 96 h of treatment (Table
II). In addition, treatment with 4 μg/ml PC-1 NF fusion protein
for 48 h decreased the mRNA levels of PCNA in RMCs, from
(8.26±1.01)x103 to (3.58±1.16)x103 copies per
million GAPDH (P<0.01).
 | Table IIConcentration- and time-dependent
inhibition of rat mesangial cell proliferation by the PC-1 NF
fusion protein. |
Table II
Concentration- and time-dependent
inhibition of rat mesangial cell proliferation by the PC-1 NF
fusion protein.
| PC-1 NF fusion
protein concentration (μg/ml) | Proliferation rate
(%) |
|---|
|
|---|
| 24 h | 48 h | 72 h | 96 h |
|---|
| 0.0 | 29.0±2.0 | 36.0±2.0 | 58.0±3.0 | 106.0±12.0 |
| 0.5 | 28.0±3.0 | 33.0±5.0 | 53.0±3.0 |
89.0±4.0a,c |
| 1.0 | 27.0±3.0 | 32.0±4.0 | 49.0±4.0 |
81.0±4.0b,c |
| 2.0 | 26.0±4.0 | 28.0±4.0 | 42.0±3.0 |
65.0±5.0b,c |
| 4.0 |
21.0±2.0b |
23.0±2.0b,c |
24.0±4.0b,c |
41.0±5.0b,c |
PC-1 NF fusion protein inhibits cell
cycle progression of RMCs and affects the mRNA levels of cell cycle
regulatory genes
PC-1 NF fusion protein treatment for 48 h reduced
the number of RMCs in S and G2/M phases, resulting in
the cells remaining at G0/G1 phases in a
concentration-dependent manner. Similarly, the PI gradually
decreased with increasing concentrations of PC-1 NF fusion protein
(Table III). Furthermore, qPCR
and immunocytochemical analysis revealed that treatment with 4
μg/ml PC-1 NF fusion protein for 48 h decreased the level of cyclin
D1 (Fig. 2A), whereas the
p21Waf1 levels increased with PC-1 NF fusion protein
treatment (Fig. 2B and Table IV). Additionally, the expression
of c-fos and c-jun decreased (Fig. 2C
and D). The expression of PKCα also gradually decreased
following treatment with increasing concentrations of PC-1 NF
fusion protein (Fig. 2E).
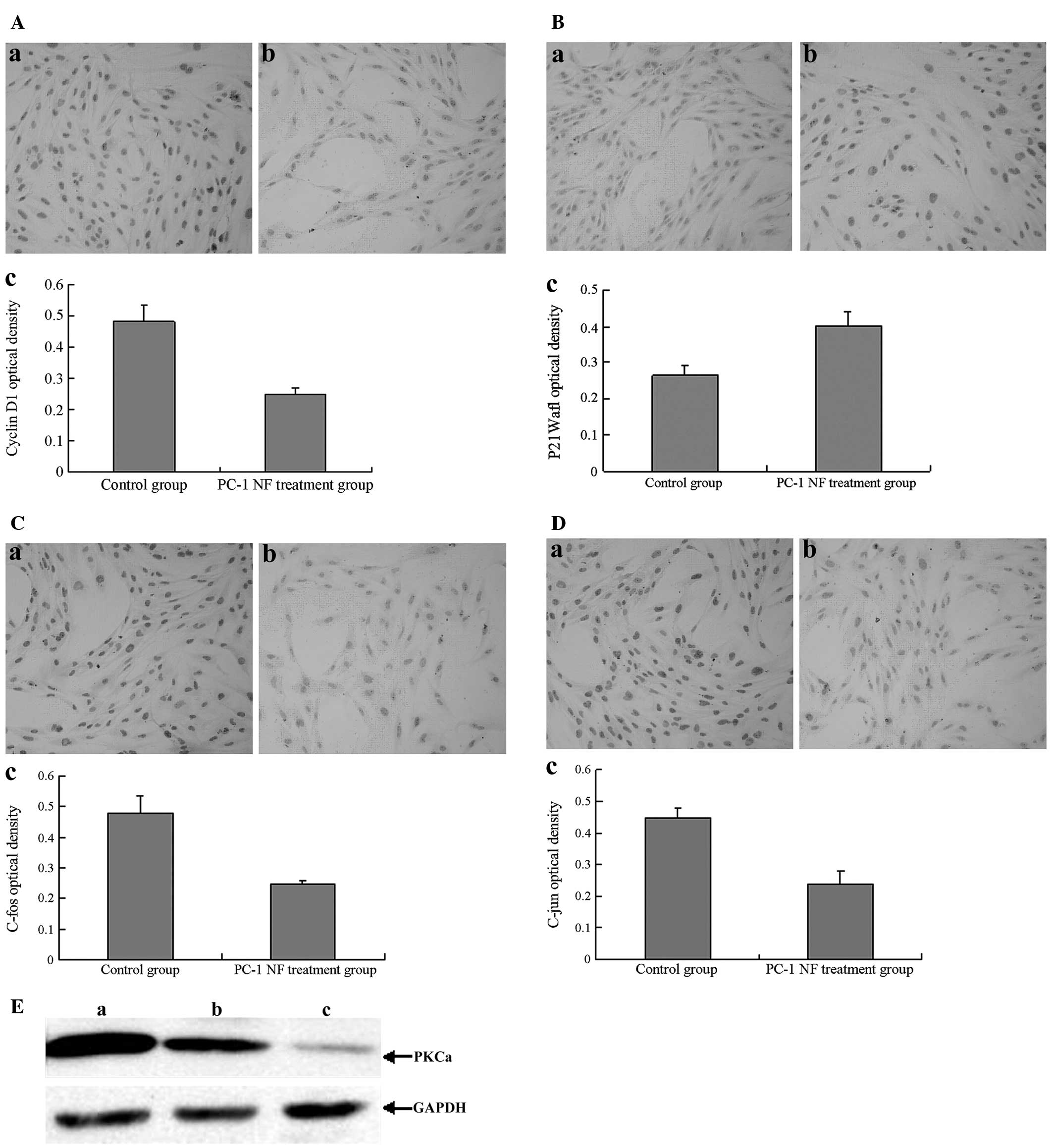 | Figure 2Effect of PC-1 NF fusion protein
treatment (48 h) on the expression of cyclin D1,
P21Waf1, c-jun, c-fos and PKCα genes in RMCs. (A)
Expression of cyclin D1 in RMCs treated with (a) 0 μg/ml and (b) 4
μg/ml PC-1 NF (magnification, ×200). (c) Quantitative analysis of
the expression of cyclin D1 in RMCs. (B) Expression of
P21Waf1 in RMCs treated with (a) 0 μg/ml and (b) 4 μg/ml
PC-1 NF (magnification, ×200). (c) Quantitative analysis of the
expression of P21Waf1 in RMCs. (C) Expression of c-jun
in RMCs treated with (a) 0 μg/ml and (b) 4 μg/ml PC-1 NF
(magnification, ×200). (c) Quantitative analysis the expression of
c-jun in RMCs. (D) Expression of c-fos in RMCs treated with (a) 0
μg/ml and (b) 4 μg/ml PC-1 NF (magnification, ×200). (c)
Quantitative analysis the expression of c-fos in RMCs. (E) Western
blot analysis of PKCα in RMCs. Culture medium alone in a flask
served as the control. GAPDH was used as an internal control to
analyze the relative level of PKCα. (a-c) RMCs treated with (a) 0,
(b) 2 and (c) 4 μg/ml PC-1 NF fusion protein. The PKCα to GAPDH
optical density ratios were 96.67% (29:30), 64.29% (18:28) and
20.69% (6:29), respectively. PC-1 NF, polycystin-1 N-terminal
fragment; RMC, rat mesangial cell; PKCα, protein kinase Cα. |
 | Table IIIEffect of PC-1 NF fusion protein
treatment (48 h) on cell cycle rate and PIs of rat mesangial
cells. |
Table III
Effect of PC-1 NF fusion protein
treatment (48 h) on cell cycle rate and PIs of rat mesangial
cells.
| PC-1NF fusion
protein concentration (μg/ml) | Cell cycle rate
(%) | PI (%) |
|---|
|
|---|
|
G0–G1 | S |
G2-M |
|---|
| 0.0 | 27.1±2.1 | 44.1±6.7 | 28.8±2.0 | 72.9 |
| 1.0 | 31.2±3.4 | 41.7±2.8 | 27.1±3.0 | 68.8 |
| 2.0 | 36.5±1.6a | 36.6±1.8a | 26.8±2.1 | 63.4 |
| 4.0 | 42.0±2.3b | 31.6±4.5b | 26.4±1.8 | 58.0 |
 | Table IVEffect of PC-1 NF fusion protein
treatment (4 μg/ml for 48 h) on the expression of cell cycle
regulatory genes in rat mesangial cells. |
Table IV
Effect of PC-1 NF fusion protein
treatment (4 μg/ml for 48 h) on the expression of cell cycle
regulatory genes in rat mesangial cells.
| mRNA levels
(copies/million GAPDH) |
|---|
|
|
|---|
| Group | Cyclin D1 |
p21Waf1 |
|---|
| Control |
(4.10±0.32)×104 |
(0.85±0.18)×102 |
| PC-1
NF-treatment |
(2.44±0.27)×104a |
(3.73±0.46)×102b |
PC-1 NF fusion protein induces the
apoptosis of RMCs and affects the levels of apoptosis regulatory
genes and proteins
Following treatment with 2 μg/ml PC-1 NF fusion
protein for 48 h, a number of previously irregularly shaped cells
became elliptical or rounded, and a number of cells were suspended
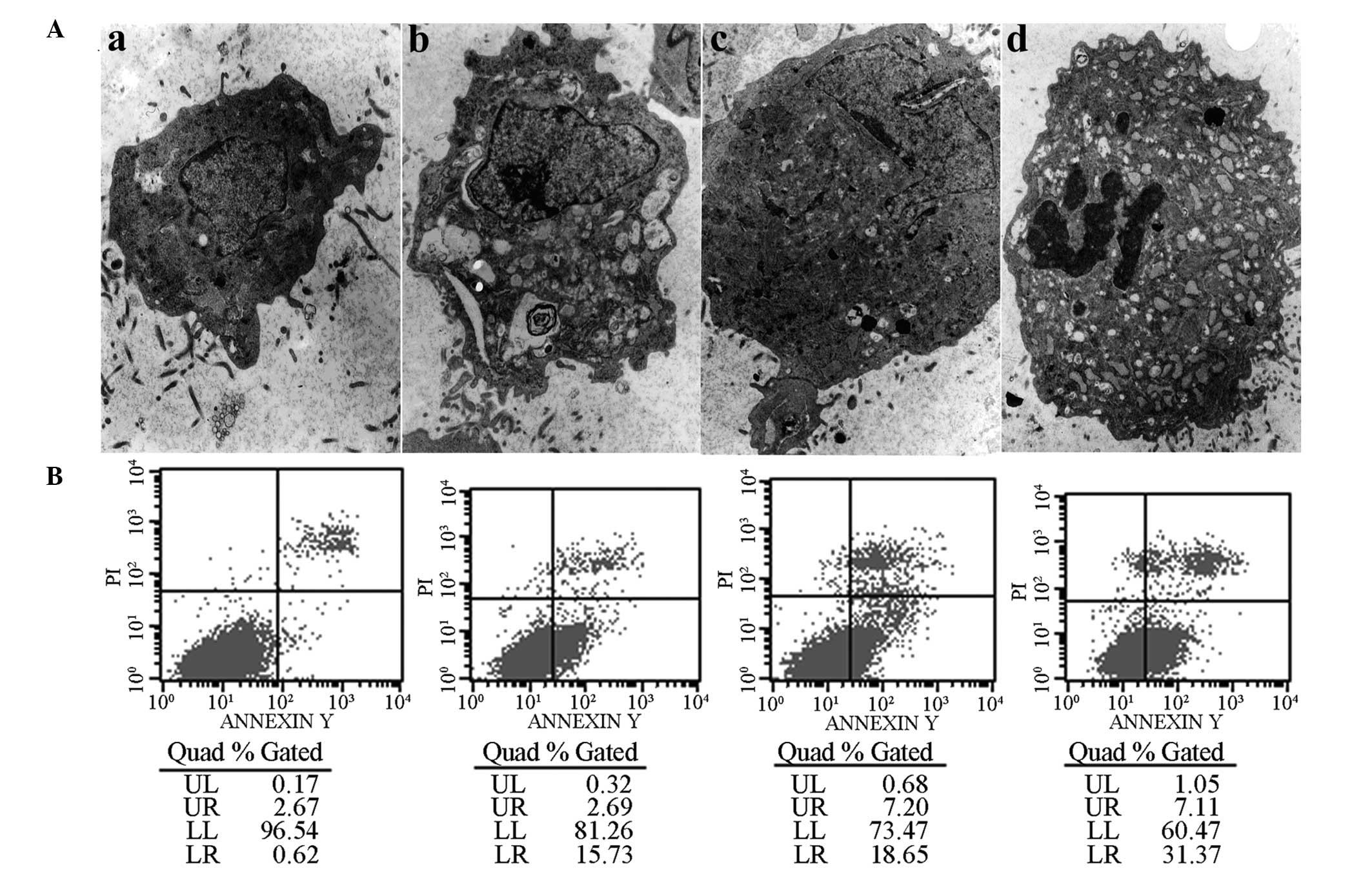
with the intact cellular membranes from adherent cells. Examination
by electron microscopy revealed typical apoptotic changes following
PC-1 NF fusion protein treatment, including the emergence of
numerous cytoplasmic vacuoles and fissures. Additionally, the
nuclei were observed to have become irregular and to contain
concentrated skirted chromatin and, in certain cells, typical
apoptotic bodies were observed (Fig.
3). AIs were detected following treatment with 1, 2 and 4 μg/ml
PC-1 NF fusion protein for 48 h and the corresponding AIs were
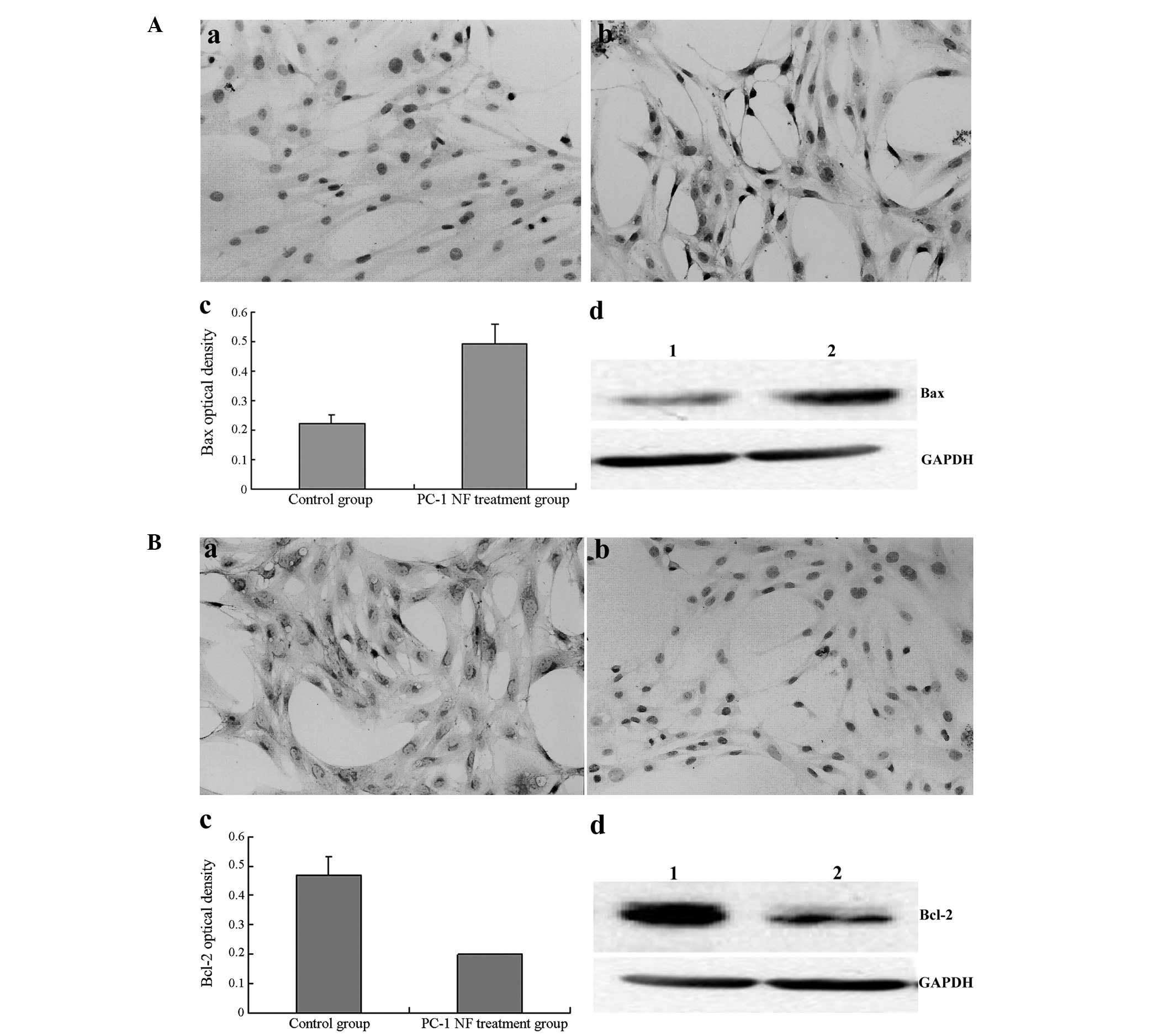
15.73, 18.65 and 31.37%, respectively. The protein expression of
the apoptosis-related genes Bax and Bcl-2 was measured. The
expression of Bax increased, whilst that of Bcl-2 decreased
following treatment with 4 μg/ml PC-1 NF fusion protein for 48 h,
which is consistent with the mRNA expression levels (Fig. 4).
PC-1 NF fusion protein induces ECM
degradation in RMCs through increasing the ratio of MMP2 to TIMP1
gene expression
After 48 h of treatment with 0, 1, 2 and 4 μg/ml
PC-1 NF fusion protein, the concentration of type IV collagen in
the supernatant was 68.62±8.11, 57.16±7.88, 49.12±6.38 and
36.76±4.49 pg/μg, respectively (Fig.
5). The TIMP1 mRNA level was significantly decreased; however
MMP2 mRNA expression levels increased significantly (Fig. 5).
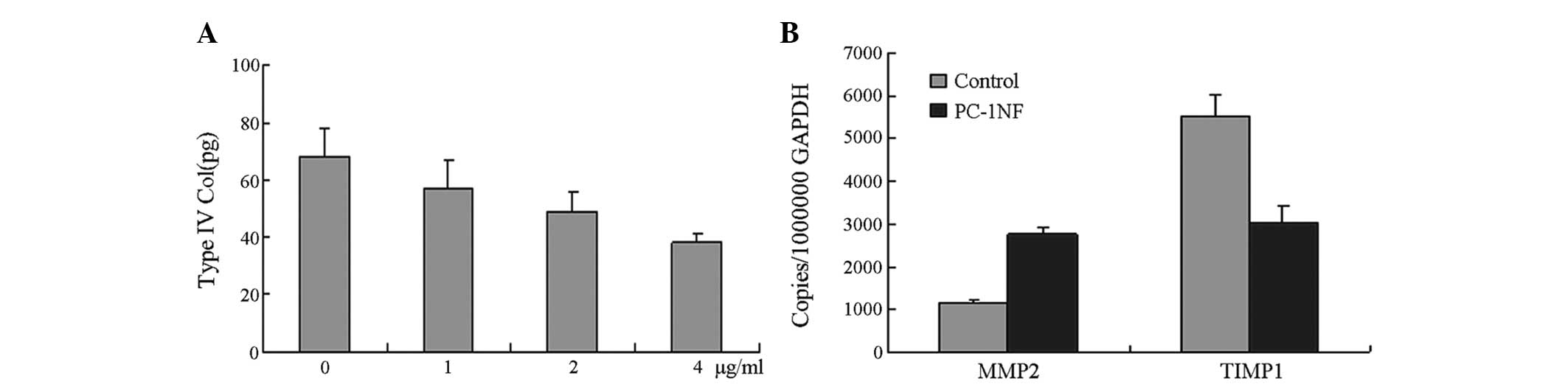 | Figure 5PC-1 NF fusion protein induces
extracellular matrix degradation in RMCs and increases the ratio of
MMP2 to TIMP1 gene expression. (A) Inhibition of type IV collagen
in RMCs treated with various concentrations of PC-1 NF fusion
protein. The inhibition of type IV collagen increased gradually
with increasing concentrations of PC-1 NF fusion protein. Following
incubation with 0, 1, 2 and 4 μg/ml PC-1 NF fusion protein for 48
h, the concentration of type IV collagen in the supernatants was
68.62±8.11, 57.16±7.88, 49.12±6.38 and 36.76±4.49 pg/μg,
respectively. (B) Effect of PC-1 NF fusion protein treatment (4
μg/ml for 48 h) on the expression of MMP2 and TIMP1 genes in RMCs.
PC-1 NF, polycystin-1 N-terminal fragment; RMC, rat mesangial cell;
MMP2, matrix metalloproteinase 2; TIMP1, tissue inhibitor of
metalloproteinase 1. |
Discussion
MsPGN is characterized by widespread mesangial cell
proliferation and the accumulation of ECM in the mesangial area. A
recent study demonstrated that the highly proliferative mesangial
cells in MsPGN rats aggravate disease progression (8). The antiproliferative activity of PC-1
has been demonstrated in Madin Darby canine kidney cell lines
(9). In a previous study, we
developed a novel PC-1 NF fusion protein that encodes part of the
LRR and CWI&SRC domains of the PC-1 extracellular region. We
demonstrated that this fusion protein may inhibit the proliferation
of ADPKD cyst-lining epithelial cells (4,5).
PC-1 may have a role in protecting against IgA nephropathy, which
is characterized by mesangial proliferation. In the present study
it was demonstrated in vitro that treatment with exogenous
PC-1 NF fusion protein reduces the proliferation of RMCs in a
concentration- and time-dependent manner. It was found that
microgram concentrations of PC-1 NF fusion protein also markedly
reduce PCNA mRNA expression. PCNA is a nuclear protein that is
expressed specifically during cell proliferation. The expression of
PCNA begins at the end of G1 phase, reaches a peak
during S phase and early G2 phase and ceases during M
and G0 phases (10).
These findings suggested that the PC-1 NF fusion protein inhibits
mesangial cell proliferation in MsPGN.
The cell cycle is the final pathway for cell
proliferation and has several checkpoints. The G1-S
checkpoint is key since this is the stage at which cells integrate
and converge signals to determine if they should initiate cell
division, enter into a resting state (G0 phase) or
undergo apoptosis. PC-1 NF fusion protein treatment increased the
number of cells at the G0/G1 phase and
correspondingly decreased the number of cells at the S phase,
indicating that the PC-1 NF fusion protein inhibition of cell
proliferation may be mediated through a regulatory effect at the
G1 phase.
Cyclin D1, encoded by the ClnD1 gene, is involved in
G1-phase regulation. Cyclin D1 controls cyclin-dependent
kinase (CDK) activity by phosphorylation and enhances the
expression of a number of genes that promote the passage of cells
through the G1-S checkpoint and, thus, the commencement
of cell division (11). The first
CDK inhibitor identified in mammalian cells, p21Waf1,
suppresses the activity of CDK or the cyclin D1-CDK complex through
dephosphorylation and therefore inhibits cell proliferation
(12). PKD1 activates the Janus
kinase/signal transducers and activators of transcription pathway,
thereby upregulating p21Waf1 and inducing cell cycle
arrest in G0/G1. An increase in the
expression of p21Waf1 has been shown to be primarily
responsible for mediating the growth-suppressing effects of PKD1 in
an experimental system (13).
C-fos and c-jun are immediate-early genes that are
associated with proliferation. The proteins encoded by these two
genes, which are located in the nucleus, combine with specific DNA
sequences and activate transcription (14). The expression of c-fos and c-jun is
associated with cell proliferation (15). PKCα activates the transcription
factor activator protein-1, leading to the upregulation of c-jun
and c-fos expression to promote cell growth (16). It has been reported that, when
increased PKCα expression is observed, there is an increase in cell
proliferation (17).
PC-1 NF fusion protein treatment decreased cyclin D1
mRNA levels but increased p21Waf1 mRNA levels in RMCs.
Additionally, PC-1 NF fusion protein treatment resulted in
decreased c-jun, c-fos and PKCα protein expression levels. These
results suggest that the PC-1 NF fusion protein regulates the cell
cycle in RMCs by inhibiting cyclin D1 expression and increasing
p21Waf1 expression to modulate the PKCα signaling
pathway. Using this mechanism, the PC-1 NF fusion protein may
prevent RMCs from entering into S phase and preserve the cells in a
resting state.
Mesangial cell apoptosis and proliferation are
dysregulated in MsPGN (18).
Dysregulation of the balance between pro- and anti-apoptotic Bcl-2
family members correlates with increased apoptosis in MsPGN
(19). The ratio of Bcl-2-Bax
heterodimers to Bax-Bax homodimers is a critical factor for
determining susceptibility to apoptosis (20). Therefore, the regulation of Bcl-2
and Bax expression may be a key mechanism underlying the PC-1 NF
fusion protein-mediated induction of apoptosis in RMCs.
It is likely that decreased degradation of ECM is an
important factor in the pathogenesis of MsPGN (21). MMPs are zinc-dependent
metalloendopeptidases that belong to the collagenase supergene
family. Primarily based on substrate specificity, they are
classified into several groups (22). MMP2 has a crucial role in the
degradation of collagen IV (23).
MMP activity is regulated by natural inhibitors, predominantly the
TIMPs (TIMP-1 to -4) (24). TIMP1
is the most important category of enzymes in ECM degradation and
has an important role in the dynamic balance between the synthesis
and degradation of the ECM, promoting tissue repair and remodeling
following pathophysiological events (24,25).
The regulation of ECM metabolism is likely the main function of
these proteolytic enzymes and their inhibitors. Following PC-1 NF
fusion protein treatment, the level of MMP2 was upregulated,
whereas the TIMP1 level was downregulated. This indicates that the
PC-1 NF fusion protein induces ECM degradation in RMCs by
increasing the ratio of MMP2 to TIMP1 gene expression. In addition,
the reduction of the ECM may contribute to the lower proliferation
and higher apoptosis rate in RMCs.
In conclusion, PC-1 NF fusion protein has been shown
to reduce proliferation and induce apoptosis in RMCs. The
antiproliferative effect may be mediated by the modulation of the
PKCα signal pathway, the inhibition of cyclin D1 expression and the
stimulation of p21Waf1 expression, thereby preventing
the passage of cells through the G1-S checkpoint. The
PC-1 NF fusion protein may also induce the apoptosis of mesangial
cells by decreasing the ratio of Bcl-2 to Bax gene expression.
Furthermore, the PC-1 NF fusion protein induced ECM degradation by
increasing the ratio of MMP2 to TIMP1 gene expression in RMCs. The
strategy of inducing cell cycle arrest and subsequent apoptosis may
contribute to the development of novel perspectives for the
treatment of MsPGN. The PC-1 NF fusion protein may provide a
protective effect and may lead to the development of a novel
therapeutic drug for the treatment of MsPGN.
Acknowledgements
This study was supported by the National Nature and
Science Foundation (No. 30330640, No. 30271523, No. 30570867)and
Xiamen Municipal Bureau of Science and Technology Foundation (grant
no. 3502Z20134015).
References
|
1
|
Sethi S and Fervenza FC:
Membranoproliferative glomerulonephritis: pathogenetic
heterogeneity and proposal for a new classification. Semin Nephrol.
31:341–348. 2011. View Article : Google Scholar
|
|
2
|
Chapin HC and Caplan MJ: The cell biology
of polycystic kidney disease. J Cell Biol. 191:701–710. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Harris PC and Torres VE: Polycystic kidney
disease. Annu Rev Med. 60:321–337. 2009. View Article : Google Scholar
|
|
4
|
Zhao HD, Mei CL, Li L, et al: Preparation
and characterization of monoclonal antibody against N-terminal
domain of polycystin 1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
20:186–190. 2004.(In Chinese).
|
|
5
|
Zhao HD, Sun TM, Wang WJ, et al:
Evaluation of polycystin-1 N-terminal peptide on the proliferation
and apoptosis of cystic lining epithilial cells in human ADPKD.
Chin J Nephrol. 21:664–668. 2005.(In Chinese).
|
|
6
|
Zheng R, Zhang Z, Lv X, et al:
Polycystin-1 induced apoptosis and cell cycle arrest in G0/G1 phase
in cancer cells. Cell Biol Int. 32:427–435. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mei CL, Zhang LM and Chen L: The
establishment of rat mesangial cell line. Chin J Nephrol Dial
Transplant. 5:90–92. 1996.(In Chinese).
|
|
8
|
Suana AJ, Tuffin G, Frey BM, et al: Single
application of low dose mycophenolate-mofetil-OX7-immunoliposomes
ameliorates experimental mesangial proliferative
glomerulonephritis. J Pharmacol Exp Ther. 337:411–422. 2011.
View Article : Google Scholar
|
|
9
|
Boletta A, Qian F, Onuchic LF, et al:
Polycystin-1, the gene product of PKD1, induces resistance to
apoptosis and spontaneous tubulogenesis in MDCK cells. Mol Cell.
6:1267–1273. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Takahashi H, Oishi Y, Chuang SS and Morii
S: Effects of tissue fixation and processing on proliferating cell
nuclear antigen (PCNA) immunohistochemistry. Acta Pathol Jpn.
42:621–623. 1992.PubMed/NCBI
|
|
11
|
Wang J, Wang Q, Cui Y, et al: Knockdown of
cyclin D1 inhibits proliferation, induces apoptosis, and attenuates
the invasive capacity of human glioblastoma cells. J Neurooncol.
106:473–484. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei J, Zhao J, Long M, et al: p21WAF1/CIP1
gene transcriptional activation exerts cell growth inhibition and
enhances chemosensitivity to cisplatin in lung carcinoma cell. BMC
Cancer. 10:6322010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhunia AK, Piontek K, Boletta A, et al:
PKD1 induces p21(waf1) and regulation of the cell cycle via direct
activation of the JAK-STAT signaling pathway in a process requiring
PKD2. Cell. 109:157–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiao X, Pham DN, Luo H and Wu J: Ran
overexpression leads to diminished T cell responses and selectively
modulates nuclear levels of c-Jun and c-Fos. J Biol Chem.
285:5488–5496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee SY, Yoon J, Lee HS, et al: The
function of heterodimeric AP-1 comprised of c-Jun and c-Fos in
activin mediated Spemann organizer gene expression. PLoS One.
6:e217962011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW
and Jeong HG: Suppression of PMA-induced tumor cell invasion by
dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and
NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol.
79:1714–1726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Francis H, Onori P, Gaudio E, et al: H3
histamine receptor-mediated activation of protein kinase Calpha
inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol
Cancer Res. 7:1704–1713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uğuz A, Gönlüşen G, Ergin M and Tuncer I:
Expression of Fas, Bcl-2 and p53 molecules in glomerulonephritis
and their correlations with clinical and laboratory findings.
Nephrology (Carlton). 10:311–316. 2005.PubMed/NCBI
|
|
19
|
Fuzio P, Ditonno P, Lucarelli G, et al:
Androgen deprivation therapy affects BCL-2 expression in human
prostate cancer. Int J Oncol. 39:1233–1242. 2011.PubMed/NCBI
|
|
20
|
Shinagawa A, Yoshida Y, Kurokawa T, et al:
The potent peptide antagonist to angiogenesis, C16Y, and cisplatin
act synergistically in the down-regulation of the Bcl-2/Bax ratio
and the induction of apoptosis in human ovarian cancer cells. Int J
Oncol. 39:1359–1364. 2011.
|
|
21
|
Stamenkovic I: Extracellular matrix
remodeling: the role of metalloproteinases. J Pathol. 200:448–464.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Selvais C, Gaide Chevronnay HP, Lemoine P,
et al: Metalloproteinase-dependent shedding of low-density
lipoprotein receptor-related protein-1 ectodomain decreases
endocytic clearance of endometrial matrix metalloproteinase-2 and
-9 at menstruation. Endocrinology. 150:3792–3799. 2009. View Article : Google Scholar
|
|
23
|
Bauvois B, Mothu N, Nguyen J, Nguyen-Khoa
T, Nöel LH and Jungers P: Specific changes in plasma concentrations
of matrix metalloproteinase-2 and -9, TIMP-1 and TGF-beta1 in
patients with distinct types of primary glomerulonephritis. Nephrol
Dial Transplant. 22:1115–1122. 2007. View Article : Google Scholar
|
|
24
|
Jacob-Ferreira AL, Lacchini R, Gerlach RF,
Passos CJ, Barbosa F Jr and Tanus-Santos JE: A common matrix
metalloproteinase (MMP)-2 polymorphism affects plasma MMP-2 levels
in subjects environmentally exposed to mercury. Sci Total Environ.
409:4242–4246. 2011. View Article : Google Scholar
|
|
25
|
Davies M, Martin J, Thomas GJ and Lovett
DH: Proteinases and glomerular matrix turnover. Kidney Int.
41:671–678. 1992. View Article : Google Scholar : PubMed/NCBI
|