Introduction
Radiation therapy has wide clinical utility,
particularly in the treatment of solid tumors (1). The application of radiation therapy
in hematological malignancies initially began in the 1920s, using
the technique of low-dose total body irradiation (LDBI) for the
treatment of low-grade malignant non-Hodgkin’s lymphoma and chronic
lymphocytic leukemia, which achieved notable efficacy (2–4).
Until the middle of the 20th century, high-dose
total body irradiation (TBI) replaced LDBI in the pretreatment of
bone marrow transplantation, establishing a classic conditioning
regimen of TBI/CY that remains in use today. TBI has an established
role as a preparative regimen for bone-marrow transplantation in
the treatment of hematological malignancies. Numerous randomized
trials have demonstrated that the clinical outcomes obtained from
the combination of TBI and cyclophosphamide are equivalent to those
based on chemotherapeutic agents, and are occasionally better than
them. Despite the therapeutic progress and the improvement in the
overall survival in recent years, this preparative regimen still
has a relatively high rate of acute and late toxicity, including
gastrointestinal toxicity, infections, hemorrhagic cystitis,
hepatic veno-occlusive disease (VOD), acute graft-versus-host
disease (aGVHD) and interstitial pneumonia (IP). Given the
limitations of radiation therapy, novel treatment strategies that
overcome acquired toxicity are urgently required.
Luckey (5) first
proposed the excitatory effect produced by low-dose irradiation
(LDR). The study’s experimental results demonstrated that moderate
LDR is not only harmless to humans, but also beneficial to life
activity by promoting fertility and reproduction, increasing
physical defense capability, producing self-adaptability and
enhancing damage repair ability. It is, however, the apparent
anti-tumor, radiation hypersensitivity and self-adaptive effects,
which were generated by LDR, that raised marked concern amongst
oncologists.
LDR is a type of irradiation with a radiation dose
of <20c Gy (low LET) and <5c Gy (high LET), with a dose rate
that ranges between 5–10c Gy/min. LDR confers significant benefits
by inducing a radio-adaptive response, where pre-irradiation with
low doses allows the body to produce an adaptive response to
subsequent high-dose irradiation, thereby reducing the radiation
damage effect caused by high doses radiation. Olivieri et al
(6) first proposed that a
low-level radiation may induce genetic self-adaptive responses in
human lymphocytes, including cytogenetic, gene mutation, cell
survival as well as immune adaptive responses.
The possible mechanisms responsible for the
difference in the induction of radio-adaptive response by LDR
between normal tissues and tumor cells have remained controversial.
Hendrikse et al (7)
demonstrated that p53 has an important role in determining the
LDR-induced adaptive response. However, evidence exists that does
not support the significance of p53 in the induction of
radioresistance by LDR. Seong et al (8) demonstrated that radio adaption or
radio sensitivity induced by LDR was independent of the expression
of p53 in the tumor cells, which may be associated with members of
the B-cell lymphoma 2 (Bcl-2) family.
In view of the biological characteristics of LDR and
the limitation caused by radiation toxicity of TBI clinically, it
is highly important that the adaptive response induced by the LDR
may be able to alleviate the subsequent high dose of TBI treatment
toxicity if combining LDR with TBI. However, whether LDR is able to
induce radioadapative responses remains dependent on several
factors, including cell types (9).
K562 is the human erythroleukemia cell line of CML
in the blastic phase (10). Bcl-2
extra large (Bcl-xl), the inhibitory apoptosis protein of the Bcl-2
family, has a similar function to Bcl-2 in inhibiting apoptosis of
tumor cells by a variety of mechanisms, including inhibiting the
decrease of the mitochondrial membrane potential (ΔΨm).
Bcl-2-associated X (Bax), the apoptosis-promoting protein, mainly
promotes the decrease of ΔΨm through the formation of retinoid X
receptor (RXR)-RXR, resulting in the subsequent induction
apoptosis. p53, the apoptosis activating gene, is involved in
apoptosis regulation through multiple pathways. K562 is the 135th
codon insertion mutation of p53 RNA, which leads to a complete loss
of function of p53. K562 has a marked resistance to chemotherapy
and radiotherapy (11).
The present study aimed to detect the anti-tumor
effect of LDR and LDR/HDR to K562 cells in vitro and to
examine their possible mechanisms with the purpose of providing
experimental evidence for the feasibility of the conditioning
regimen, TBI combined with LDR, as a pre-transplant conditioning
regimen in the treatment of leukemia.
Materials and methods
Cell lines and culture conditions
The human cell line K562 was a gift from Jiangsu
Institute of Hematology, Suzhou University First Hospital (Jiangsu,
China). The cells were cultured in RPMI-1640 (Gibco-BRL, Carlsbad,
CA, USA) supplemented with 10% heat inactivated fetal bovine serum
(FBS; Gibco-BRL), 4 mM glutamine (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA), 50 U/ml penicillin and 50 μg/ml streptomycin
(Gibco-BRL) at 37°C in a humidified atmosphere with 5%
CO2. The cells were screened routinely to verify the
lack of mycoplasma contamination and used in the long phase of
growth.
Experimental classification
The K562 cells in the long phase of growth were
divided into four groups (ten subgroups), including a blank control
group (0 Gy; BC group), a low-dose radiation group (LDR group), a
high-dose radiation group (6 Gy, HDR group) and a low-dose
radiation combined with high-dose radiation group (LDR/HDR group).
The LDR groups were re-divided into four subsets according to the
irradiation dose: 0.08 Gy, 0.2 Gy, 0.5 Gy and 0.8 Gy. LDR/HDR
groups were divided into four subsets: 0.08 Gy/6 Gy, 0.2 Gy/6 Gy,
0.5 Gy/6 Gy and 0.8 Gy/6 Gy. Each dosage group was assayed in
triplicate.
Irradiation conditions
The K562 cells were cultivated in flasks, covered
with a 1.5 cm lucite plate and irradiated at the different doses
with 6 MV X-rays (Clinac 23EX, Varian, Palo Alto, CA, USA) using a
20 cm × 20 cm irradiation field and 100 cm of source skin distance,
with dose rates of 0.05 Gy/min for LDR and 2 Gy/min for HDR. LDR
was administered 12 h prior to HDR. The cell cultures were
maintained in cultivation and harvested at the time-points of 24,
48, 72, 96 and 120 h following irradiation at 37°C in 5%
CO2 with saturated humidity.
Measurement of apoptosis
Apoptosis was examined using a fluorescein
isothiocyanate (FITC)-labeled Annexin V/propidium iodide (PI)
apoptosis detection kit (BD Pharmingen, San Diego, CA, USA)
according to the manufacturer’s instructions. Briefly,
1×106 K562 cells were harvested and washed twice with
cold phosphate-buffered saline (PBS). The cells were then
resuspended in 250 μl 1× binding buffer. Next, 5 μl Annexin V-FITC
and 5 μl PI were added. Flow cytometric analysis was performed
immediately following staining. The data acquisition and analysis
were performed on a fluorescence-activated cell scanner (FACScan)
flow cytometer (Becton Dickinson, San Jose, CA, USA). Cells in the
early stages of apoptosis were Annexin V-positive and PI-negative,
whereas the cells in the late stages of apoptosis were both Annexin
V and PI-positive.
Western blot analysis
To determine the pre- and post radiation expression
of Bcl-xl, Bax and p53 proteins, western blot analysis was
performed. The cells were harvested from the plates, and aliquots
of cell extracts were separated by 12% SDS-PAGE. The proteins were
then transferred to a nitrocellulose membrane and incubated
overnight at 4°C with the following rabbit polyclonal antibodies:
Anti-p53, anti-Bcl-xl, anti-Bax (Cell Signaling Technology, Inc.,
Beverly, MA, USA) and anti-β-actin antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), respectively. The
membranes were then washed and incubated with alkaline phosphatase
conjugated secondary antibodies in TBST (Tris-buffered saline with
Tween-20) for 2 h and developed using a nitro blue
tetrazolium/5-bromo-4-chloro-3-indolyl phosphate color substrate
(Promega Corporation, Madison, WI, USA). The density of the protein
bands on the membrane were scanned and analyzed with an image
analyzer (Labworks software; UVP, Inc., Upland, CA, USA).
Assay of ΔΨm
The ΔΨm was determined by flow cytometry using
J-aggregate-forming lipophilic cationic probe JC-1
(5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol
carbocyanine iodide) following the manufacturer’s instructions
(Molecular Probes, Eugene, OR, USA). The ΔΨm was estimated by flow
cytometry following staining with JC-1 fluorescent dye. When the
cell is in a normal state, ΔΨm is high and JC-1 predominantly
appears as red fluorescence. When the cell is in an apoptotic or
necrotic state, the ΔΨm is reduced and JC-1 appears as a monomer
indicated by green fluorescence. A change in fluorescence from red
to green indicates a decrease in the ΔΨm. A total of
5×105/ml K562 cells in six-well plates were treated with
various concentrations of curcumin for 24 h, then washed with PBS
and incubated in the dark with JC-1 working solution for 20 min at
37°C. The cells were washed with PBS and resuspended in 500 μl PBS.
The stained cells were analyzed by flow cytometry to determine the
change in fluorescence from red to green.
Assay of caspase-3 activity
Caspase-3 activity was assessed using the caspase-3
colorimetric assay kit (Sigma-Aldrich, St. Louis, MO, USA),
following the manufacturer’s instructions. This assay is based on
the detection of the amount of
N-acetyl-Asp-Glu-Val-Asp-p-nitroanilide
(Ac-DEVD-p-NA) substrate cleaved by cell lysates to release
the colored p-NA molecule. In the present study, PANC-1
cells were exposed to punctata extract (100 μg/ml; Sigma-Aldrich)
or staurosporine (0.1 μg/ml). Following radiation, the cells were
washed in PBS and suspended in lysis buffer [50 mM hydroxyethyl
piperazineethanesulfonic acid (HEPES) pH 7.4, 5 mM cholamidopropyl
dimethylammonio-1-propanesulfonate (CHAPS) and 5 mM dithiothreitol
(DTT)] for 15 min at a concentration of 107 cells per
100 μl buffer. The lysed cells were centrifuged at 16,000 × g, 4°C
for 15 min. The protein concentrations in lysates were determined
using the Bradford assay. Equal amounts of protein (20 μg) from
each sample were added to wells containing the assay buffer (20 mM
HEPES, pH 7.4, 0.1% CHAPS, 5 mM DTT, 2 mM EDTA), followed by 10 μl
of Ac-DEVD-p-NA (20 mM), bringing the total volume of each
well to 100 μl. The caspase-3 activity was assessed by measuring
the optical density (OD) at 405 nm using an enzyme mark instrument
(Multiskan MK3; Thermo Labsystems, Waltham, MA, USA). All of the
experiments were performed in duplicate and repeated at least three
times.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The significance of the differences between groups was determined
using one-way analysis of variance and the Student-Newman-Keuls
q-test for intergroup comparison using SPSS statistical software
version 13 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
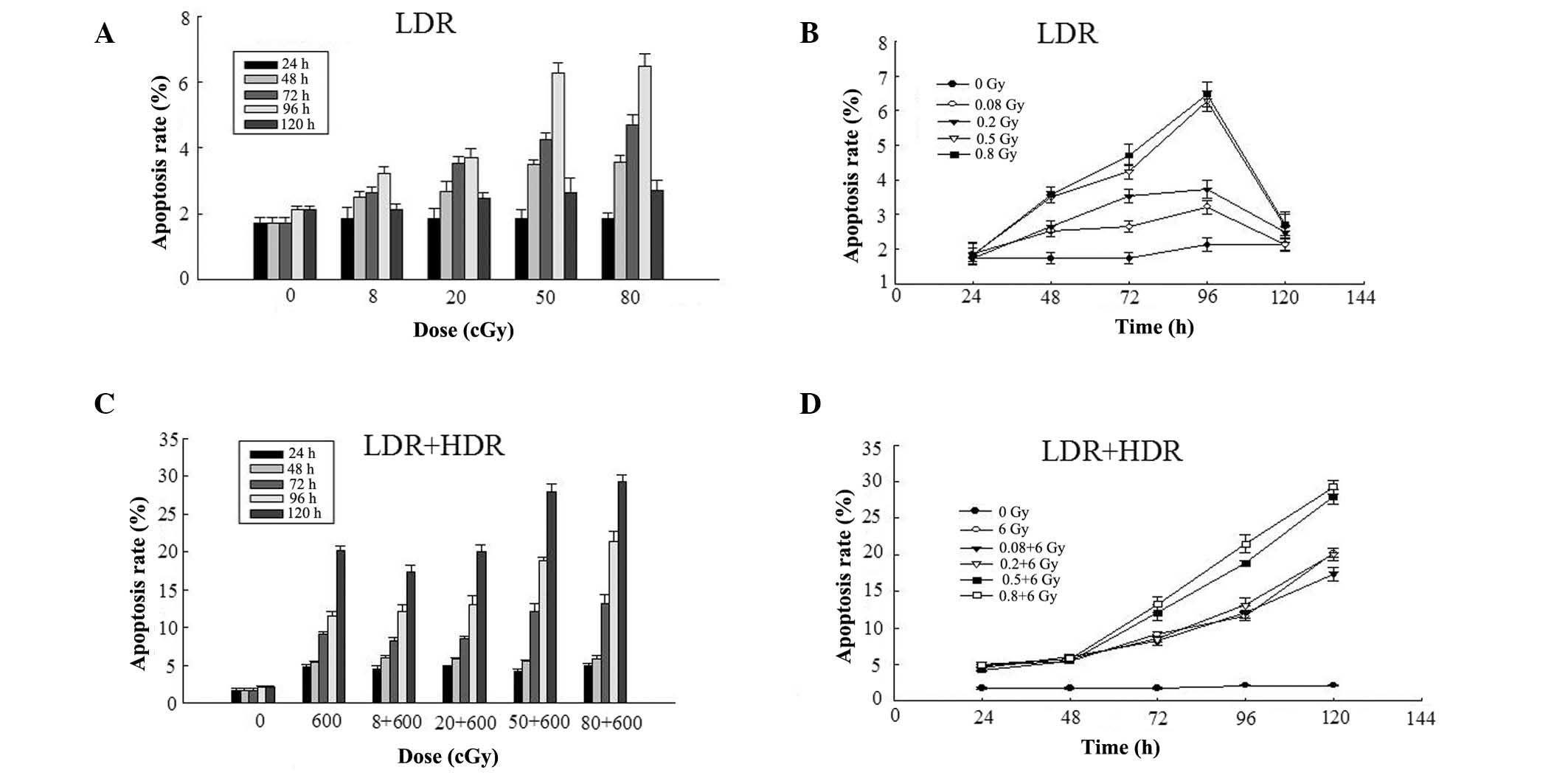
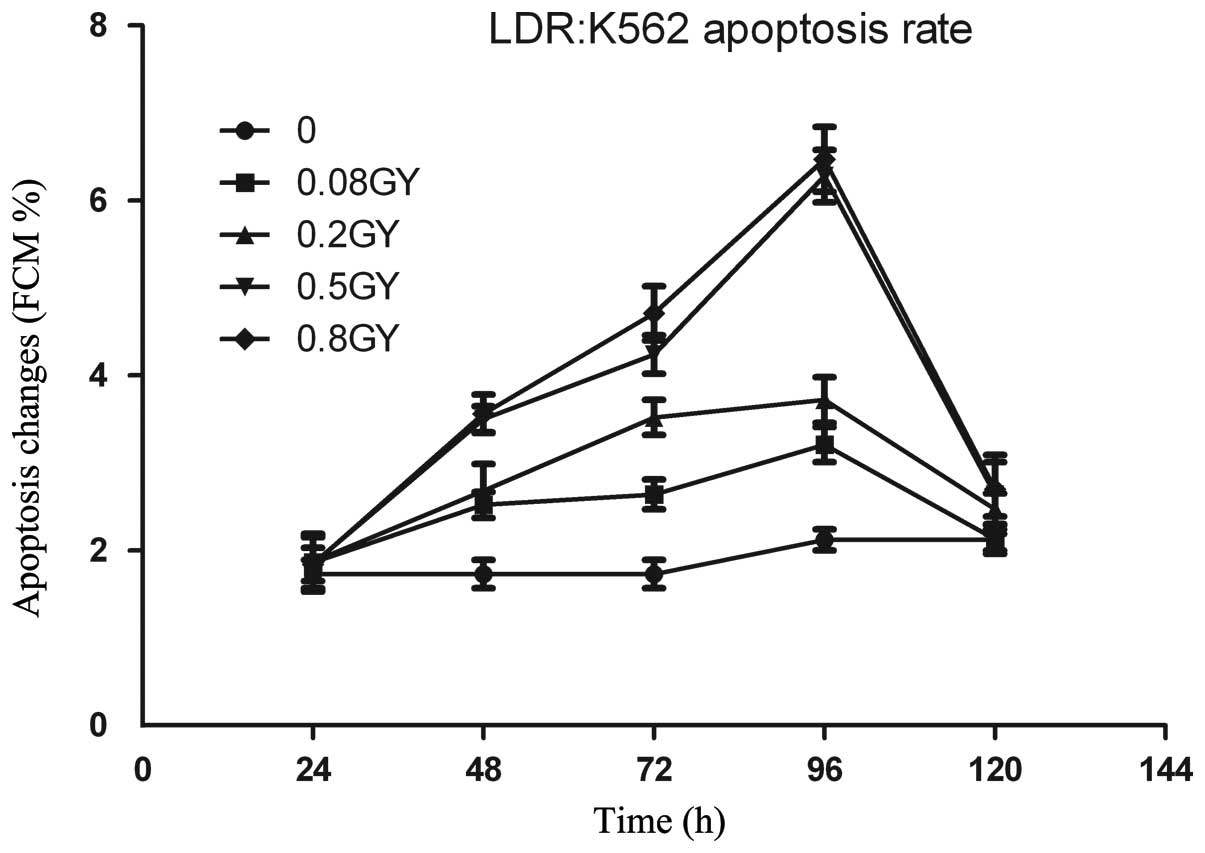
Effect of LDR on apoptosis in K562
cells
Compared with the control group, the number of
apoptotic K562 cells in all of the LDR groups increased marginally
at 24 and 120 h following radiation; however, the difference was
not statistically significant (P>0.05). Notably, the apoptotic
rate of K562 cells in all of the LDR groups increased markedly at
48, 72 and 96 h, with statistically significant differences when
compared with the control group. The apoptotic rate of K562 cells
increased in the LDR group with statistical significance compared
with the control group (P<0.01). In the 0.5 Gy group, the
apoptotic rate of K562 cells increased to 4.24±0.22% at 72 h,
6.28±0.30% at 96 h, 4.71±0.31% at 72 h and 6.47±0.37% at 96 h
following irradiation. The 0.8 Gy group exhibited statistically
significant differences at different time-points (P<0.05)
following irradiation. The apoptotic rate of K562 cells increased
to 2.52±0.15% at 48 h, 2.64±0.17% at 72 h, 3.21±0.20% at 96 h in
the 0.08 Gy group, and 2.68±0.31% at 48 h, 3.52±0.20% at 72 h,
3.72±0.26% at 96 h in the 0.2 Gy group following irradiation,
exhibiting no statistically significant differences at each
identical time-point (P>0.05; Fig.
1).
Effect of LDR/HDR on apoptosis in K562
cells
The apoptotic rate of K562 cells markedly increased
at 24 h following radiation in the HDR group, peaking at 120 h
(17.38±0.92%~29.27±0.93%), with statistically significant
differences when compared with the control group (P<0.01). The
apoptotic rate of K562 cells in the two LDR/HDR groups (0.08 Gy+6
Gy, 0.2 Gy+6 Gy) demonstrated no statistically significant
differences when compared with the HDR group at identical time
points (P>0.05). Notably, the apoptotic rate of the K562 cells
in the LDR/HDR groups (0.5 Gy+6 Gy, 0.8 Gy+6 Gy) enhanced
significantly with the dose increase of LDR in the other two
LDR/HDR groups at 72 h, peaking at 120 h (27.91±1.07%~29.27±0.93%)
following radiation in comparison with the HDR groups at the same
time points (P<0.01; Fig.
1).
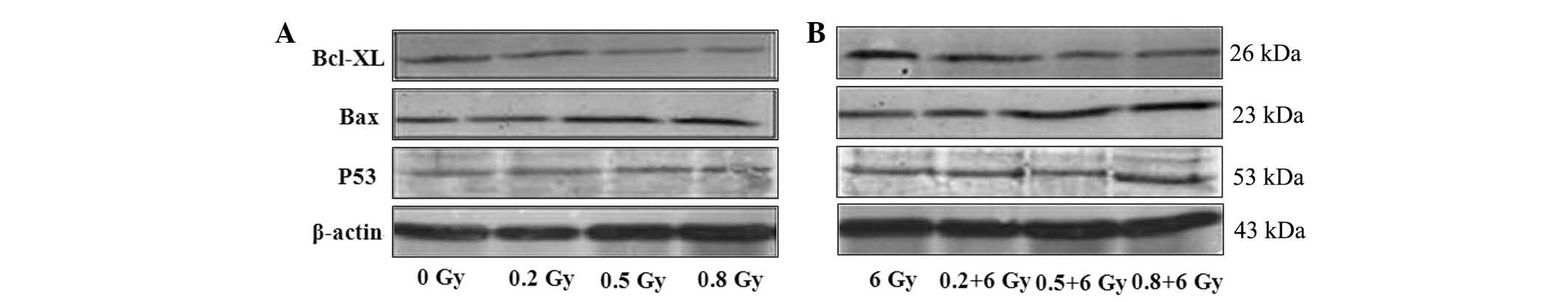
Bcl-xl protein expression is
downregulated by LDR alone or in combination with HDR
(LDR/HDR)
The expression of Bcl-xl protein in K562 cells
changed in a dose-dependent manner at 24 h following radiation.
Western blot analysis showed that the expression levels of Bcl-xl
protein were 2.65±0.30 (0.5 Gy) and 2.07±0.31 (0.8 Gy) at 24 h
following radiation, which were evidently lower than those in the
0.2 Gy radiation (3.44±0.18) and the blank control (3.96±0.08;
P<0.05) groups. The Bcl-xl protein expression was reduced even
further in the combined LDR/HDR group (6 Gy) as compared with that
in the LDR group in K562 cells (Fig.
2; Table I).
 | Table IThe gray scale of Bcl-xl at 24 h
following low-dose radiation. |
Table I
The gray scale of Bcl-xl at 24 h
following low-dose radiation.
| Dose (c Gy) | 0 | 20 | 50 | 80 | 600 | 20+600 | 50+600 | 80+600 |
|---|
| The gray value of
Bcl-xl | 3.96±0.08 | 3.44±0.18 | 2.65±0.30 | 2.07±0.31 | 2.59±0.48 | 2.40±0.24 | 1.67±0.30 | 1.19±0.08 |
Bax protein expression is upregulated by
LDR alone or in combination with HDR
The Bax protein expression in K562 cells changed in
a dose-dependent manner. Using western blot analysis, the
expression of Bax in all of the LDR groups exhibited a significant
difference compared with the control group (0 Gy) at 24 h following
radiation. However, in the combined LDR/HDR group (6 Gy), the Bax
protein expression levels were notably higher in the K562 cells
than those in either the LDR or HDR groups at 24 h following
radiation (P<0.01; Fig. 2).
Wild-type p53 protein expression is
unaltered by LDR or LDR/HDR treatment
At 24 h following radiation, the p53 protein levels
in K562 cells demonstrated no significant differences between
either the LDR groups or the LDR/HDR group (P>0.05; Fig. 2).
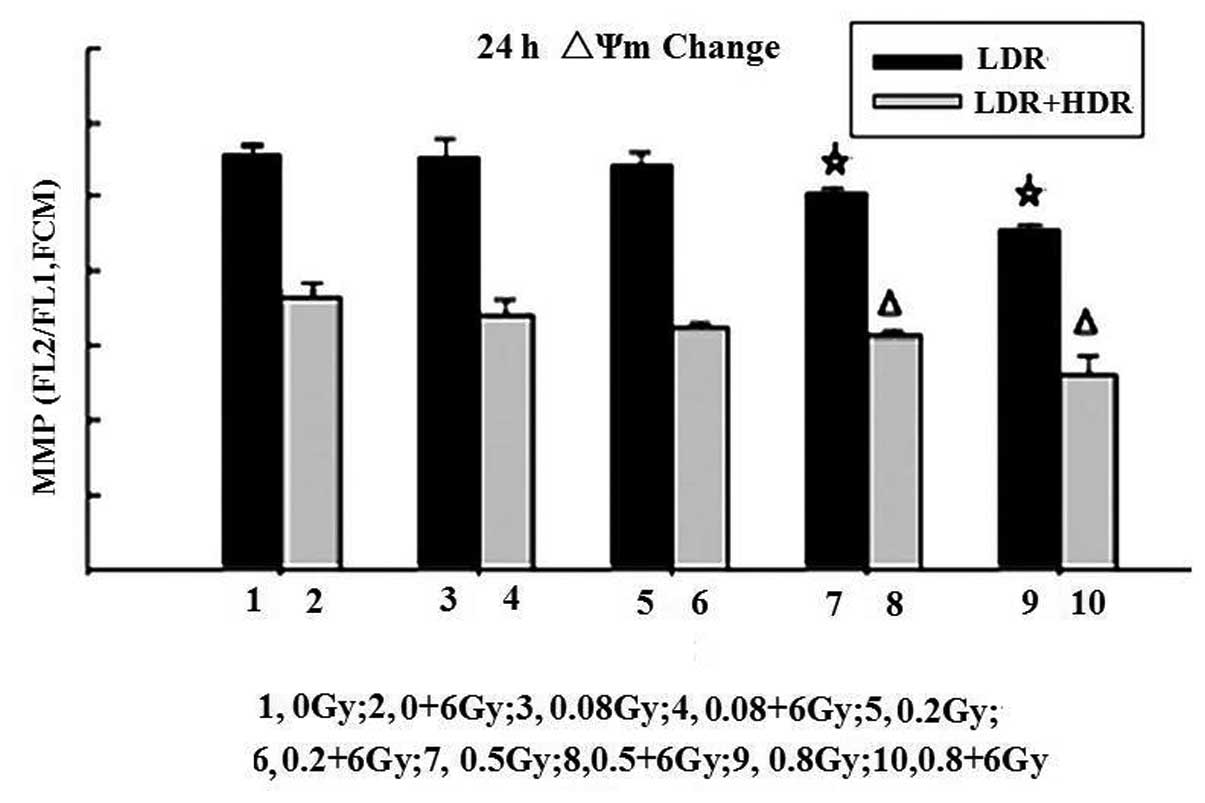
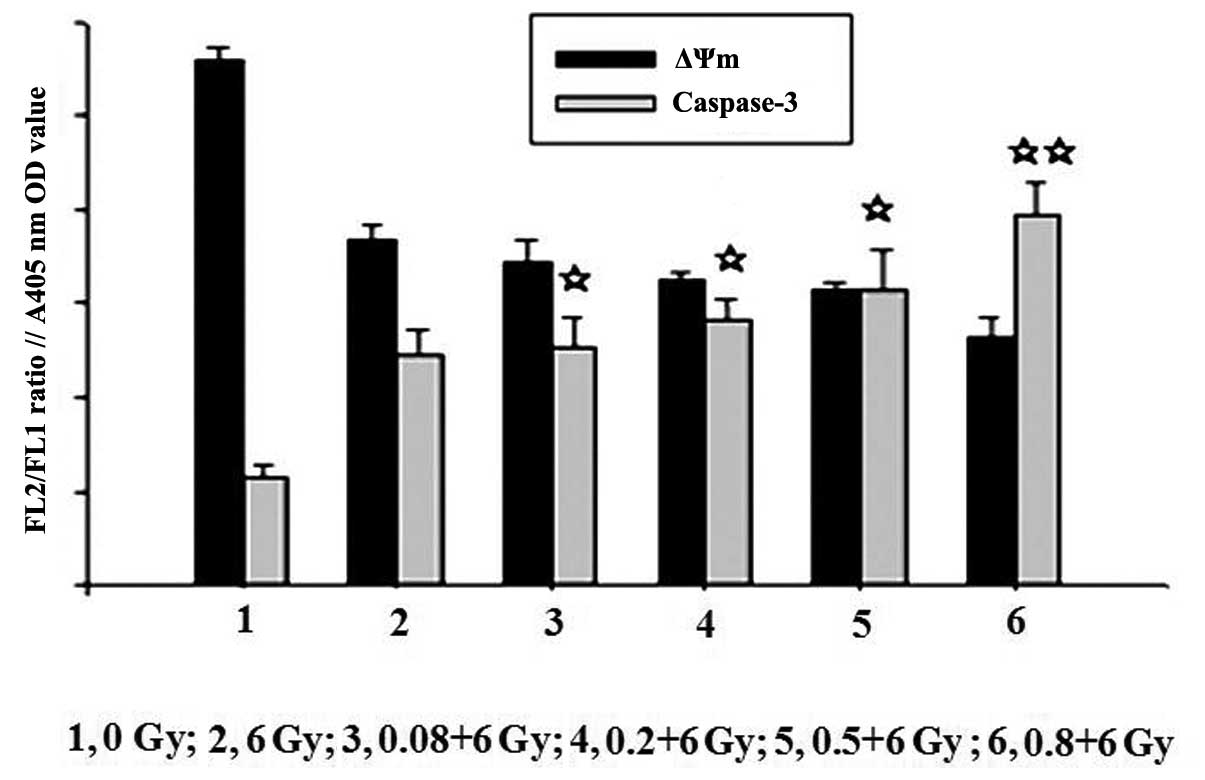
Change in ΔΨm by LDR or LDR/HDR
One of the early critical events in apoptosis is the
loss/disruption of ΔΨm in cells, which eventually causes the
initiation and activation of apoptotic cascades. The present study
examined the ΔΨm in K562 cells by flow cytometry with JC-1
staining. Following 24 h, the results demonstrated that treatment
of K562 cells with LDR resulted in a decreasing number of green
fluorescence-positive cells (indicating loss of ΔΨm) in a
dose-dependent manner. The ratios of red to green fluorescence in
the 0.5 Gy (5.03±0.08) and 0.8 Gy (4.58±0.07) groups were
significantly different compared with those in the control
(5.60±0.11), (0.08 Gy) 5.57±0.20 and 0.2 Gy (5.45±0.17) groups
(P<0.05). A higher proportion of green fluorescence-positive
cells was detected in the LDR/HDR-treated K562 cells compared with
the LDR cells (P<0.01). These results indicated that LDR or
LDR/HDR induced a loss of ΔΨm in K562 cells at 24 h following
radiation, which was more evident in the LDR/HDR group than that in
the LDR group (Fig. 3).
Measurement of caspase-3 activities
Caspase-3 activation is a crucial component in the
apoptotic signaling cascade. To further elucidate the mechanism of
cell apoptosis induced by irradiation, a caspase-3 colorimetric
assay was conducted to assess the levels of caspase-3 activation in
cells at 24 h following LDR or LDR/HDR treatments. LDR mildly
increased caspase-3 activity with a statistically significant
difference among the LDR groups (P<0.05). Furthermore, when the
K562 cell cultures were co-treated with LDR and HDR, the activity
of caspase-3 was increased significantly compared with that in the
cells treated with HDR alone (P<0.05; Figs. 4 and 5).
Discussion
LDR is able to induce a number of biological effects
on the tissues of the body or cell cultures, including radiation
homeostasis, adaptive response, hyper-sensitivity and bystander
effect (12). Experimental studies
suggested that the efficacy of LDBI is mainly attributed to the
mechanisms of immune enhancement (13–18),
induction of apoptosis (19,20),
intrinsic hypersensitivity (21)
and contribution of antioxidant enzymes to low-radiation doses
(22). These effects are not
mutually exclusive, and it is quite plausible that more than one
mechanism is functional at the same time, or that they all work
simultaneously.
Numerous human tumor cell lines exhibit a low-dose
hyper-sensitivity to radiation, known as hyperradiosensitivity
(HRS). This is most commonly manifested <0.5 Gy as a clear
deviation from the standard linear-quadratic cell survival response
extrapolated from higher doses back to 0 Gy. It is accompanied by
an increase in radioresistance between 0.5 and 1 Gy, known as
increased radioresistance. Hypersensitivity to radiation is one of
the leading mechanisms involved in the induction of tumor cell
apoptosis. Enns et al (23)
examined the response of human A549 lung carcinoma and T98G glioma
cells to γ-radiation from doses of 0–2 Gy delivered at 0.18 and
0.22 Gy/min, and observed that there was a marked hypersensitivity
at radiation doses <0.5 Gy. In this study it was also suggested
that low-dose hypersensitivity is associated with p53-dependent
apoptosis. A possible explanation is that at very low-acute doses,
cells do not to upregulate radioprotective repair mechanisms to
repair damage and instead, apoptosis is initiated (24). Furthermore, other evidence
supported the existence of a correlation between the enhanced
low-dose cell death and apoptosis. It was demonstrated that
low-dose HRS is likely to be a measure of the apoptosis of
radiation-damaged G2-phase-specific cells that evade early G2-phase
checkpoint arrest (25). A
three-component model of hypersensitivity was proposed that
consisted of damage recognition, signal transduction and DNA damage
repair. The foundation of the model is a rapidly occurring
dose-dependent pre-mitotic cell cycle checkpoint that is specific
to cells exposed to irradiation whilst in G2 phase (26). Olive and Durand (27) studied the apoptotic mechanisms of
hematopoietic cell lines, finding that they generally undergo rapid
apoptosis (within hours) following radiation, in contrast to cells
of non-hematopoietic origin, which are more likely to be
characterized by delayed apoptosis (within days). Tolerance for DNA
damage appears to be reduced in cells capable of rapid apoptosis,
and those hematopoietic cells are sensitized to ionizing radiation
when their apoptotic response mechanisms are fully functional. This
rapid apoptotic response demonstrates minor sensitivity to the cell
cycle phase or radiation dose rate.
Various therapeutic strategies have been used for
the treatment of CML. These include cytotoxic agents, including
hydroxyurea, interferon-α (IFN-α)-based regimens, tyrosine kinase
inhibitors (TKI) and allogeneic hematopoietic stem cell
transplantation (allo-HSCT). The cumulative evidence generated thus
far demonstrated that CML remains a difficult malignancy to treat,
with the median survival ranging between 3–4 years. Despite the
introduction of targeted therapies, such as TKI drugs, the
prognosis of CML has not changed to a large extent. As a result,
novel effective treatments are urgently required.
Takahashi et al (28) previously found that small doses of
X-rays accelerated the process of cell death, using a dye exclusion
test in MOLT-4 cells of human T-cell leukemic origin, in which
radiation-induced cell death has been well characterized to proceed
via apoptosis. Chen and Sakai (29) also reported that by administering
0.2 Gy of X-rays 12 h prior to a challenge with 5 Gy irradiation,
the process of apoptosis in human leukemic MOLT-4 cells was
accelerated. The acceleration was associated with an increase in
caspase-3 activity, a disruption of the mitochondrial transmembrane
potential and an accumulation of p53 proteins. This finding is in
contrast to the radiation-adaptive responses in which a small dose
of pre-irradiation would induce certain radiation resistance and
decrease the cell death following radiation with higher doses. The
studies described above performed in vitro have demonstrated
that LDR may induce apoptosis in leukemia cells containing the
wild-type p53 gene. This radiation-induced apoptosis requires
further study.
The present study demonstrated that the increase in
the apoptotic rate of K562 cells following LDR exhibits a well
defined dose-dependent response. The proportion apoptotic K562
cells, particularly when exposed to 0.5 or 0.8 Gy, was higher than
in the other exposure groups. This possibly results from the high
expression of Bcl-xl protein in K562 cells, which, to a certain
extent, contributes to radioresistance, whereas the p53 mutation in
K562 cells leads to no evident retardation in the G1 phase.
Apoptosis of K562 cells under LDR increased following 48 h and
lasted until 96–120 h post-irradiation, characterized by delayed
apoptosis and longer apoptosis duration. Furthermore, this type of
apoptosis in K562 cells had no correlation with the p53 mutation
status, suggesting the possible involvement of a p53-independent
apoptotic pathway, which belongs to a slow-response cell
population, only progressing through mitosis before apoptosis would
have occurred. Waldman et al (30) reported that in the absence of p21
or p53 in certain tumor cells DNA damage caused cell cycle arrest
in the G2-like stage, but cells additionally proceeded through S
phase without undergoing mitosis. As a result, the cells acquired
grossly deformed, polyploid nuclei and subsequently died through
apoptosis. This apoptosis, triggered by DNA-damaging agents that
are able to trigger S/M uncoupling, is a mechanism of the
non-p53-dependent-apoptotic pathway. In the present study, it was
identified that HDR combined with LDR enhanced the lethal effect to
K562 cells, leading to an increased apoptotic rate and extending
cell apoptosis by the same apoptosis pathway as Waldman et
al previously reported. The Bcl-2 gene family has a crucial
regulatory role in cell apoptosis. High expression of Bcl-2 family
proteins in tumor cells is an important factor in the resistance to
chemoradiation and consequently, there is a high correlation with
clinical prognosis in treatment. The Bcl-xl gene is also an
apoptosis-inhibiting gene in the Bcl-2 gene family. Jiang et
al (31) previously observed
that pre-exposure to 0.075 Gy of X-ray prior to 4 Gy of X-rays
induced a higher apoptotic effect and an increased expression of
apoptosis-associated genes, p53 and Bax, along with a lower
expression of anti-apoptosis gene Bcl-2, in tumor cells (lung
carcinoma cell line NCI-H446, glioma cell line U251,
erythroleukemia cell line K562 and acute promyelocytic leukemia
cell line HL60) than those in the tumor cells exposed to 4 Gy of
X-rays alone. In the present study, LDR significantly decreased the
expression of the Bcl-xl protein and increased the expression of
Bax in a dose-dependent manner, and lowering the Bcl-xl/Bax ratio
promoted the apoptosis of K562 cells. When the Bcl-xl protein
expression was high, the K562 cells exhibited resistance to common
chemoradiation. However, when exposed to LDR, the expression of
Bcl-xl protein was downregulated, resulting in DNA damage and the
induction of cell apoptosis. Of note, LDR/HDR further promoted the
reduction of Bcl-xl protein and the production of Bax protein in
K562 cells, leading to a higher apoptotic rate compared with HDR
alone.
The activation of the mitochondria-mediated
apoptotic pathway is essential for programmed cell death.
Mitochondrial membrane permeability and the alteration of membrane
potentials are essential aspects in the apoptotic process,
resulting in release of CytoC and initiation of apoptosis by
activating the caspase reaction cascade. Yu et al (32) also discovered that LDR exposure
(0.0075 Gy) increased the therapeutic efficacy of cyclophosphamide
(CTX) to S180 sarcoma cells. The apoptosis of tumor cells increased
significantly following LDR. The cell cycle was more significantly
arrested upon exposure to LDR followed by CTX as compared with that
resulting from exposure to CTX chemotherapy only. This was due to
LDR+CTX inducing greater cytochrome c levels and caspase-3 activity
than CTX chemotherapy only in tumor cells. The present study
demonstrated that LDR significantly decreased the ΔΨm and increased
caspase-3 activity. Furthermore, in combination with HDR, the ΔΨm
in K562 cells was severely diminished and higher caspase-3 activity
was present, indicating that LDR was able to enhance the radiation
efficacy of HDR. Notably, no evident change in the level of p53
protein expression occurred following combined LDR/HDR. It was
therefore assumed that this result was likely owing to a mutation
of p53 in K562 cells.
In conclusion, LDR was able to induce apoptosis of
K562 cells and enhanced the lethal effect of HDR on K562 cells
combined with HDR. It provided evidence for the feasibility of the
conditioning regimen of TBI combined with LDR, as a pre-transplant
conditioning regimen in the treatment of leukemia. However, to
achieve this synergistic effect, a dosage of LDR between 0.5 Gy and
0.8 Gy is required. The possible mechanism of action is through the
p53-independent apoptosis pathway. K562 cells would undergo DNA
damage with LDR, which initiates the mitochondria-mediated
apoptotic pathway, including upregulation of Bax protein expression
and downregulation of Bcl-xl protein expression, resulting in the
breakdown of ΔΨm. The outer membrane of mitochondria becomes
permeable to cytochrome c, and when cytochrome c is released to the
cytosol, it triggers caspase cascade reactions. It is suggested
that LDR in combination with HDR, when administered at the
appropriate dosage, is able to increase the apoptotic index of HDR
by accelerating apoptosis as well as increasing apoptosis duration
and incidence.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30770916, 81071831,
81372424 and 81372916); the Department of Science and Technology of
Jiangsu Province (no. BK20131131); and the Deapartment of Science
and Technology of Xuzhou (no. XM12B029).
References
|
1
|
Li J, Chen F, Cona MM, et al: A review on
various targeted anticancer therapies. Target Oncol. 7:69–85. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nwokocha CR, Nwokocha M, Mounmbegna P, et
al: Proteins and liver function changes in rats following
cumulative total body irradiations. West Indian Med J. 61:773–777.
2012.PubMed/NCBI
|
|
3
|
Walch J, Tettenborn B, Weber J and
Hundsberger T: Radiation-induced cavernoma after total body
irradiation and haematopoietic stem cell transplantation in an
adult patient suffering from acute myeloid leukaemia. Case Rep
Neurol. 5:91–97. 2013. View Article : Google Scholar
|
|
4
|
Nomura T, Sakai K, Ogata H and Magae J:
Prolongation of life span in the accelerated aging klotho mouse
model, by low-dose-rate continuous γ irradiation. Radiat Res.
179:717–724. 2013.PubMed/NCBI
|
|
5
|
Luckey TD: Atomic bomb health benefits.
Dose Response. 6:369–382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olivieri G, Bodycote J and Wolff S:
Adaptive response of human lymphocytes to low concentrations of
radioactive thymidine. Science. 223:594–597. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hendrikse AS, Hunter AJ, Keraan M and
Blekkenhorst GH: Effects of low dose irradiation on TK6 and U937
cells: induction of p53 and its role in cell-cycle delay and the
adaptive response. Int J Radiat Biol. 76:11–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seong J, Kim SH, Pyo HR, Chung EJ and Suh
CO: Effect of low-dose irradiation on induction of an apoptotic
adaptive response in the murine system. Radiat Environ Biophys.
40:335–339. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joksic G and Petrović S: Lack of adaptive
response of human lymphocytes exposed in vivo to low doses of
ionizing radiation. J Environ Pathol Toxicol Oncol. 23:195–206.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Czyz M and Szuławska A: Induced
differentiation of the K562 leukemic cell line. Postepy Hig Med
Dosw (Online). 59:82–97. 2005.(In Polish).
|
|
11
|
Damiano JS, Hazlehurst LA and Dalton WS:
Cell adhesion-mediated drug resistance (CAM-DR) protects the K562
chronic myelogenous leukemia cell line from apoptosis induced by
BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation.
Leukemia. 15:1232–1239. 2001. View Article : Google Scholar
|
|
12
|
Ito M, Shibamoto Y, Ayakawa S, Tomita N,
Sugie C and Ogino H: Low-dose whole-body irradiation induced
radioadaptive response in C57BL/6 mice. J Radiat Res. 48:455–460.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hosoi Y: Antitumor effects by low dose
total body irradiation. Yakugaku Zasshi. 126:841–848. 2006.(In
Japanese).
|
|
14
|
Safwat A: The role of low-dose total body
irradiation in treatment of non-Hodgkin’s lymphoma: a new look at
an old method. Radiother Oncol. 56:1–8. 2000.
|
|
15
|
Hashimoto S, Shirato H, Hosokawa M, et al:
The suppression of metastases and the change in host immune
response after low-dose total-body irradiation in tumor-bearing
rats. Radiat Res. 151:717–724. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu SZ, Han ZB and Liu WH: Changes in
lymphocyte reactivity to modulatory factors following low dose
ionizing radiation. Biomed Environ Sci. 7:130–135. 1994.PubMed/NCBI
|
|
17
|
Nogami M, Huang JT, James SJ, Lubinski JM,
Nakamura LT and Makinodan T: Mice chronically exposed to low dose
ionizing radiation possess splenocytes with elevated levels of
HSP70 mRNA, HSC70 and HSP72 and with an increased capacity to
proliferate. Int J Radiat Biol. 63:775–783. 1993. View Article : Google Scholar
|
|
18
|
Safwat A: The immunobiology of low-dose
total-body irradiation: more questions than answers. Radiat Res.
153:599–604. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knoops L, Haas R, de Kemp S, et al: In
vivo p53 response and immune reaction underlie highly effective
low-dose radiotherapy in follicular lymphoma. Blood. 110:1116–1122.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sgouros G, Knox SJ, Joiner MC, Morgan WF
and Kassis AI: MIRD continuing education: Bystander and low
dose-rate effects: are these relevant to radionuclide therapy? J
Nucl Med. 48:1683–1691. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heyer BS, MacAuley A, Behrendtsen O and
Werb Z: Hypersensitivity to DNA damage leads to increased apoptosis
during early mouse development. Genes Dev. 14:2072–2084.
2000.PubMed/NCBI
|
|
22
|
Bravard A, Luccioni C, Moustacchi E and
Rigaud O: Contribution of antioxidant enzymes to the adaptive
response to ionizing radiation of human lymphoblasts. Int J Radiat
Biol. 75:639–645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Enns L, Bogen KT, Wizniak J, Murtha AD and
Weinfeld M: Low-dose radiation hypersensitivity is associated with
p53-dependent apoptosis. Mol Cancer Res. 2:557–566. 2004.PubMed/NCBI
|
|
24
|
Mitchell CR and Joiner MC: Effect of
subsequent acute-dose irradiation on cell survival in vitro
following low dose-rate exposures. Int J Radiat Biol. 78:981–990.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krueger SA, Joiner MC, Weinfeld M,
Piasentin E and Marples B: Role of apoptosis in low-dose
hyper-radiosensitivity. Radiat Res. 167:260–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Marples B, Wouters BG, Collis SJ, Chalmers
AJ and Joiner MC: Low-dose hyper-radiosensitivity: a consequence of
ineffective cell cycle arrest of radiation-damaged G2-phase cells.
Radiat Res. 161:247–255. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olive PL and Durand RE: Apoptosis: an
indicator of radiosensitivity in vitro? Int J Radiat Biol.
71:695–707. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takahashi K, Inanami O, Hayashi M and
Kuwabara M: Protein synthesis-dependent apoptotic signalling
pathway in X-irradiated MOLT-4 human leukaemia cell line. Int J
Radiat Biol. 78:115–124. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Z and Sakai K: Enhancement of
radiation-induced apoptosis by preirradiation with low-dose X-rays
in human leukemia MOLT-4 cells. J Radiat Res. 45:239–243. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Waldman T, Lengauer C, Kinzler KW and
Vogelstein B: Uncoupling of S phase and mitosis induced by
anticancer agents in cells lacking p21. Nature. 381:713–716. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jiang H, Li W, Li X, Cai L and Wang G:
Low-dose radiation induces adaptive response in normal cells, but
not in tumor cells: in vitro and in vivo studies. J Radiat Res.
49:219–230. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu HS, Xue HW, Guo CB, et al: Low dose
radiation increased the therapeutic efficacy of cyclophosphamide on
S(180) sarcoma bearing mice. J Radiat Res. 48:281–288. 2007.
View Article : Google Scholar : PubMed/NCBI
|