Introduction
Gastric cardiac adenocarcinomas (GCAs) are derived
from the most proximal region of the stomach immediately adjoining
the esophagus. GCA is one of the most common forms of malignant
cancer in China. Epidemiological studies have revealed that the
incidence of GCA has been increasing every year, and is rapidly
becoming one of the leading causes of malignant tumors worldwide
(1–3). Although there has been great progress
in the diagnosis and treatment of GCA, the five-year survival rate
remains at <24%. Therefore, there is an urgent requirement for
the development of functional biomarkers for the early diagnosis of
GCA and improved survival rates.
Midkine (MK) was originally identified as a retinoic
acid-inducible gene in murine embryonic carcinoma cells. MK is
known to be involved in early embryogenesis. In adults, low levels
of MK are detectable in kidney tissue (4). However, MK expression is frequently
upregulated in numerous solid tumors, including gastric cancer
(5,6). Deregulation of MK expression is
associated with tumor invasion and a poor prognosis (7), indicating that MK may be involved in
tumorigenesis.
MK is involved in the regulation of a large number
of cellular activities. MK binds heparin to promote proliferation,
survival and migration by activating cell growth signaling
pathways. The interaction of MK with anaplastic lymphoma kinase
(ALK) leads to the activation of the phosphoinositide-3 kinase/Akt
and mitogen-activated protein kinase pathways. MK induces neurite
outgrowth and migration by interacting with β1-integrin (8). In addition, MK tightly binds
proteoglycans (PGs), including syndecan-1 [also known as cluster of
differentiation (CD)138] (9).
Syndecan-1 has a role in cell growth and differentiation by
interacting with several growth factors through its extracellular
heparan sulfate (HS) glycosaminoglycan chains (10). The differentiation and
morphogenesis of vertebrate embryos depends on the interaction of
syndecan-1 with MK (9). Syndecan-1
is also involved in cell adhesion and migration by controlling
cell-cell and cell-extracellular matrix interactions (11). The correlation between syndecan-1
and tumor development has been a controversial subject. It has been
reported that the loss of syndecan-1 facilitates the migration of
metastatic tumor cells, and is correlated with a poor prognosis in
patients with head and neck, non-small cell lung and hepatocellular
carcinomas (12,13). Furthermore, another study has
indicated that the overexpression of syndecan-1 is correlated with
a poor outcome in patients with cancer (14). However, further studies are
required to confirm the role of syndecan-1 in tumor development and
progression.
A concomitant increase in MK expression and a
decrease in syndecan-1 expression in certain cancer types prompted
the present study to examine the expression levels of MK and
syndecan-1 in the same GCA tissue samples, and the exploration of
the roles of MK and syndecan-1 in GCA development, invasion and
metastasis. The present study assessed the expression levels of MK
and syndecan-1 in 72 surgically obtained GCA tissue samples and 40
normal gastric cardia tissue samples, and assessed the correlation
between MK and syndecan-1 expression and the clinicopathological
features of GCA.
Materials and methods
Patients and tissue samples
A total of 72 paraffin-embedded GCA tissue samples
were obtained from the Department of Pathology of the First
Affiliated Hospital of Henan University of Science and Technology
(Luoyang, China). The paraffin-embedded samples had been surgically
resected and pathologically diagnosed between 2007 and 2009. The
research protocol and the consent forms were approved by the
Institutional Committee for the Protection of Human Subjects. The
72 GCA samples had been extracted from 56 male and 16 female
patients. The average age of the patients was 55.4±7.56 years. A
total of 40 samples of normal tissue were obtained from 28 males
and 12 females, with an average age of 55.3±8.3 years. None of the
patients received radiotherapy or chemotherapy treatment prior to
the surgery.
Reagents and antibodies
Rabbit anti-human MK polyclonal antibody was
obtained from Santa Cruz Biotechnology, Inc., (bs-1849R; Dallas,
TX, USA) and mouse anti-syndecan-1 monoclonal antibody was obtained
from Beijing Zhongshan Jinqiao Biotechnology (ZM-0459; Beijing,
China). Anti-rabbit and anti-rat streptavidin-peroxidase (SP)
high-sensitivity kits were purchased from Fuzhou Maixin
Biotechnology (KIT-9710; Fujian, China).
Immunohistochemistry and criteria for
analysis of results
Three 4-μm thick slices cut from the formalin-fixed,
paraffin-embedded tissue blocks were used for immunohistochemical
staining. Known positive tissue sections served as positive
controls. All immunohistochemical data were evaluated by two
pathologists separately. MK and syndecan-1 staining was visible on
the cell membrane and/or in the cytoplasm. The presence of clear
yellow-brown granules in the cytoplasm or on the cell membrane was
considered as positive staining for MK and syndecan-1.
Semi-quantitative evaluation was performed, with scores as follows:
0, <10% positive cells (−); 1, 10–49% positive cells (+); 2,
50–75% positive cells (++); and 3, >75% positive cells (+++)
displaying cytoplasmic and membrane-associated immunoreactivity.
Scores of 1, 2 and 3 were interpreted as positive (+) staining in
all tissues.
Statistical analysis
Statistical analysis was performed using the SPSS
17.0 statistical software package (SPSS, Inc., Chicago, IL, USA).
The χ2 or Fisher’s exact probability tests were applied
for the analysis of qualitative variables, and the Spearman’s rank
correlation coefficient was used to analyze the correlation between
MK and syndecan-1. P<0.05 was used to indicate a statistically
significant difference. Overall survival curves were generated
using the Kaplan-Meier method, and the differences between the
groups were analyzed using the log-rank test.
Results
Expression levels of MK and syndecan-1 in
GCA and atypical hyperplasia tissues
Immunohistochemical staining showed that MK and
syndecan-1 were expressed on the cell membranes and/or in the
cytoplasm. Among the GCA samples, 76.4% (55/72) exhibited positive
staining for MK (Fig. 1A), whereas
only 5% (2/40) of the normal gastric cardiac specimens showed
positive staining for MK (P<0.05) (Fig. 1B). Positive staining for syndecan-1
was observed in 100% (40/40) of the normal gastric cardiac
specimens (Fig. 2A), whereas 38.9%
(28/72) of GCA tissue specimens showed syndecan-1 staining
(P<0.05) (Fig. 2B). Significant
differences were observed between normal cardiac mucosa and GCA
tissue samples for MK and syndecan-1 levels (P<0.05) (Table I). These data demonstrate that the
expression levels of MK had increased, whereas those of syndecan-1
had decreased in progressive GCA, indicating that MK and syndecan-1
have significant roles in the tumorigenesis of GCA.
 | Table IExpression levels of MK and syndecan-1
in various gastric cardiac mucosal tissue samples.a |
Table I
Expression levels of MK and syndecan-1
in various gastric cardiac mucosal tissue samples.a
| MK, n (%) | | | Syndecan-1, n
(%) | | |
|---|
|
| | |
| | |
|---|
| Group | Negative | Positive | χ2 | P-value | Negative | Positive | χ2 | P-value |
|---|
| Normal cardiac mucosa
(n=40) | 38 (95.0) | 2 (5.0) | 52.437 | <0.01 | 0 (0.0) | 40 (100.0) | 40.261 | <0.01 |
| GCA (n=72) | 17 (24.6) | 55 (76.4) | | | 44 (61.1) | 28 (38.9) | | |
Association between the
clinicopathological features of GCA and the expression levels of MK
or syndecan-1
MK expression levels were positively correlated with
metastases of the lymph node (χ2, 8.50; P<0.05) and
stromal invasion (χ2, 5.073; P<0.01). MK expression
was not associated with age, gender, tumor size or the degree of
histological differentiation of GCA (P>0.05) (Table II). Logistic regression analysis
showed that the MK levels were closely associated with metastases
to the lymph nodes (P=0.007) and the depth of serosal membrane
invasion (P=0.018) (Table III).
Univariate analysis showed that the expression levels of syndecan-1
were not correlated with age, gender or tumor size (P>0.05), but
that they were negatively correlated with the degree of
histological differentiation of GCA (χ2, 6.768;
P<0.05), stromal invasion (χ2, 7.182; P<0.01) and
metastases of the lymph nodes (χ2, 10.979; P<0.01)
(Table II). Similarly, logistic
regression analysis demonstrated that syndecan-1 levels were not
associated with metastases to the lymph nodes (P=0.271) or the
degree of differentiation (P=0.307), but that they were closely
associated with the depth of serosal membrane invasion (P=0.021)
(Table IV). Therefore, MK
expression levels were correlated with serosal membrane invasion
and lymph node metastases, while syndecan-1 expression levels were
correlated with serosal membrane invasion only.
 | Table IIAssociation between
clinicopathological features of GCA and expression of MK or
syndecan-1. |
Table II
Association between
clinicopathological features of GCA and expression of MK or
syndecan-1.
| MK | Syndecan-1 |
|---|
|
|
|
|---|
| Factors | Positive, n | % | P-value | Positive, n | % | P-value |
|---|
| Gender |
| Male (n=56) | 45 | 80.4 | 0.152 | 22 | 39.3 | 0.897 |
| Female (n=16) | 10 | 62.5 | | 6 | 37.5 | |
| Age (years) |
| <60 (n=34) | 28 | 82.4 | 0.256 | 16 | 47.1 | 0.554 |
| ≥60 (n=38) | 27 | 71.1 | | 12 | 31.6 | |
| Depth of
invasion |
| No stromal
invasion (n=25) | 15 | 60.0 | 0.017 | 15 | 60.0 | 0.007 |
| Invaded stroma
(n=47) | 40 | 85.1 | | 13 | 27.7 | |
| Degree of
differentiation |
| High (n=11) | 9 | 81.8 | 0.894 | 8 | 72.7 | 0.034 |
| Moderate
(n=45) | 34 | 75.6 | | 16 | 35.6 | |
| Low (n=16) | 12 | 75.0 | | 4 | 25.0 | |
| Lymph node
metastasis |
| Positive
(n=43) | 38 | 88.4 | 0.004 | 10 | 23.3 | 0.001 |
| Negative
(n=29) | 17 | 58.6 | | 18 | 62.1 | |
 | Table IIILogistic regression analysis for the
association between MK levels and lymph node metastasis or serosal
membrane invasion. |
Table III
Logistic regression analysis for the
association between MK levels and lymph node metastasis or serosal
membrane invasion.
| | | | | | | 95% CI for Exp
(B) |
|---|
| | | | | | |
|
|---|
| Parameter | B | S.E. | Wald | df | P-value | Exp (B) | Lower | Upper |
|---|
| Lymph node
metastasis | −1.723 | 0.641 | 7.232 | 1 | 0.007 | 0.178 | 0.051 | 0.627 |
| Serosal membrane
invasion | 1.483 | 0.629 | 5.550 | 1 | 0.018 | 4.404 | 1.283 | 15.119 |
 | Table IVLogistic regression analysis for the
association between syndecan-1 levels and differentiation, lymph
node metastasis or serosal membrane invasion. |
Table IV
Logistic regression analysis for the
association between syndecan-1 levels and differentiation, lymph
node metastasis or serosal membrane invasion.
| | | | | | | 95% CI for Exp
(B) |
|---|
| | | | | | |
|
|---|
| Parameter | B | S.E. | Wald | df | P-value | Exp (B) | Lower | Upper |
|---|
| Differentiation
status | 0.532 | 0.536 | 2.363 | 2 | 0.307 | 1.972 | 0.735 | 5.899 |
| Lymph node
metastasis | 0.621 | 0.545 | 1.210 | 1 | 0.271 | 1.821 | 0.626 | 5.299 |
| Serosal membrane
invasion | −1.284 | 0.556 | 5.331 | 1 | 0.021 | 0.277 | 0.093 | 0.824 |
Expression levels of MK and syndecan-1
are inversely correlated
The correlation between the expression levels of MK
and syndecan-1 was assessed for the 72 cases of GCA by Spearman’s
rank correlation analysis. It was revealed that the expression
levels of MK were negatively correlated with those of syndecan-1.
Increases in the levels of MK led to concomitant decreases in the
levels of syndecan-1 (r=−0.352; P<0.01) (Table V).
 | Table VCorrelation between the expression of
MK and syndecan-1.a |
Table V
Correlation between the expression of
MK and syndecan-1.a
| MK, n | |
|---|
|
| |
|---|
| Syndecan-1 | − | + | ++ | +++ | Total |
|---|
| − | 5 | 9 | 13 | 17 | 44 |
| + | 2 | 2 | 1 | 3 | 8 |
| ++ | 4 | 2 | 2 | 2 | 10 |
| +++ | 6 | 1 | 2 | 1 | 10 |
| Total | 17 | 14 | 18 | 23 | 72 |
Association between the prognosis of GCA
and the expression levels of MK or syndecan-1
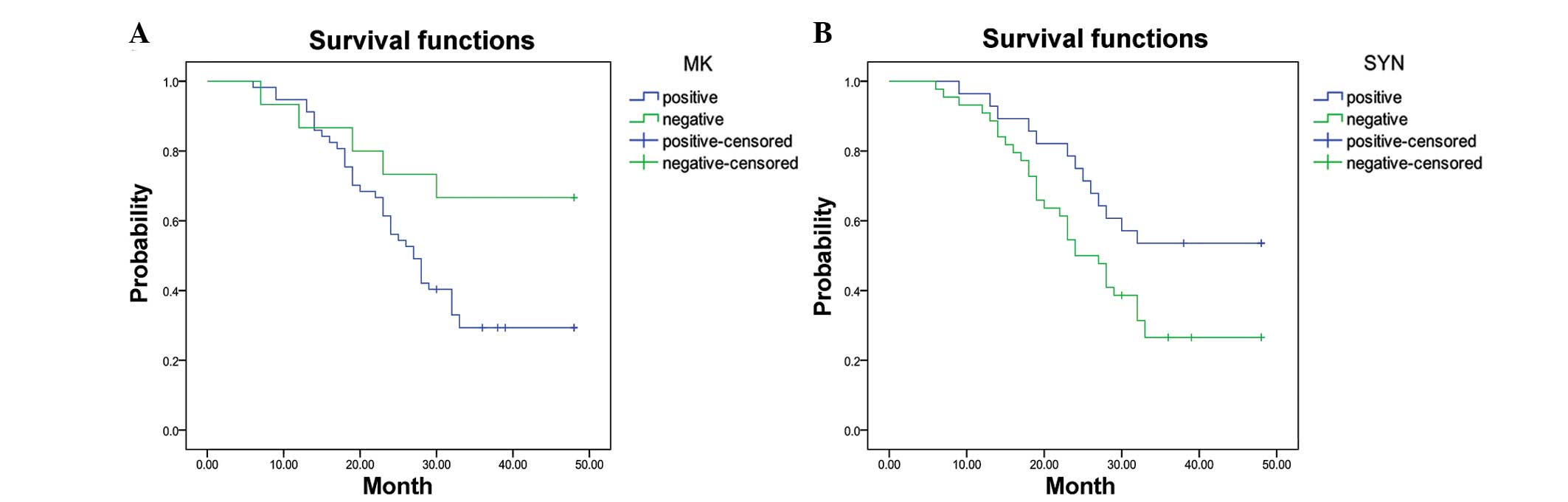
Among the 72 cases of GCA, the median survival time
for patients with increased MK expression was 27 months [95%
confidence interval (CI), 23.3–30.7 months]. The three-year
survival rate of patients with high levels of MK (29.8%) was
significantly lower than that of patients with low levels of MK
(66.8%) (P=0.029) (Fig. 3A). The
median survival time of patients with low expression levels of
syndecan-1 was 24 months (95% CI, 19.7–28.3 months). The three-year
survival rate for patients with low expression levels of syndecan-1
(27.3%) was significantly lower than that for the group with high
syndecan-1 levels (53.6%) (P=0.028; Fig. 3B).
Discussion
GCA is a malignant carcinoma that arises in the
gastric cardiac tissue. It is one of the most common malignant
tumors of the digestive tract in Northern China. The incidence of
gastric GCA has increased rapidly (1), whereas that of distal gastric
adenocarcinoma has steadily decreased (2,3).
Epidemiological and population cohort studies have shown that risk
the factors and the clinical and pathological characteristics of
GCA are distinctly different from those of gastric cancers that
arise in the distal parts, indicating that GCA is a separate
disease (15–17). As early symptoms of GCA are
non-specific and lack sensitive and specific biomarkers for early
diagnosis, the majority of patients are diagnosed at advanced
stages; therefore, the five-year survival rate for patients with
GCA does not exceed 24%, even with comprehensive treatment. Thus,
to improve the early diagnosis of GCA, it is important to
investigate the molecular mechanisms underlying the development and
progression of GCA and to identify possible biomarkers. A number of
studies have analyzed the expression of MK or syndecan-1 in distal
gastric adenocarcinoma; however, few studies have explored this in
GCA (18–20).
Human MK can bind syndecan proteoglycan family
members, receptor-type tyrosine kinase-ξ, low-density lipoprotein,
receptor-related protein and ALK. MK binds tightly to the syndecan
proteins, including syndecan-1, 3 and 4, through HS (9). Deregulation of MK is associated with
the pathogenesis of numerous diseases, including cancer (7,21).
MK promotes carcinogenesis by enhancing fibrinolysis, cell
transformation, migration, cell survival and anti-apoptotic
activity through a molecular mechanism that remains to be
elucidated (22). The
overexpression of MK is common in numerous malignant tumor types
and particularly in advanced cancer types, including esophageal
(23), gastric (1,24),
colorectal (25), liver (26), pancreatic, lung (27) and breast (28) cancer and neuroblastoma (29). The overexpression of MK is closely
correlated with tumor development and progression (30); however, expression levels of MK in
GCA have yet to be reported.
In the present study, the overexpression of MK was
present in 55 out of 72 tumor samples from patients with GCA
(76.4%), which was significantly higher than for normal tissue
samples. These results are consistent with the 65–80% positive
rates for gastric cancer (20,31).
Furthermore, the MK overexpression rates in the lymph node
metastasis and stromal invasion groups were significantly higher
than in the groups with no lymph node metastasis (χ2,
8.5; P<0.05) and no stromal invasion (χ2, 5.073;
P<0.01). Logistic regression analysis revealed that high MK
expression was associated with lymph node metastases (P=0.007) and
the depth of serosal membrane invasion (P=0.018). Notably, the
increased expression of MK in the tumor tissue was correlated with
a shorter median survival time and lower three-year survival rates
compared with patients with low MK expression levels. These results
indicate that the overexpression of MK in GCA may promote the
proliferation of cancer cells and contribute to cancer invasion and
metastasis, which is associated with late clinical stages and a
poor prognosis for patients with GCA.
Syndecans are transmembrane proteoglycans that carry
covalently bound HS side chains. There are four members in this
family, syndecan-1, -2, -3 and -4, which are encoded by different
genes. Syndecan-1 (CD138) is an important component of the plasma
membrane (32); it is a
transmembrane HS proteoglycan (HSPG) that is mainly expressed in
the epithelial cells. HS is a polysaccharide that usually occurs in
the form of HSPG. The HS chains allow for the interaction with a
variety of regulatory factors. Syndecan-1 binds to a variety of
growth factors, including MK, through its HS side chains to
regulate cell growth, differentiation, adhesion and migration, as
well as cell-cell and cell-extracellular matrix interactions
(33). The association between
syndecan-1 expression and tumor development is not currently clear.
A number of studies have indicated that syndecan-1 inhibits tumor
development and is absent or present at extremely low levels in
most solid malignant tumors, including head and neck squamous cell,
cervical, gastrointestinal and liver cancer (12,13).
As syndecan-1 is a cell surface adhesion molecule, complete loss or
reduction of syndecan-1 may facilitate the migration of metastatic
cells. Therefore, decreased syndecan-1 expression may be indicative
of aggressive malignant behavior (34). By contrast, certain studies have
demonstrated that syndecan-1 promotes metastasis in rat lung
squamous cell cancer (13).
Moreover, its expression levels are increased in pancreatic,
gastric and breast cancer. Thus, increased expression of syndecan-1
is correlated with tumor invasion, metastasis and a poor prognosis
(35).
The results of the present study showed that
syndecan-1 was expressed in 100% of the normal cardiac mucosa
tissue samples, whereas only 38.95% of the GCA samples
(χ2, 40.261; P<0.01) were positive for syndecan-1.
These observations indicate that a loss of syndecan-1 expression is
associated with the development of GCA. The expression levels of
syndecan-1 were associated with the degree of differentiation. A
total of 72.7, 35.6 and 25% of cells expressed syndecan-1 in the
samples with high, medium and low degrees of differentiation,
respectively. Statistical analysis revealed that low expression
levels of syndecan-1 were significantly correlated with the degree
of differentiation, presence of lymph node metastases, stromal
invasion of GCA, short median survival time and low three-year
survival rate (P<0.01). Logistic regression analysis showed that
syndecan-1 expression was correlated only with serosal membrane
invasion. These results indicate that syndecan-1 expression may be
a prognostic marker for patients with GCA. However, other studies
have indicated that patients with gastric cancer with low
epithelial syndecan-1 expression levels have poor overall survival
rates (36). In addition, high
stromal syndecan-1 expression levels have been shown to correlate
with decreased epithelial syndecan-1 expression, which led to
significantly reduced survival times in females (36). Thus, further evaluation of the
expression levels of epithelial and stromal syndecan-1 in GCA
tissue is required to ascertain its prognostic value.
Furthermore, the statistical analysis performed in
the present study showed a strong inverse correlation between MK
and syndecan-1 (r=−0.352, P<0.01). The overexpression of MK was
frequently detected in GCA tissue in which syndecan-1 was absent,
indicating that MK and syndecan-1 are involved in the development
and progression of GCA. The concomitant loss of syndecan-1 and
overexpression of MK may promote the development of GCA. Loss of
syndecan-1 on the tumor cell surface may be due to increased
enzymatic activity of heparanase, which degrades the extracellular
HS chains of syndecan-1. This loss of HS chains may negatively
affect the interaction of syndecan-1 with heparin-binding growth
factors, including fibroblast growth factor (FGF), hepatocyte
growth factor and vascular endothelial growth factor (37). Numerous studies have demonstrated
that basic FGF (bFGF) is inactive when HS is present on the cell
surface membrane or in the extracellular matrix, but its biological
activity is restored when it is released from the cell surface
following hydrolysis of HS by acetyl-heparanase (38,39).
The mechanism by which syndecan-1 and MK impact the
development of GCA remains to be elucidated. Although the
statistical analysis performed in this study showed that there is a
correlation between the expression of MK and syndecan-1 in GCA
tissue, the functional link between these two molecules may be
affected by interactions with other factors, as MK binds to a wide
variety of receptors. The interaction with these receptors may
exert a synergistic affect on the biological activity of MK. Thus,
further studies are required to enhance our understanding of the
mechanisms by which MK and syndecan-1 interact with each other.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. U1204819).
References
|
1
|
Wang LD, Qin YR, Fan ZM, et al:
Comparative genomic hybridization: comparison between esophageal
squamous cell carcinoma and gastric cardia adenocarcinoma from a
high-incidence area for both cancers in Henan, northern China. Dis
Esophagus. 19:459–467. 2006. View Article : Google Scholar
|
|
2
|
Devesa SS, Blot WJ and Fraumeni JF Jr:
Changing patterns in the incidence of esophageal and gastric
carcinoma in the United States. Cancer. 83:2049–2053. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Botterweck AA, Schouten LJ, Volovics A,
Dorant E and van Den Brandt PA: Trends in incidence of
adenocarcinoma of the oesophagus and gastric cardia in ten European
countries. Int J Epidemiol. 29:645–654. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kadomatsu K and Muramatsu T: Midkine and
pleiotrophin in neural development and cancer. Cancer Lett.
204:127–143. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao ZQ, Yang S and Lu HS: Expression of
midkine and vascular endothelial growth factor in gastric cancer
and the association of high levels with poor prognosis and
survival. Mol Med Rep. 5:415–419. 2012.PubMed/NCBI
|
|
6
|
Huang Y, Cao G, Wang H, Wang Q and Hou Y:
The expression and location of midkine in gastric carcinomas of
Chinese patients. Cell Mol Immunol. 4:135–140. 2007.PubMed/NCBI
|
|
7
|
Muramatsu T: Midkine, a heparin-binding
cytokine with multiple roles in development, repair and diseases.
Proc Jpn Acad Ser B Phys Biol Sci. 86:410–425. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muramatsu T: Midkine and pleiotrophin: two
related proteins involved in development, survival, inflammation
and tumorigenesis. J Biochem. 132:359–371. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitsiadis TA, Salmivirta M, Muramatsu T,
et al: Expression of the heparin-binding cytokines, midkine (MK)
and HB-GAM (pleiotrophin) is associated with epithelial-mesenchymal
interactions during fetal development and organogenesis.
Development. 121:37–51. 1995.
|
|
10
|
Bernfield M, Kokenyesi R, Kato M, et al:
Biology of the syndecans: a family of transmembrane heparan sulfate
proteoglycans. Annu Rev Cell Biol. 8:365–393. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Teng YH, Aquino RS and Park PW: Molecular
functions of syndecan-1 in disease. Matrix Biol. 31:3–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anttonen A, Kajanti M, Heikkilä P,
Jalkanen M and Joensuu H: Syndecan-1 expression has prognostic
significance in head and neck carcinoma. Br J Cancer. 79:558–564.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirabayashi K, Numa F, Suminami Y, et al:
Altered proliferative and metastatic potential associated with
increased expression of syndecan-1. Tumour Biol. 19:454–463. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harada K, Masuda S, Hirano M and Nakanuma
Y: Reduced expression of syndecan-1 correlates with histologic
dedifferentiation, lymph node metastasis, and poor prognosis in
intrahepatic cholangiocarcinoma. Hum Pathol. 34:857–863. 2003.
View Article : Google Scholar
|
|
15
|
Wu X, Chen VW, Andrews PA, Ruiz B and
Correa P: Incidence of esophageal and gastric cancers among
Hispanics, non-Hispanic whites and non-Hispanic blacks in the
United States: subsite and histology differences. Cancer Causes
Control. 18:585–593. 2007. View Article : Google Scholar
|
|
16
|
Wijetunge S, Ma Y, DeMeester S, et al:
Association of adenocarcinomas of the distal esophagus,
‘gastroesophageal junction,’ and ‘gastric cardia’ with gastric
pathology. Am J Surg Pathol. 34:1521–1527. 2010.PubMed/NCBI
|
|
17
|
Xiao ZY, Ru Y, Sun JT, et al: Expression
of CDX2 and villin in gastric cardiac intestinal metaplasia and the
relation with gastric cardiac carcinogenesis. Asian Pac J Cancer
Prev. 13:247–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ding GC, Ren JL, Chang FB, et al: Human
papillomavirus DNA and P16(INK4A) expression in concurrent
esophageal and gastric cardia cancers. World J Gastroenterol.
16:5901–5906. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu YQ, Ye ZY, Tao HQ, et al: Relationship
between cell adhesion molecules expression and the biological
behavior of gastric carcinoma. World J Gastroenterol. 14:1990–1996.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Obata Y, Kikuchi S, Lin Y, et al: Serum
midkine concentrations and gastric cancer. Cancer Sci. 96:54–56.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakamoto K and Kadomatsu K: Midkine in the
pathology of cancer, neural disease, and inflammation. Pathol Int.
62:445–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Michikawa M, Kikuchi S, Muramatsu H,
Muramatsu T and Kim SU: Retinoic acid responsive gene product,
midkine, has neurotrophic functions for mouse spinal cord and
dorsal root ganglion neurons in culture. J Neurosci Res.
35:530–539. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimada H, Nabeya Y, Okazumi S, et al:
Increased serum midkine concentration as a possible tumor marker in
patients with superficial esophageal cancer. Oncol Rep. 10:411–414.
2003.PubMed/NCBI
|
|
24
|
Xu YY, Mao XY, Song YX, et al: Midkine
confers Adriamycin resistance in human gastric cancer cells. Tumour
Biol. 33:1543–1548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krzystek-Korpacka M, Diakowska D,
Grabowski K and Gamian A: Tumor location determines midkine level
and its association with the disease progression in colorectal
cancer patients: a pilot study. Int J Colorectal Dis. 27:1319–1324.
2012. View Article : Google Scholar
|
|
26
|
Hung YJ, Lin ZH, Cheng TI, Liang CT, Kuo
TM and Kao KJ: Serum midkine as a prognostic biomarker for patients
with hepatocellular carcinoma. Am J Clin Pathol. 136:594–603. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao G, Nie Y, Lv M, He L, Wang T and Hou
Y: ERβ-mediated estradiol enhances epithelial mesenchymal
transition of lung adenocarcinoma through increasing transcription
of midkine. Mol Endocrinol. 26:1304–1315. 2012.
|
|
28
|
Ibusuki M, Fujimori H, Yamamoto Y, et al:
Midkine in plasma as a novel breast cancer marker. Cancer Sci.
100:1735–1739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reiff T, Huber L, Kramer M, Delattre O,
Janoueix-Lerosey I and Rohrer H: Midkine and Alk signaling in
sympathetic neuron proliferation and neuroblastoma predisposition.
Development. 138:4699–4708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fabri L, Maruta H, Muramatsu H, et al:
Structural characterisation of native and recombinant forms of the
neurotrophic cytokine MK. J Chromatogr. 646:213–225. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao ZQ, Yang S, Lu HS, et al: Expression
of midkine and vascular endothelial growth factor in gastric cancer
and the association of high levels with poor prognosis and
survival. Mol Med Rep. 5:415–419. 2012.PubMed/NCBI
|
|
32
|
Teng YH, Aquino RS and Park PW: Molecular
functions of syndecan-1 in disease. Matrix Biol. 31:3–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fux L, Ilan N, Sanderson RD and Vlodavsky
I: Heparanase: busy at the cell surface. Trends Biochem Sci.
34:511–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang MF, Zhu YQ, Chen ZF, et al:
Syndecan-1 and E-cadherin expression in differentiated type of
early gastric cancer. World J Gastroenterol. 11:2975–2980. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conejo JR, Kleeff J, Koliopanos A, et al:
Syndecan-1 expression is up-regulated in pancreatic but not in
other gastrointestinal cancers. Int J Cancer. 88:12–20. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wiksten JP, Lundin J, Nordling S, et al:
Epithelial and stromal syndecan-1 expression as predictor of
outcome in patients with gastric cancer. Int J Cancer. 95:1–6.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vlodavsky I, Friedmann Y, Elkin M, et al:
Mammalian heparanase: gene cloning, expression and function in
tumor progression and metastasis. Nat Med. 5:793–802. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Naggi A, Casu B, Perez M, et al:
Modulation of the heparanase-inhibiting activity of heparin through
selective desulfation, graded N-acetylation, and glycol splitting.
J Biol Chem. 280:12103–12113. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kato M, Wang H, Kainulainen V, et al:
Physiological degradation converts the soluble syndecan-1
ectodomain from an inhibitor to a potent activator of FGF-2. Nat
Med. 4:691–707. 1998. View Article : Google Scholar : PubMed/NCBI
|