Introduction
Previous studies have established that chronic
exposure to solar radiation leads to skin damage (1,2).
Ultraviolet (UV) radiation, which can be categorized into UVA, UVB
and UVC according to wavelength, has the potential to cause DNA
damage, leading to sunburn and skin cancer (3). UVC radiation has the shortest
wavelength and thus emits the highest energy levels (4), but the majority of UVC from sunlight
is absorbed by the atmosphere, in particular the ozone layer, so is
not a threat to health. UVA and UVB, however, reach the skin by
penetrating the atmosphere (5),
and in hair follicles, androgenetic alopecia is a result of
UV-induced photo-aggravated dermatitis (6). Additionally, UV represses growth and
cycling of hair follicles and follicular melanogenesis in
vitro (7).
microRNAs (miRNAs) are small, non-coding RNAs that
regulate mRNA translation (8,9) and
have been implicated in the regulation of apoptosis, survival and
differentiation (9). UV radiation
has been demonstrated to regulate miRNAs in various types of cell
(10,11), including melanocytes, in which
UV-induced miR-145, miR-148 and miR-25 regulate pigmentation by
repressing Myo5a and MITF (12,13).
miR-125b and miR-22 promote cell survival by targeting p38α and
PTEN following UV irradiation (10,14).
Additionally, Pothof et al (11) implicated miRNA-mediated gene
interference in the UV-induced DNA damage response. In other
studies, miRNA expression in UV-irradiated mouse epidermis and
human keratinocytes was profiled via microarray analysis (15,16).
However, despite these studies, changes in miRNA expression in
response to UV radiation remain unclear in human dermal papilla
cells. Therefore, in the present study, global miRNA expression in
UVB-irradiated human dermal papilla cells was profiled and
bioinformatics were utilized to identify putative miRNA target
genes and their associated biological functions. The data from the
current study may provide insights into a novel mechanism of
UV-dependent damage in human dermal papilla cells.
Materials and methods
Cell culture and UVB irradiation
Normal human dermal papilla cells (nHDPs) were
obtained from Cell Engineering For Origin (Seoul, Korea). nHDPs
were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA),
5,000 U/ml penicillin G and 5,000 μg/ml streptomycin. The cells
were incubated at 37˚C in 5% CO2 humidity.
Cells were exposed to UVB radiation using a G8T5E
lamp (Sankyo Denki, Toshima, Japan). Doses were measured with a UV
light meter (Lutron UV-340; Lutron Electronic Enterprise Co., Ltd.,
Taipei, Taiwan). nHDPs were seeded in 60-mm culture dishes and
incubated for 24 h, then washed and resuspended in
phosphate-buffered saline (PBS) prior to exposure to UVB. The cells
were placed in fresh medium following irradiation. Non-exposed
control samples were maintained in the dark under the same
conditions.
RNA extraction and miRNA microarray
All materials were obtained from Agilent
Technologies (Santa Clara, CA, USA) unless otherwise stated. Total
RNA was extracted using RiboEx™ (Geneall, Seoul, Korea) according
to the manufacturer’s instructions. RNA stability was confirmed
using the Bioanalyzer 2100. Nucleic acid purity was calculated from
A260/A280 and A260/A230 ratios using the MaestroNano
spectrophotometer (Maestrogen, Las Vegas, NV, USA). miRNA
expression profiles were analyzed using the SurePrint G3 Human
v16.0 miRNA 8x60K Microarray kit (based on miRBase release 19.0),
which included 1,368 probes representing 1,205 human miRNAs. Total
RNA (100 ng) was dephosphorylated using calf intestine alkaline
phosphatase (CIP) and denatured by heat inactivation with dimethyl
sulfoxide (DMSO). The dephosphorylated RNA was labeled with pCp-Cy3
using T4 RNA ligase. Unlabeled pCp-Cy3 was removed using the Micro
Bio-Spin P-6 column (Bio-Rad, Hercules, CA, USA). Labeled RNA was
dried and resuspended in Hi-RPM hybridization buffer prior to
hybridization with the microarray at 55˚C and 20 rpm for 20 h in an
Agilent Microarray Hybridization Oven (Agilent Technologies, Santa
Clara, CA, USA). Following hybridization, microarray slides were
washed with wash buffers 1 and 2 and then scanned using an Agilent
SureScan Microarray Scanner (Agilent Technologies). The scanned
image was quantitated using Agilent Feature Extraction Software
(version 10.7; Agilent Technologies). The data were analyzed using
GeneSpring GX version 11.5 software.
miRNA target gene prediction and gene
ontology (GO) analysis
The target genes of miRNAs that exhibited altered
expression levels in response to UVB exposure were predicted using
TargetScan (http://www.targetscan.org). The
target genes were predicted from the high context score percentile
(50–100) in the conserved and nonconserved database. The predicted
target genes underwent GO analysis to identify their associated
biological functions.
Cell viability
nHDPs were seeded into a 96-well plate at a density
of 5x103 cells/well and incubated for 24 h. The cells
were irradiated with different doses of UV (0–400
mJ/cm2) and incubated for another 24 h. The cells were
then incubated with 0.5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
dissolved in DMSO for 1 h. The absorbance at 490 nm was measured
with an iMark Microplate Absorbance reader (Bio-Rad).
Cell cycle analysis
nHDPs were plated on 60-mm tissue culture dishes at
a density of 2x106 cells/plate and then grown until 70%
confluent, prior to irradiation with different doses of UVB (0–400
mJ/cm2). Following the 24-h incubation, the cells were
trypsinized and fixed with 70% ethanol for 18 h at 4˚C. The cells
were then resuspended in 1 ml propidium iodide (PI) staining
solution (50 μg/ml PI, 0.1 μg/ml RNase, and 0.05% Triton X-100 in
PBS) for 1 h. The stained cells were analyzed using a FACSCalibur
flow cytometer (BD Biosciences, San Jose, CA, USA). A minimum of
10,000 events were collected in each analysis. The various cell
cycle populations were determined by ModFit LT (Verity Software
House, Topsham, ME, USA).
Reactive oxygen species (ROS)
staining
Intercellular ROS levels were measured using
2′,7′-dichlorofluorescein diacetate (DCF-DA) (Sigma-Aldrich) as
previously described (17). nHDPs
were plated onto 60-mm tissue culture dishes at a density of
2x106 cells/plate. The cells were irradiated with UVB
and then incubated for 24 h prior to staining with 20 μM DCF-DA for
30 min. The stained cells were analyzed via flow cytometry using
the FACSCalibur flow cytometer.
Statistical analysis
Statistical significance was determined by the
Student’s t-test and data were subjected to global normalization.
P<0.05 was considered to indicate a statistically significant
difference.
Results
UVB irradiation decreases cell viability
by increasing the occurrence of cell cycle arrest or apoptosis in
nHDPs
Agents such as UV radiation, that induce DNA damage
in mammalian cells, trigger growth arrest, cell cycle arrest, and
apoptosis by regulating the ATM-p53 pathway (18,19).
However, different types of cell exhibit varied responses to
equivalent UV doses (5,20–23).
Therefore, in the current study, to determine how nHDPs respond to
UV irradiation, these cells were exposed to 0–400 mJ/cm2
of UVB radiation for 24 h, prior to a cell viability assessment
(Fig. 1A). Cell viability was
significantly reduced to 70.34% following exposure to 50
mJ/cm2 of UVB compared with that following exposure to 0
mJ/cm2. As previous studies have shown that UVB
radiation regulates cell viability and apoptosis by increasing ROS
production (24,25), intracellular ROS was examined using
DCF-DA in the current study. The results demonstrated that ROS
production increased in cells exposed to 50 mJ/cm2 UVB
compared with those exposed to 0 mJ/cm2 (Fig. 1B). Analysis of cell cycle
progression in cells that were exposed to 0, 25 and 50
mJ/cm2 UVB for 24 h revealed that G1-phase cell cycle
arrest was induced by 25 mJ/cm2 UVB irradiation
(Fig. 1C). Notably, the frequency
of sub-G1 cells, which represent apoptotic cells, was increased in
nHDPs exposed to 50 mJ/cm2 UVB compared with those
exposed to 0 mJ/cm2.
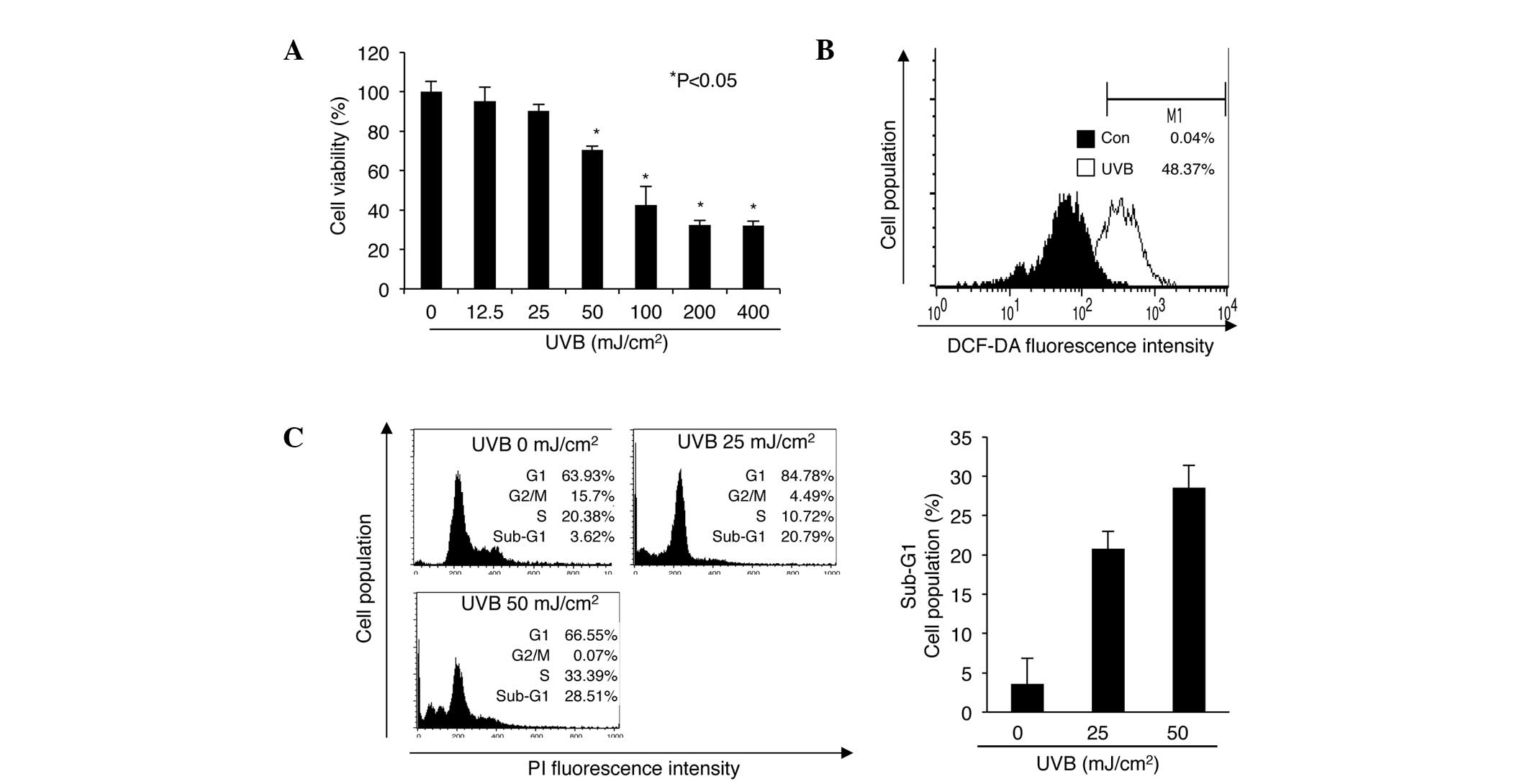 | Figure 1Effect of UVB irradiation on cell
viability, ROS production and the cell cycle in nHDPs. (A) Effect
of UVB radiation on nHDP viability. Cell viability was measured by
MTT assay. Results are presented as the mean ± standard error of
the percentage of control OD of triplicate samples.
*P<0.05 vs. 0 mJ/cm2 UVB irradiation. (B)
Effect of UVB on the levels of ROS in nHDPs. Different cell
populations are represented by different colors (black, 0
mJ/cm2 UVB; white, 50 mJ/cm2 UVB). (C) Effect
of UVB on the cell cycle in nHDPs. The distributions of cells of
different populations in the different stages of the cell cycle
were analyzed by flow cytometry using PI-stained nHDPs. The
frequency of cells in the sub-G1 phase is presented as the mean ±
standard error of the percentage of the gated cell population of
triplicate samples. UVB, ultraviolet B; DCF-DA,
2′,7′-dichloroflorescein diacetate; PI, propidium iodide; ROS,
reactive oxygen species; nHDP, normal human dermal papilla cell;
OD, optical density. |
miRNA expression is altered by UVB
irradiation
To analyze UVB-dependent changes in miRNA expression
in nHDPs, miRNA microarray analysis was performed using arrays
containing 1,368 probes representing 1,205 human miRNAs. Total RNA
was extracted from non-irradiated and 50 mJ/cm2
UVB-irradiated nHDPs. Data from each sample were subjected to
global normalization. To obtain refined data, miRNAs were selected
and further considered as a result of flag-present filtration in
the data file, indicating that the sensitivity of the selected
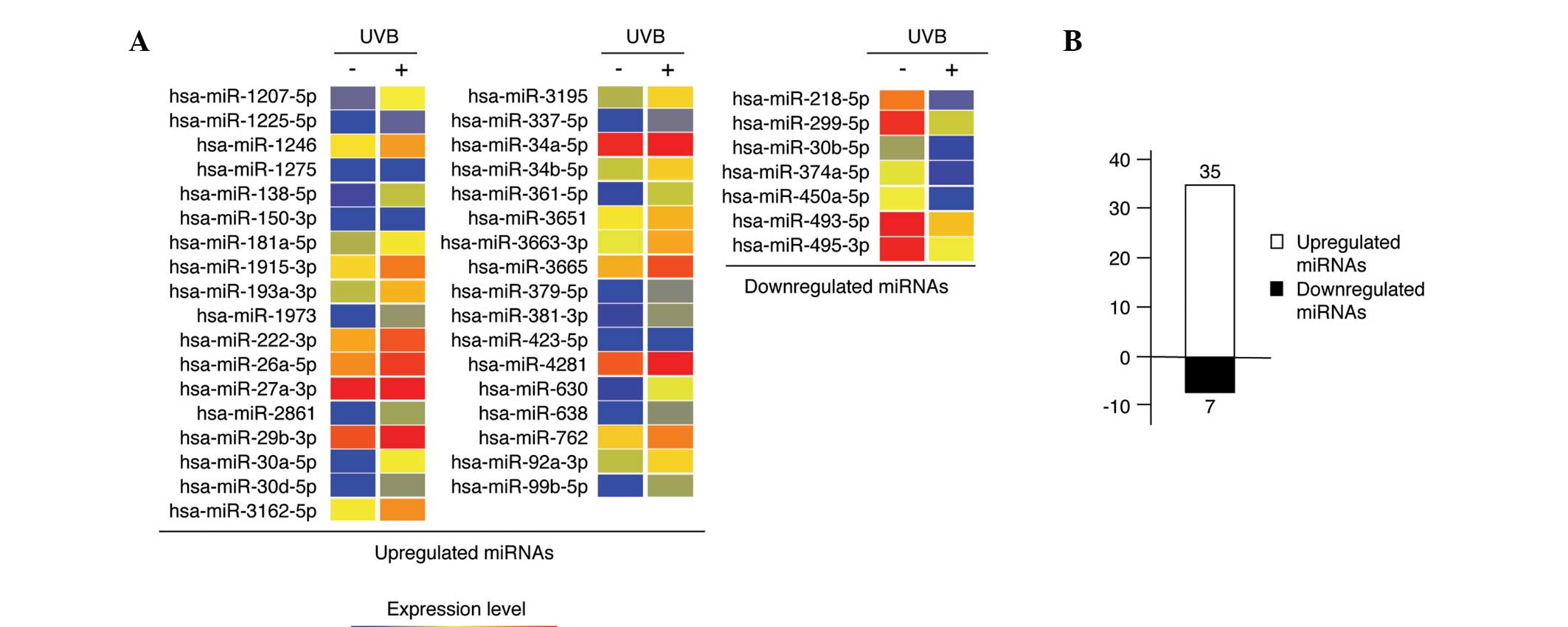
miRNAs were sufficient for the microarray. A total of 183 miRNAs
were selected for further analysis. miRNAs from 50
mJ/cm2 UVB-irradiated nHDPs that were upregulated (n=35)
and downregulated (n=7) at least 1.5-fold compared with those of
the non-irradiated control nHDPs were identified (Fig. 2), and lists of these 42 miRNAs are
presented in Tables I and II.
 | Table ImiRNAs upregulated at least 1.5-fold
in UVB-irradiated nHDPs. |
Table I
miRNAs upregulated at least 1.5-fold
in UVB-irradiated nHDPs.
| miRNA | Fold change | Chr |
|---|
| hsa-miR-1207-5p | 2.00 | chr8 |
| hsa-miR-1225-5p | 1.62 | chr16 |
| hsa-miR-1246 | 1.79 | chr2 |
| hsa-miR-1275 | 1.76 | chr6 |
| hsa-miR-138-5p | 1.80 | chr3 |
| hsa-miR-150-3p | 2.24 | chr19 |
|
hsa-miR-181a-5p | 1.56 | chr1 |
|
hsa-miR-1915-3p | 2.19 | chr10 |
|
hsa-miR-193a-3p | 2.32 | chr17 |
| hsa-miR-1973 | 2.18 | chr4 |
| hsa-miR-222-3p | 2.07 | chrX |
| hsa-miR-26a-5p | 2.08 | chr3 |
| hsa-miR-27a-3p | 1.90 | chr19 |
| hsa-miR-2861 | 2.42 | chr9 |
| hsa-miR-29b-3p | 2.25 | chr1 |
| hsa-miR-30a-5p | 4.03 | chr6 |
| hsa-miR-30d-5p | 2.28 | chr8 |
|
hsa-miR-3162-5p | 2.20 | chr11 |
| hsa-miR-3195 | 1.85 | chr20 |
| hsa-miR-337-5p | 1.62 | chr14 |
| hsa-miR-34a-5p | 1.64 | chr1 |
| hsa-miR-34b-5p | 1.76 | chr11 |
| hsa-miR-361-5p | 2.12 | chrX |
| hsa-miR-3651 | 1.55 | chr9 |
|
hsa-miR-3663-3p | 2.16 | chr10 |
| hsa-miR-3665 | 2.34 | chr13 |
| hsa-miR-379-5p | 2.09 | chr14 |
| hsa-miR-381-3p | 1.51 | chr14 |
| hsa-miR-423-5p | 1.78 | chr17 |
| hsa-miR-4281 | 2.09 | chr5 |
| hsa-miR-630 | 2.35 | chr15 |
| hsa-miR-638 | 2.55 | chr19 |
| hsa-miR-762 | 1.89 | chr16 |
| hsa-miR-92a-3p | 1.74 | chr13 |
| hsa-miR-99b-5p | 3.28 | chr19 |
 | Table IImiRNAs downregulated at least
1.5-fold in UVB-irradiated nHDPs. |
Table II
miRNAs downregulated at least
1.5-fold in UVB-irradiated nHDPs.
| miRNA | Fold change | Chr |
|---|
|
hsa-miR-1207-5p | 2.00 | chr8 |
|
hsa-miR-1225-5p | 1.62 | chr16 |
| hsa-miR-1246 | 1.79 | chr2 |
| hsa-miR-1275 | 1.76 | chr6 |
| hsa-miR-138-5p | 1.80 | chr3 |
| hsa-miR-150-3p | 2.24 | chr19 |
|
hsa-miR-181a-5p | 1.56 | chr1 |
GO analysis of UVB-specific putative
miRNA target genes in nHDPs
To identify the cellular functions associated with
UVB-mediated changes in the levels of miRNA expression, a
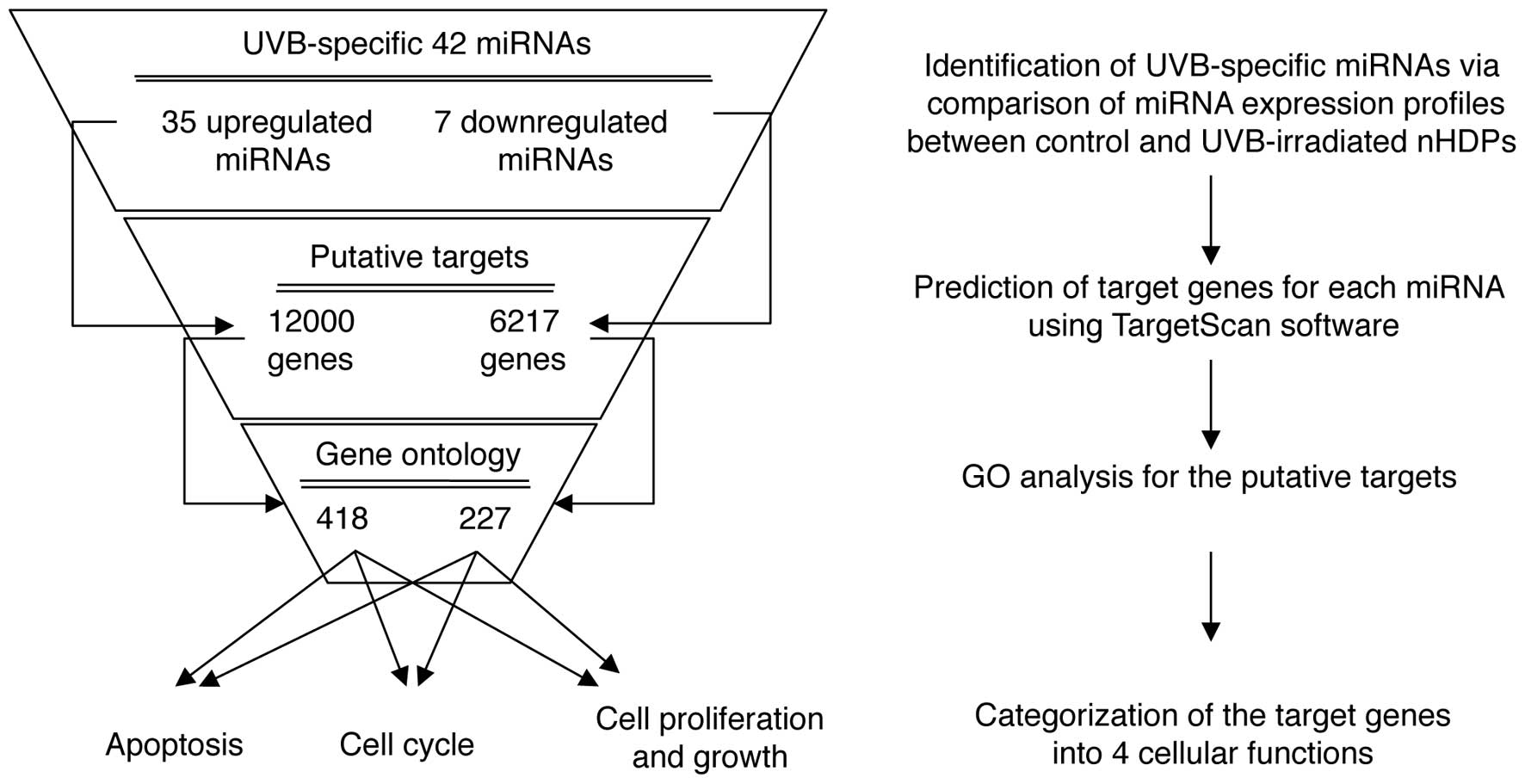
multi-step bioinformatics scheme was used to predict putative miRNA
target genes and their biological functions (Fig. 3). TargetScan, a sequence-based
miRNA target prediction database, was used to identify 18,217
putative target genes for the up- and downregulated miRNAs. The
analysis revealed 12,000 potential targets for the 35 upregulated
miRNAs and 6,217 targets for the 7 downregulated miRNAs.
Subsequently, the putative target genes involved in UV-mediated
cellular damage, including the cell cycle, apoptosis, cell growth
and proliferation, were screened. Using a GO web-based tool, AmiGO,
the gene lists for the above four GO terms (cell cycle, apoptosis,
cell growth and proliferation) were obtained, and these genes were
compared with the putative target genes. The overlapping genes in
the two groups were then identified (Fig. 3). Among the deregulated 42 miRNAs
(Table I and II), the majority of miRNAs were altered
>2.0-fold. Thus, the five most upregulated and downregulated
miRNAs were selected to obtain more representative biological
meanings for the putative genes. The predicted target genes of the
top 5 most differentially upregulated and downregulated miRNAs in
the UVB-irradiated HDPs are categorized by their functions in
Tables III and IV, respectively.
 | Table IIIPredicted target genes of the top
five most differentially upregulated miRNAs in UVB-irradiated
nHDPs. |
Table III
Predicted target genes of the top
five most differentially upregulated miRNAs in UVB-irradiated
nHDPs.
| Target genes and
functions |
|---|
|
|
|---|
| miRNA | Cell cycle | Apoptosis | Cell growth and
proliferation |
|---|
| hsa-miR-30a-5p | APBB2, RHOB, EPHB2,
NF1 NOTCH2, PNN, TSC1, RECK, CUL3, CUL2, BCL10, MTBP, TBRG1,
TBRG1 | ACTC1, CASP3, GJA1,
HTT, IL1A, IL2RA, IL17A, MAP3K5, MLL, NAIP, PTGER3, ATXN1, TFDP1,
TIA1, UNC5C, TNFSF9, TNFRSF10D, BCL10, ATG12, EBAG9, ARHGEF6, ATG5,
BCL2L11, EDAR, TRIM35, SIRT1, TCTN3, CECR2, SH3KBP1, DDIT4, C8orf4,
AVEN, BIRC6, TRIB3, NLRP3, RFFL, TICAM1, PRUNE2, RNF144B,
BCL2L15 | ADRA1D, ADRA2A,
ADRB2, IL7, TLX1, BNC1, DDX11, CAMK2D, JAG2, KRAS, LIFR, LYN, MAFG,
PDGFRB, PPP2CA, SOX9, VIPR1, CDC7, CUL3, SOCS1, CREG1, NOV, SOCS3,
EBAG9, CFDP1, ENOX2, C19orf10, BIRC6, CDCA7 |
| hsa-miR-99b-5p | MCC, RASSF4 | DFFB, NLRP2,
RNF144B | BMPR2, IGF1R,
STAT5B |
| hsa-miR-638 | CDKN2B, MCC,
MPHOSPH1, XAF1 | CLU, ATN1,
TNFRSF11B, PAK2, TNFRSF1B, ATG5, CIDEB, SAP30BP, XAF1, UBE2Z,
FAM130A1, RHOT2, RFFL | CD47, CDK2, CLU,
CTF1, GAP43, IFNG, IL11, LIF, LIFR, PAK2, TRAF5, VEGFA, HOXB13,
BRD8 |
| hsa-miR-2861 | - | - | - |
| hsa-miR-630 | RHOB, CDKN2B, CYLD,
NOTCH2, RB1CC1, WWOX, ZAK, C11orf82, LIN9 | XIAP, DOCK1, EP300,
GJA1, PAX3, TP63, ATG12, SLK, SMNDC1, NCKAP1, CKAP2, CROP, ZAK,
DDIT4, AVEN, ZMAT3, TP53INP1, C11orf82 | BMPR2, KLF5, FOXO1,
IGF1R, CYR61, IL7, SSR1, TDGF1, TGFBR2, CDC7, SOCS2, RBM9, CCDC88A,
ZMAT3 |
 | Table IVPredicted target genes of the top
five most differentially downregulated miRNAs in UVB-irradiated
nHDPs. |
Table IV
Predicted target genes of the top
five most differentially downregulated miRNAs in UVB-irradiated
nHDPs.
| Target genes and
functions |
|---|
|
|
|---|
| miRNA | Cell cycle | Apoptosis | Cell growth and
proliferation |
|---|
| hsa-miR-218-5p | TRIM13, CDK6,
CDKN1B, KHDRBS1, CETN2, MAPRE2, RCC1, FOXN3, PYHIN1, DCC, E2F2,
SENP5, FANCD2, MAPRE3, SEPT2, SASH1, CLASP1, SPECC1L, SH3BP4,
EGFL6, PDCD4, HLA, QB1, HPGD, BIRC5, MCC, NEDD9, ZAK, ANLN, PPP1CB,
PPP1CC, RIF1, RCBTB1, FANCI, SMPD3, PCNP, CCND1, SIAH2, BRCA1,
TACC1, PTP4A1, MAP9, RASSF5, PARD6B, CCNA2, CCND3, LYK5,
RASSF2 | - | NAMPT, CDKN1B,
NET1, SOCS4, CTGF, FLT1, KIT, KRT6A, LIF, LIFR, MST1R, NEDD9,
NODAL, NRAS, PAK2, C20orf20, BIRC6, PURA, APPA2, BNC1, SHC1, SSR1,
TRAF5, YEATS4, CUL3, SOCS3, SOCS6, HTRA3, CD47, SOCS5 |
|
hsa-miR-450a-5p | RCC1, EGFR, CD2AP,
XAF1 | - | - |
| hsa-miR-299-5p | CDKN1A, MAEA, SMC2,
CTCF, SPIN1, POLS, CEP110, FOXN3, GADD45A, AHR, SENP5 , FANCD2,
SIRT2, SEP2, CCNDBP1, CD2AP, RABGAP1, KIF11, NF1, NPAT, ZAK,
MPHOSPH8, PPP1CB, CEP55, MTUS1, RAD21, RAP1A, AVPI1, SIAH1, PTP4A1,
CDC7, PPAPDC1B, MCM8, TBRG1, CDC16, CCNG1, CCNG2, CCNH, AURKB,
HDAC4 | - | IGFBP3, IL8RB, NOV,
SIRPG, SSR1, TDGF1, CDC7, SOCS6, CD86, SOCS5 |
|
hsa-miR-374a-5p | CDK6, SPIN1, POLS,
ZWINT, ADCYAP1, ESCO2, SLC5A8, GADD45A, AHR, CCRK, CD2AP, NIPBL,
EGFL6, APPL1, GAS1, MTBP, LIN9, ANXA1, HELLS, HPGD, FLJ44060, ING1,
LOH11CR2A, MCM2, MCM6, MLH1, NBN, ATM, NEDD9, NEK2, NF1, ZAK, ANLN,
ERBB2IP, MAPK1, MAPK6, MAPK7, PCNP, PTEN, SPC25, ARHGAP20, CCND1,
NCAPG, PAPD5, BMP2, NEK4, TACC1, TFDP1, TP53BP2, SUV39H2, MAP9,
CHAF1B, RECK, MCM8, PARD6B, TBRG1, CDC14A, BCL10, STARD13, CCNE2,
KNTC1 | - | NAMPT, DNAJA2,
UBE2E3, CHAD, CLU, SOCS4, HES1, IGFBP3, IGFBP7, IL7, CXCL10, KRAS,
LIFR, MYC, NEDD9, PAK2, CRIM1, PURA, BCL2, PAPPA2, CXCL5, SLAMF1,
BTC, TBX3, TSHR, VEGFA, YEATS4, SOCS6, CIAO1, NTN1, CD47,
SOCS5 |
| hsa-miR-495-3p | CDKN1A, MAEA, SMC2,
CTCF, SPIN1, POLS, CEP110, FOXN3, GADD45A, AHR, SENP5, FANCD2,
SIRT2, SEP2, CCNDBP1, CD2AP, RABGAP1, KIF11, NF1, NPAT, ZAK,
MPHOSPH8, PPP1CB, CEP55, MTUS1, RAD21, RAP1A, AVPI1, SIAH1, CD2AP,
SH3BP4, CADM1, ANAPC13, GAS1, LIN9, UHRF1, HHEX, TMPRSS11A, ING1,
JAG2, LIG4, AD2L1, MCC, MCM2, MCM3, MCM7, NBN, EDD9, PNN, ANLN,
XRN1, TXNL4B, TIPIN, PPP1CB, RIF1, RCBTB1, PPP6C, CCAR1, SEP11,
BIN3, ERBB2IP, KLK10, PCNP, PTCH1, PTEN, SPC25, ARHGAP20, RAD17,
RAP1A, CCND1, NCAPG, PAPD5, SIAH1, BMP2, NEK4, TACC1, BUB1, TFDP1,
WEE1, EVI5, HMGA2, CDC7, RASSF5, CUL4B, PARD6G, JUB, CDC14A, RUNX3,
PCAF, BCL10, UBA3, CCNT2, PKMYT1, CCNE2, ZNF830, CCPG1, WTAP, MDC1,
HDAC4, DLGAP5, MTSS1, RB1CC1, CDC2, TLK1, CDC6, DLEC1, CASP8AP2,
SEP7 | - | CDKN1B, NET1,
TNFSF13B, MORF4L1, ESM1, FGFR1OP, SOCS4, ADM, ADRB2, DDX11, S1PR3,
FGF7, TMEM97, ID4, IFNG, IGF1, IGFBP3, IL6, IL8RB, IL11, LIFR,
NAP1L1, NDN, NEDD9, NRAS, CRIM1, FGFRL1, TIPIN, C19orf10, HTRA1,
CXCL5, SSR1, KLF5, TGFB2, TRAF5, VEGFA, HMGA2, CAMK2D, CDC7,
BRMS1L, CREG1, FGF17, NRP1, SOCS3, SOCS6, CD47, MORF4L2 |
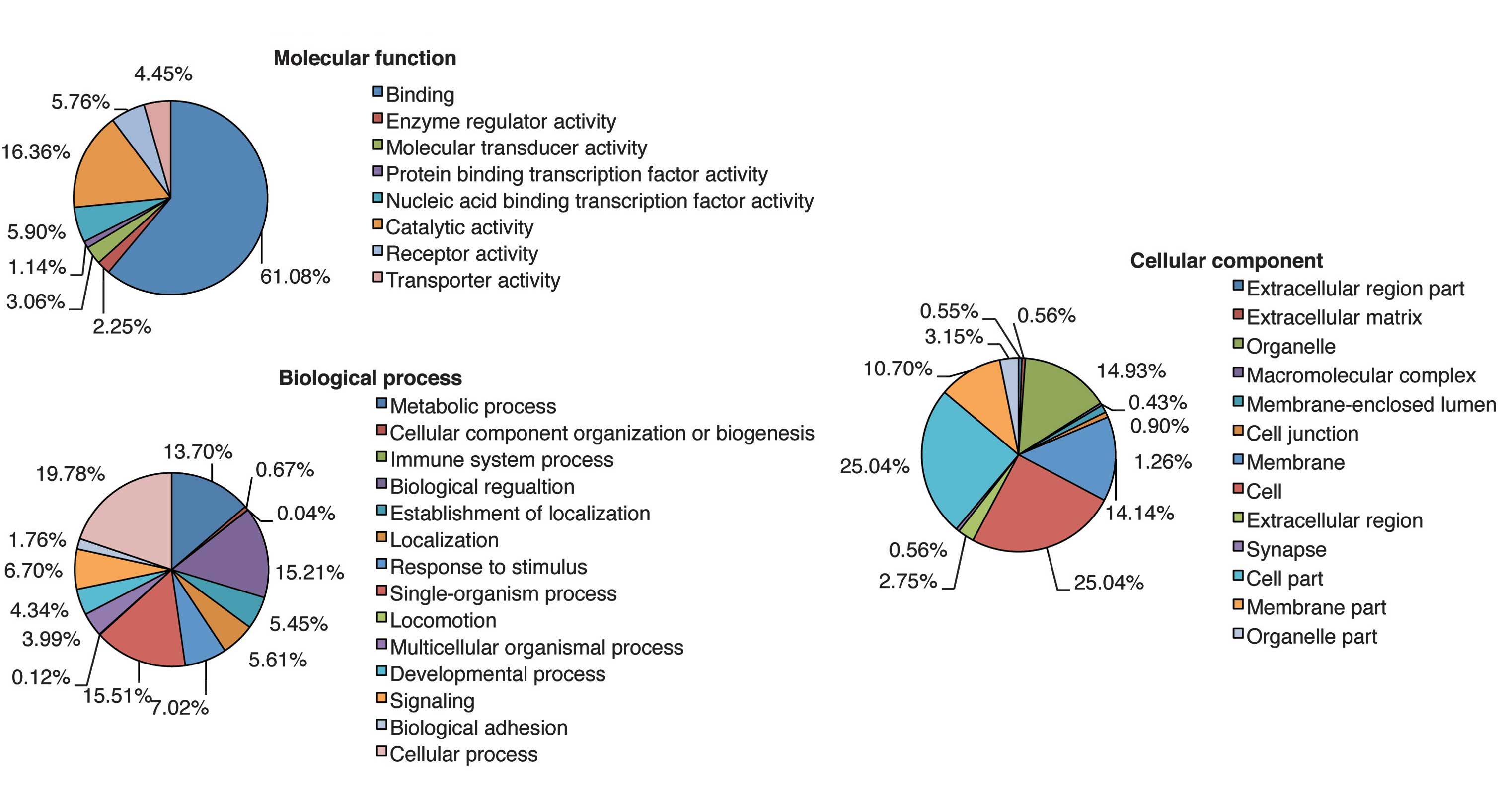
Each putative target gene was subjected to GO
analysis to reveal its cellular function. GO was analyzed using
three significant categories as follows: Molecular function,
biological process and cellular component. The analysis revealed a
wide distribution of cellular functions, which are presented in
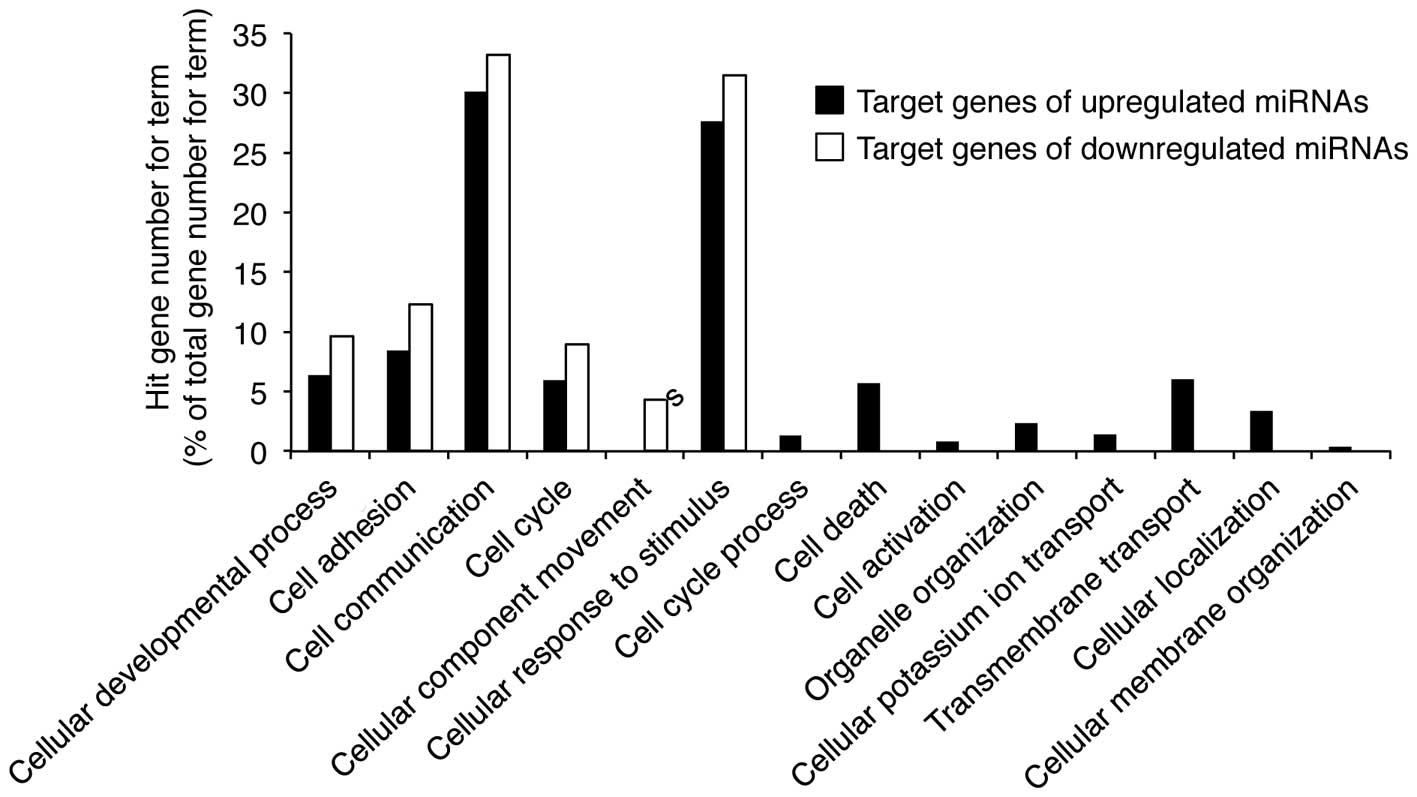
Fig. 4. Cellular process, which
comprised the largest percentage of the biological process category
in Fig. 4, was analyzed in greater
detail. The highest percentage of gene functions were in the
cellular process category, which encompasses the processes analyzed
in Fig. 5. In particular, the
target genes of miRNAs up- and downregulated by UVB radiation are
associated with cellular responses to stimuli and cell
communication. These results suggest that UVB-induced growth arrest
and apoptosis may be mediated by miRNAs involved in cellular
stimulation and communication in nHDPs.
Discussion
Sunlight is a multi-wavelength light that modulates
cellular signaling in the skin (3). UV radiation from sunlight is known to
induce DNA damage in types of skin cell such as keratinocytes and
fibroblasts (26), but the
UV-mediated cellular effects in nHDPs have not yet been reported.
In the current study it was determined that UVB radiation represses
nHDP growth via cell cycle arrest and apoptosis, and that it can
induce ROS generation. miRNAs have been demonstrated to have a
major influence over the control of UVB-induced growth repression
in a previous study (27).
Therefore, in the present study, the miRNA expression profile in
nHDPs following UVB-irradiation was analyzed. A total of 42 miRNAs
whose expression in nHDPs changed at least 1.5-fold following UVB
irradiation were identified. One of the upregulated miRNAs
identified in the current study was miR-30a-5p, which has been
reported to modulate cell growth by targeting the denticleless
protein homolog, a gene implicated in S phase and UVB-induced
growth arrest (28). miR-34a-5p
and miR-34b-5p, which were upregulated 1.6- and 1.8-fold in the
current study, respectively, are induced by DNA damage in various
types of cell (29,30), and by p53, which is activated by
DNA break-induced ataxia telangiectasia mutated activation
(29). Overall, the results of the
current study indicate that, in nHDPs, UVB regulates specific
miRNAs in order to regulate cell growth and death.
In addition, the present study identified putative
target genes of the up- and downregulated miRNAs, and categorized
their reported biological functions by GO into cell cycle,
apoptosis and cell growth and proliferation categories. UV
irradiation of various types of cell, including keratinocytes,
melanocytes and dermal fibroblasts, can regulate cell fate via the
intrinsic apoptosis pathway (26,31,32).
Consistent with this, the results of the present study demonstrated
that the miRNAs that were upregulated by UVB irradiation targeted
intrinsic apoptosis pathway-related genes, including NAIP (targeted
by miR-30a-5p), XAF1 (targeted by miR-638) and XIAP (targeted by
miR-630). In addition, miRNAs induced by UVB exposure were
demonstrated to regulate core cell cycle regulators including
cyclins, cyclin dependent kinases (CDKs) and CDK inhibitors. For
example, upregulated miRNAs targeted CDC7 (targeted by miR-30a-5p
and miR-630) and CDK2 (targeted by miR-638), while downregulated
miRNAs targeted CDKN1A (targeted by miR-299-5p), CDKN1B (targeted
by miR-218-5p and miR-495-3p) and CCNA2 (targeted by
miR-218-5p).
Overall, data of the current study demonstrated that
miRNA expression in nHDPs was altered in response to UVB exposure.
This furthers the current understanding of the cellular mechanisms
mediating the response to UVB exposure, whilst providing insight
into the processes involved in sunlight-induced hair and skin
aging.
Acknowledgements
This study was supported by a grant from the Korean
Health Technology R&D Project (grant no. HN13C0075), Ministry
of Health & Welfare, Republic of Korea. Dr Seunghee Bae was
supported by the KU Research Professor Program of Konkuk
University.
References
|
1
|
Schreiber MM, Moon TE and Bozzo PD:
Chronic solar ultraviolet damage associated with malignant melanoma
of the skin. J Am Acad Dermatol. 10:755–759. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helfrich YR, Sachs DL and Voorhees JJ:
Overview of skin aging and photoaging. Dermatol Nurs. 20:177–184.
2008.PubMed/NCBI
|
|
3
|
Muller HK and Woods GM: Ultraviolet
radiation effects on the proteome of skin cells. Adv Exp Med Biol.
990:111–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee YK, Cha HJ, Hong M, Yoon Y, Lee H and
An S: Role of NF-κB-p53 crosstalk in ultraviolet A-induced cell
death and G1 arrest in human dermal fibroblasts. Arch Dermatol Res.
304:73–79. 2012.
|
|
5
|
Xu H, Yan Y, Li L, Peng S, Qu T and Wang
B: Ultraviolet B-induced apoptosis of human skin fibroblasts
involves activation of caspase-8 and -3 with increased expression
of vimentin. Photodermatol Photoimmunol Photomed. 26:198–204. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Trüeb RM: Is androgenetic alopecia a
photoaggravated dermatosis? Dermatology. 207:343–348. 2003.
|
|
7
|
Lu Z, Fischer TW, Hasse S, et al:
Profiling the response of human hair follicles to ultraviolet
radiation. J Invest Dermatol. 129:1790–1804. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cha HJ, Shin S, Yoo H, et al:
Identification of ionizing radiation-responsive microRNAs in the
IM9 human B lymphoblastic cell line. Int J Oncol. 34:1661–1668.
2009.PubMed/NCBI
|
|
9
|
Wan G, Mathur R, Hu X, Zhang X and Lu X:
miRNA response to DNA damage. Trends Biochem Sci. 36:478–484. 2011.
View Article : Google Scholar
|
|
10
|
Tan G, Shi Y and Wu ZH: MicroRNA-22
promotes cell survival upon UV radiation by repressing PTEN.
Biochem Biophys Res Commun. 417:546–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pothof J, Verkaik NS, van IJcken W, et al:
MicroRNA-mediated gene silencing modulates the UV-induced
DNA-damage response. EMBO J. 28:2090–2099. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dynoodt P, Mestdagh P, Van Peer G, et al:
Identification of miR-145 as a key regulator of the pigmentary
process. J Invest Dermatol. 133:201–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu Z, He J, Jia X, et al: MicroRNA-25
functions in regulation of pigmentation by targeting the
transcription factor MITF in Alpaca (Lama pacos) skin
melanocytes. Domest Anim Endocrinol. 38:200–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan G, Niu J, Shi Y, Ouyang H and Wu ZH:
NF-κB-dependent microRNA-125b up-regulation promotes cell survival
by targeting p38α upon ultraviolet radiation. J Biol Chem.
287:33036–33047. 2012.
|
|
15
|
Zhou BR, Xu Y and Luo D: Effect of UVB
irradiation on microRNA expression in mouse epidermis. Oncol Lett.
3:560–564. 2012.PubMed/NCBI
|
|
16
|
Zhou BR, Xu Y, Permatasari F, et al:
Characterization of the miRNA profile in UVB-irradiated normal
human keratinocytes. Exp Dermatol. 21:317–319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim YJ, Cha HJ, Nam KH, Yoon Y, Lee H and
An S: Centella asiatica extracts modulate hydrogen
peroxide-induced senescence in human dermal fibroblasts. Exp
Dermatol. 20:998–1003. 2011. View Article : Google Scholar
|
|
18
|
Smith ML, Ford JM, Hollander MC, et al:
p53-mediated DNA repair responses to UV radiation: studies of mouse
cells lacking p53, p21, and/or gadd45 genes. Mol Cell Biol.
20:3705–3714. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meek DW: The p53 response to DNA damage.
DNA Repair (Amst). 3:1049–1056. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lewis DA, Yi Q, Travers JB and Spandau DF:
UVB-induced senescence in human keratinocytes requires a functional
insulin-like growth factor-1 receptor and p53. Mol Biol Cell.
19:1346–1353. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
El-Abaseri TB, Putta S and Hansen LA:
Ultraviolet irradiation induces keratinocyte proliferation and
epidermal hyperplasia through the activation of the epidermal
growth factor receptor. Carcinogenesis. 27:225–231. 2006.
View Article : Google Scholar
|
|
22
|
Takasawa R, Nakamura H, Mori T and Tanuma
S: Differential apoptotic pathways in human keratinocyte HaCaT
cells exposed to UVB and UVC. Apoptosis. 10:1121–1130. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chainiaux F, Magalhaes JP, Eliaers F,
Remacle J and Toussaint O: UVB-induced premature senescence of
human diploid skin fibroblasts. Int J Biochem Cell Biol.
34:1331–1339. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bossi O, Gartsbein M, Leitges M, Kuroki T,
Grossman S and Tennenbaum T: UV irradiation increases ROS
production via PKCdelta signaling in primary murine fibroblasts. J
Cell Biochem. 105:194–207. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Scharffetter-Kochanek K, Wlaschek M,
Brenneisen P, Schauen M, Blaudschun R and Wenk J: UV-induced
reactive oxygen species in photocarcinogenesis and photoaging. Biol
Chem. 378:1247–1257. 1997.PubMed/NCBI
|
|
26
|
Pustisek N and Situm M: UV-radiation,
apoptosis and skin. Coll Antropol. 35(Suppl 2): 339–341. 2011.
|
|
27
|
Schneider MR: MicroRNAs as novel players
in skin development, homeostasis and disease. Br J Dermatol.
166:22–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baraniskin A, Birkenkamp-Demtroder K,
Maghnouj A, et al: MiR-30a-5p suppresses tumor growth in colon
carcinoma by targeting DTL. Carcinogenesis. 33:732–739. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu H and Gatti RA: MicroRNAs: new players
in the DNA damage response. J Mol Cell Biol. 3:151–158. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato M, Paranjape T, Müller RU, et al: The
mir-34 microRNA is required for the DNA damage response in vivo in
C. elegans and in vitro in human breast cancer cells.
Oncogene. 28:2419–2424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamauchi T, Adachi S, Yasuda I, et al:
Ultra-violet irradiation induces apoptosis via mitochondrial
pathway in pancreatic cancer cells. Int J Oncol. 39:1375–1380.
2011.PubMed/NCBI
|
|
32
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|