Introduction
In the human endometrium, uterine leukocytes undergo
cyclic changes in cell number during the menstrual cycle. Uterine
natural killer (uNK) cells comprise 70% of all decidual leukocytes
during the secretory phase, when implantation occurs, and during
early pregnancy (1,2). At the initiation of embryo
implantation and placentation, uNK cells interact with the
extravillous trophoblasts and fetal cells that invade the uterus,
where they remove and replace the smooth muscle of maternal spiral
arteries (3). This ultimately
converts small, coiled vessels into wider channels that are able to
provide nutrients to the developing fetus (4).
The initial contact between the blastocyst and
uterus occurs through adhesion of the embryonic trophectoderm to
the uterine epithelium (5). During
implantation, the epithelium is said to become ‘receptive’
(6). In addition, the
transformation of endometrial stromal cells from small, densely
packed cells to large polygonal cells with an open vesicular
nucleus is one of the characteristic features of decidualization
(7). These findings suggest that
endometrial epithelial and stromal cells are important in
implantation and decidualization. Furthermore, a previous study
demonstrated that epithelial STAT3 controlled stromal function via
a paracrine mechanism (8),
indicating that there is epithelial-stromal crosstalk during
implantation.
Implantation marks a transition stage in pregnancy,
in which the blastocyst assumes a fixed position and establishes an
altered physiological interaction with the uterus. The paracrine
effects of uNK cells stimulate stromal fibroblasts to produce
chemokines and cytokines, which support trophoblast migration
during implantation. They also upregulate interleukin (IL)-15 and
IL-15Rα in stromal fibroblasts that may establish an environment
for uNK cells to promote cell proliferation and recruitment into
the uterus (9). There is strong
evidence that implantation and early pregnancy are not a single
event, and do not occur simultaneously (10). Thus, in order to investigate the
effects of uNK cell paracrine signaling on these processes as a
whole, a co-culture system consisting of epithelial and stromal
cells was created. Furthermore, the regulation of trophoblast
invasion and modification of the spiral arteries, was investigated
(11).
Materials and methods
Ethical approval
All subjects understood and signed the informed
consent form prior to participation. Experimental protocols were
approved by the Ethics Committee of the Dongfang Hospital Human
Ethics Committee, Beijing, China (no. 2011090201).
Tissue collection
Decidual tissues were obtained from ten healthy
females undergoing an elective termination of a normal pregnancy at
between seven and eight weeks of gestation, as determined by the
last menstrual period.
Endometrial tissues were collected from biopsies
taken during the proliferative phase of the menstrual cycle of
females undergoing laparoscopy for benign disease (Dongfang
Hospital of Beijing University of Chinese Medicine, Beijing,
China). The exclusion criteria were hormonal stimulation, cancerous
lesions and irregular menstrual bleeding. There were six volunteers
and two endometrial biopsies per volunteer were obtained. Samples
from three of the females (six biopsies) were used for the for
microarray experiments and samples from the remaining three females
(six biopsies) were used for the reverse transcription-quantitative
polymerase chain reaction and enzyme-linked immunosorbent assay
experiments. Of the two samples taken from each patient, one sample
was used as a control and the other was used in the experimental
group.
uNK cell isolation
uNK cells were purified as previously described
(2). Briefly, decidual tissues
were thoroughly washed with Ca2+- and
Mg2+-free Hank’s balanced salt solution (HBSS)
containing 100 U/ml penicillin and 100 g/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA), cut into fragments of 1–2
mm3 using two scalpels and digested for 1 h at 37°C with
gentle agitation in HBSS with 0.1% (w/v) collagenase I (Gibco-BRL,
Carlsbad, CA, USA). Cell suspensions were layered over
Ficoll-Hypaque medium (General Electric, Fairfield, CT, USA) and
centrifuged at 800 × g for 25 min. Cells at the interface were
washed twice in RPMI-1640 media with 10% fetal calf serum (FCS) and
antibiotics. Following incubation for 20 min at 4°C with anti-CD56
micro beads (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany),
cells were washed in washing buffer [phosphate-buffered saline
(PBS), EDTA 2 mM and 0.5% bovine serum albumin(w/v)] and loaded
onto a manual cell separation (MS) column in a MiniMACS magnet
(MiniMACSTM Separator System; Miltenyi Biotec GmbH). The
MS column was flushed three times and CD56+ cells were
flushed according to the manufacturer’s instructions. The purity of
the uNK cells was >90% CD56+CD3− according
to flow cytometric analysis. The uNK cells were cultured in
RPMI-1640 media with 1% FCS and 10 ng/ml IL-15 (R&D Systems
Inc., Minneapolis, MN, USA).
uNK cell-secretion medium production
uNK cell-secretion medium was prepared using 200 μl
RPMI-1640 media with 1% FCS and IL-15 (10 ng/ml) containing
5×105 of the purified uNK cells and placed into the
upper chamber of a 0.4-μm pore hanging cell culture insert (EMD
Millipore, Billerica, MA, USA) in a 24-well tissue plate, with
1,300 μl of the same media excluding cells in the lower chamber.
Germeyer et al (9) showed
that soluble factors from uterine leucocytes had significant
effects on endometrial cell gene expression. Thus, a hanging cell
culture insert was used so that soluble molecules from the uNK
cells were able pass through the filter into the lower chamber,
without cells being in direct contact. The control medium comprised
1,500 μl RPMI-1640 media with 1% FCS and 10 ng/ml IL-15. This was
used for subsequent experiments. Following incubation for 24 h at
37°C, the uNK cell-secretion medium from the lower chamber and the
control media were collected. To reduce interassay variability, the
media from several batches was pooled for subsequent experiments
and frozen at −80°C. Cells in the upper chamber were collected and
the cell viability was measured using a live/dead viability kit
(Invitrogen Life Technologies, Carlsbad, CA, USA). Only uNK cell
samples containing <35% of dead cells following overnight
incubation were used for subsequent experiments.
Endometrial stromal and epithelial cell
isolation
Human endometrial tissue was dissociated into single
cells using 0.1% (w/v) collagenase I (Life Technologies, Carlsbad,
CA, USA) for 50–60 min at 37°C. Cell suspensions were filtered
using a 40-μm sieve to separate undigested myometrial tissue and
debris. Further dissociation of the filtrate was prevented by
Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (no Phenol Red;
Gibco-BRL) with 10% FBS (Gibco-BRL). To remove erythrocytes, the
cells were resuspended in 4 ml DMEM/F12 with 1% FCS, layered over
Ficoll-Paque PLUS (General Electric) and centrifuged for 25 min at
800 × g. Endometrial cells were removed from the Ficoll-Paque PLUS
medium interface, washed three times and resuspended in 1 ml
DMEM/F12 with 1% FCS. Leukocytes were removed with CD45-coated
Dynabeads (Invitrogen Life Technologies). Purified stromal and
epithelial cell suspensions were then obtained by a further round
of magnetic bead sorting using Collection Epithelial Enrich
Dynabeads (Invitrogen Life Technologies). Epithelial and stromal
cell preparations were >95% pure.
Stromal cells were cultured in DMEM/F12 with 10% FBS
and an antibiotic-antimycotic agent (100 U/ml penicillin, 100 g/ml
streptomycin, 10 μg/ml gentamicin 0.25 μg/ml amphotericin B; Life
Technologies). Epithelial cells were cultured in serum-free
bronchial epithelial cell growth medium (final volumes: 2 ml bovine
pituitary extract, 0.5 ml insulin, 0.5 ml HC, 0.5 ml GA-1000, 0.5
ml retinoic acid, 0.5 ml transferrin, 0.5 ml triiodothyronine, 0.5
ml epinephrine and 0.5 ml hEGF; Lonza, Walkersville, MD, USA) and
an antibiotic-antimycotic agent. The isolated stromal and
epithelial cells were separately seeded into six-well plates with 3
ml culture medium per well. Each well contained stromal or
epithelial cells from a single patient. After two weeks, cells were
passaged into 25 cm2 cell culture flasks. Following
this, stromal cells were passaged every 4–5 days and epithelial
cells were passaged every 9–10 days.
Co-culture system
On day 20 following endometrial stromal and
epithelial cell generation, cells of each type were seeded onto a
Nunc UpCell Surface membrane (Thermo Labsystems, Santa Rosa, CA,
USA). The co-culture system was built on these
temperature-responsive cell culture surfaces, according to the
manufacturer’s instructions. In brief, when cells reached 80%
confluence, all medium was aspirated and 500 μl fresh medium was
added. The membrane was then placed on top of the stromal cell
layer. The Nunc UpCell Surface was maintained at 20°C for 13 min.
The membrane and cell layer were then carefully removed from the
Nunc UpCell Surface using forceps. The membrane with the attached
cell layer was transferred facing downwards onto the epithelial
cell surface. Fresh medium was added and samples were incubated at
37°C for 40 min. A further 1 ml of medium was added to the top of
the membrane and the membrane was withdrawn from the cell layer.
The ratio of stromal to epithelial cells was 1:1, and every
co-culture system was built using stromal and epithelial cells from
the same participant. Co-cultured cells were maintained in DMEM/F12
with 1% FCS.
Co-culture system treatment
Each group, control and uNK cell, contained six
co-culture systems. All the groups were washed twice with PBS and
placed in serum-free DMEM for 16 h prior to subsequent experiments.
DMEM was replaced by 80% uNK cell-secretion medium and 20% DMEM in
the uNK cell group, whilst the control group was treated with 80%
control medium and 20% DMEM. Following incubation for 6 h, the
cells and media from each group were collected. Three pairs of
co-culture systems were used for the microarray studies and three
pairs for the RT-qPCR experiments.
Microarray experiments
Total RNA was extracted from the endometrial cells
in each co-culture system. RNA was purified with the RNeasy Mini
kit (Qiagen, Hilden, Germany), according to the manufacturer’s
instructions. The microarray analysis was performed using the
GeneChip® 3′ IVT Express kit (Affymetrix Inc., Santa
Clara, CA, USA). Briefly, total RNA underwent reverse
transcription, first strand cDNA synthesis, double strand DNA,
in vitro transcription, cRNA synthesis and fragmentation
(12). Samples were hybridized
onto GeneChip PrimeView Human Gene Expression Array (Affymetrix
Inc.). This array covers >36,000 transcripts and variants.
Following 16 h hybridization at 45°C, arrays were washed on
Fluidics Station 450 (Affymetrix, Inc.) and were scanned with
Scanner 3000 (Affymetrix, Inc.) in order to obtain quantitative
gene expression levels. The control and uNK cell groups were
processed simultaneously throughout. Three chips were analyzed for
each group.
RT-qPCR analysis
A total of four differentially expressed genes were
selected for validation of the results from the microarray
experiments using RT-qPCR. Cells from the control and uNK cell
groups were washed twice with PBS, and total RNA was extracted
using TRIzol (Life Technologies). Reverse transcription was
performed with 8 μl of total RNA per 20 μl reaction using a
standard cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan). The
RT-qPCR primer sequences for target genes were self-designed by
this group and ordered from Invitrogen. Primer sequences for target
genes are shown in Table I.
 | Table ISequences of primers for reverse
transcription-quantitative polymerase chain reaction. |
Table I
Sequences of primers for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
5′g3′ | Length (bases) | Amplicon (bp) |
|---|
| CXCL10 | F:
CTTTCTGACTCTAAGTGGCATTC | 23 | 176 |
| R:
CACCCTTCTTTTTCATTGTAGCAA | 24 | |
| CXCL11 | F:
TATTACTATCTGTGGTTACGGTGGAG | 26 | 269 |
| R:
GCACTTTTGCCAGTATCCCAT | 21 | |
| IL-15 | F:
TGGCTGCTGGAAACCC | 16 | 123 |
|
R:CACAAGTAGCACTGGATGGAAAT | 23 | |
| SAMD9L | F:
GCCTTATCTCCACCTGTTTCTTAG | 24 | 300 |
| R:
TGGGATGGCATTCCTTGAC | 19 | |
| GAPDH | F:
GAGCCAAAAGGGTCATCATCT | 21 | 231 |
| R:
AGGGGCCATCCACAGTCTTC | 20 | |
For each RT-qPCR experiment, the typical thermal
cycling conditions included an initial activation step at 95°C for
5 min, 40 cycles at 95°C each for 30 sec, 56°C for 20 sec and 72°C
for 30 sec. PCR reactions were performed on ABI Prism 7700 Sequence
Detection system (Applied Biosystems Life Technologies, Foster
City, CA, USA). cDNA concentration was normalized to that of GAPDH.
The target mRNA expression was analyzed using the 2−ΔΔCt
algorithm.
ELISA experiments
IL-15 was analyzed using a commercially available
ELISA kit (ELH-IL-15, RayBiotech, China) in the uNK cell-secretion
medium prior to its use in the co-culture system experiments and in
supernatants of the co-culture systems that had been treated with
control media or with uNK cell-secretion medium. The analysis was
conducted according to the manufacturer’s instructions. Assays were
performed in triplicate and concentrations of IL-15 (pg/ml) were
compared with standard curves. To determine the quantities of IL-15
secreted by the co-culture systems, the starting IL-15 content of
the uNK cell-secretion medium was subtracted. The sensitivity of
the kit was 10 pg/ml.
Statistical analysis
Data were analyzed using analysis of variance using
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). In the
microarray experiments, median fold change ratios between the
control and uNK cell groups were derived for each transcript, and
genes that were up- or downregulated with a fold change >1 and
P<0.001 were selected. In RT-qPCR and ELISA analysis, data are
presented as the median ± standard error of the mean. P<0.01 was
considered to indicate a statistically significant difference.
Graphs of the data were produced using Microsoft Excel
software.
Results
Microarray experiments
Gene expression profiling using a microarray was
used to compare transcript expression in the endometrial co-culture
systems treated with either control medium or uNK cell-secretion
medium.
This analysis identified 155 upregulated genes that
exhibited a change of >2-fold in the median expression level in
response to uNK cell-secretion medium. No transcript was
downregulated >2-fold (Table
II). However, certain genes in uNK cell groups were upregulated
with a 1.0–2.0-fold change, compared with the control group.
Previous studies have shown that these genes have an important
functions in uNK cells. For instance, the co-culture system treated
with uNK cell-secretion medium showed increased expression of
interleukin (IL)15RA (1.6-fold) (13), vascular endothelial growth factor
(VEGF)-C (1.35-fold) (14),
intercellular adhesion molucule (ICAM) 1 (1.66-fold) (15), superoxide dismutase (SOD)2
(1.09-fold), caspase (CASP)1 (1.85-fold), nuclear factor erythroid
2-related factor 3 (NFE2L3) (1.37-fold), interferon γ receptor 1
(IFNGR1; 1.10-fold) (16), major
histocompatibility complex (MHC) class I polypeptide-related
sequence A (1.12-fold) and MHC class I polypeptide-related sequence
B (1.34-fold) (3,17) and showed decreased expression of
IGFBP3 (1.04-fold) (P<0.001).
 | Table IIUpregulated transcripts altered
>2-fold. |
Table II
Upregulated transcripts altered
>2-fold.
| Gene | Fold change | Gene ID | Description |
|---|
| Cytokines/
Chemokines |
| CXCL10 | 11.1 | 3627 | Chemokine (C-X-C
motif) ligand 10 |
| CXCL11 | 4.2 | 6373 | Chemokine (C-X-C
motif) ligand 11 |
| IL-15 | 2.6 | 3600 | Interleukin 15 |
| IL-7 | 2.1 | 3574 | Interleukin 7 |
| Immunological
factors |
| NLRC5 | 3.8 | 84166 | NLR family, CARD
domain containing 5 |
| FAM111A | 3.4 | 63901 | Family with
sequence similarity 111, member A |
| ACTR2 | 2.6 | 10097 | ARP2 actin-related
protein 2 homolog (yeast) |
| HSPH1 | 2.2 | 10808 | Heat shock protein
1 |
| IFIT5 | 2.2 | 24138 | Interferon-induced
protein with tetratricopeptide repeats 5 |
| UVRAG | 2.1 | 7405 | UV radiation
resistance associated gene |
| Apoptotic
protein |
| RASSF6 | 2.2 | 166824 | Ras association
(RalGDS/AF-6) domain family member 6 |
| Tryptophan
metabolism |
| IDO1 | 3.6 | 3620 | Indoleamine
2,3-dioxygenase 1 |
| Signaling
factors |
| GBP2 | 3.2 | 2634 | Guanylate binding
protein 2, interferon-inducible |
| UACA | 3.1 | 55075 | Uveal autoantigen
with coiled-coil domains and ankyrin repeats |
| IFIT3 | 2.8 | 3437 | Interferon-induced
protein with tetratricopeptide repeats 3 |
| ALCAM | 2.8 | 214 | Activated leukocyte
cell adhesion molecule |
| CGA | 2.6 | 1081 | Glycoprotein
hormones, α polypeptide |
| EDNRA | 2.5 | 1909 | Endothelin receptor
type A |
| WASF2 | 2.2 | 10163 | WAS protein family,
member 2 |
| IL6ST | 2.1 | 3572 | Interleukin 6
signal transducer |
| USP15 | 2.1 | 9958 | Ubiquitin specific
peptidase 15 |
| MIER1 | 2.1 | 57708 | Mesoderm induction
early response 1 homolog |
| RGS12 | 2.0 | 6002 | Regulator of
G-protein signaling 12 |
| Transcription |
| STAT1 | 3.7 | 6772 | Signal transducer
and activator of transcription 1 |
| IRF1 | 3.6 | 3659 | Interferon
regulatory factor 1 |
| IRF9 | 3.2 | 10379 | Interferon
regulatory factor 9 |
| IFI16 | 3.2 | 3428 | Interferon,
γ-inducible protein 16 |
| ATRX | 3.1 | 546 | Alpha
thalassemia/mental retardation syndrome X-linked |
| TCERG1 | 2.5 | 10915 | Transcription
elongation regulator 1 |
| ZNF644 | 2.4 | 84146 | Zinc finger protein
644 |
| ZEB1 | 2.2 | 6935 | Zinc finger E-box
binding homeobox 1 |
| SAFB2 | 2.1 | 9667 | Scaffold attachment
factor B2 |
| PRDM2 | 2.1 | 7799 | PR domain
containing 2 |
| Nucleotide
metabolism |
| NUFIP2 | 3.0 | 57532 | Nuclear fragile X
mental retardation protein interacting protein 2 |
| PAPOLA | 2.2 | 57532 | Poly(A) polymerase
alpha |
| EIF4G1 | 2.3 | 10914 | Eukaryotic
translation initiation factor 4γ, 1 |
| CRCP | 2.5 | 1981 | CGRP receptor
component |
| AGGF1 | 2.6 | 27297 | Angiogenic factor
with G patch and FHA domains 1 |
| XRN2 | 2.1 | 55109 | 5′-3′
exoribonuclease 2 |
| Enzyme
activity |
| GBP4 | 6.8 | 115361 | Guanylate binding
protein 4 |
| GBP5 | 4.8 | 115362 | Guanylate binding
protein 5 |
| INPP4B | 4.8 | 8821 | Inositol
polyphosphate-4-phosphatase, type II |
| GBP7 | 4.7 | 388646 | Guanylate binding
protein 7 |
| SETD2 | 4.2 | 29072 | SET domain
containing 2 |
| GBP1 | 3.9 | 2633 | Guanylate binding
protein 1 |
| PSMB9 | 3.3 | 5698 | Proteasome
(prosome, macropain) subunit, β type, 9 |
| WASL | 3.3 | 8976 | Wiskott-Aldrich
syndrome-like |
| PUS7L | 3.0 | 83448 | Pseudouridylate
synthase 7 homolog-like |
| C1R | 2.9 | 715 | Complement
component 1 |
| TAP1 | 2.8 | 6890 | Transporter 1,
ATP-binding cassette, sub-family B (MDR/TAP) |
| FAF2 | 2.7 | 23197 | Fas associated
factor family member 2 |
| UBE2L6 | 2.7 | 9246 |
Ubiquitin-conjugating enzyme E2L 6 |
| PARP14 | 2.7 | 54625 | Poly (ADP-ribose)
polymerase family, member 14 |
| PARP9 | 2.7 | 83666 | Poly (ADP-ribose)
polymerase family, member 9 |
| PSME4 | 2.5 | 23198 | Proteasome
(prosome, macropain) activator subunit 4 |
| OTUD4 | 2.5 | 54726 | OTU domain
containing 4 |
| BIRC6 | 2.4 | 57448 | Baculoviral IAP
repeat containing 6 |
| ARHGAP21 | 2.4 | 57584 | Rho GTPase
activating protein 21 |
| DTX3L | 2.4 | 151636 | Deltex 3-like |
| RARRES3 | 2.3 | 5920 | Retinoic acid
receptor responder 3 |
| MGEA5 | 2.3 | 10724 | Meningioma
expressed antigen 5 |
| DCAF8 | 2.3 | 50717 | DDB1 and CUL4
associated factor 8 |
| GPD2 | 2.3 | 2820 |
Glycerol-3-phosphate dehydrogenase 2 |
| UHMK1 | 2.3 | 127933 | U2AF homology motif
(UHM) kinase 1 |
| PDP1 | 2.2 | 54704 | Pyruvate
dehyrogenase phosphatase catalytic subunit 1 |
| USP10 | 2.2 | 9100 | Ubiquitin specific
peptidase 10 |
| COIL | 2.2 | 8161 | Coilin |
| UFL1 | 2.2 | 23376 | UFM1-specific
ligase 1 |
| USP1 | 2.1 | 7398 | Ubiquitin specific
peptidase 1 |
| G2E3 | 2.1 | 55632 | G2/M-phase specific
E3 ubiquitin protein ligase |
| NF1 | 2.0 | 4763 | Neurofibromin
1 |
| PHACTR2 | 2.0 | 9749 | Phosphatase and
actin regulator 2 |
| Transporters |
| TPR | 4.4 | 7175 | Translocated
promoter region, nuclear basket protein |
| APOL6 | 3.2 | 80830 | Apolipoprotein L,
6 |
| GCC2 | 2.8 | 9648 | GRIP and
coiled-coil domain containing 2 |
| APOL3 | 2.4 | 80833 | Apolipoprotein L,
3 |
| CPNE3 | 2.4 | 8895 | Copine III |
| USO1 | 2.2 | 8615 | USO1 vesicle
docking protein homolog |
| SLC38A1 | 2.2 | 81539 | Solute carrier
family 38, member 1 |
| NIPAL1 | 2.1 | 152519 | NIPA-like domain
containing 1 |
| KIAA1033 | 2.1 | 23325 | KIAA1033 |
| NUPL1 | 2.1 | 9818 | Nucleoporin like
1 |
| Structural
factors |
| MIS18BP1 | 3.9 | 55320 | MIS18 binding
protein 1 |
| CDC27 | 3.3 | 996 | Cell division cycle
27 homolog |
| CALD1 | 3.1 | 800 | Caldesmon 1 |
| LIMA1 | 3.0 | 51474 | LIM domain and
actin binding 1 |
| SLMAP | 2.7 | 7871 | Sarcolemma
associated protein |
| TLN1 | 2.7 | 7094 | Talin 1 |
| EZR | 2.5 | 7430 | Ezrin |
| ITGB1 | 2.5 | 3688 | Integrin, β1 |
| APPL1 | 2.5 | 26060 | Adaptor protein,
phosphotyrosine interaction, PH domain and leucine zipper
containing 1 |
| RTP4 | 2.5 | 64108 | Receptor
(chemosensory) transporter protein 4 |
| WAPAL | 2.4 | 23063 | Wings apart-like
homolog |
| PRRC2C | 2.4 | 23215 | Proline-rich
coiled-coil 2C |
| CASC5 | 2.4 | 57082 | Cancer
susceptibility candidate 5 |
| ENC1 | 2.3 | 8507 | Ectodermal-neural
cortex 1 |
| KIF14 | 2.2 | 9928 | Kinesin family
member 14 |
| SPTBN1 | 2.2 | 6711 | Spectrin, β,
non-erythrocytic 1 |
| MYO6 | 2.2 | 4646 | Myosin VI |
| ODF2L | 2.1 | 57489 | Outer dense fiber
of sperm tails 2-like |
| DYNC1H1 | 2.1 | 1778 | Dynein, cytoplasmic
1, heavy chain |
| PPP4R2 | 2.1 | 151987 | Protein phosphatase
4, regulatory subunit 2 |
| SRPR | 2.0 | 6734 | Signal recognition
particle receptor |
| Kinase |
| WNK1 | 4.0 | 65125 | WNK lysine
deficient protein kinase 1 |
| PRKDC | 3.5 | 5591 | Protein kinase,
DNA-activated, catalytic polypeptide |
| IQGAP1 | 3.0 | 8826 | IQ motif containing
GTPase activating protein 1 |
| CMPK2 | 2.8 | 129607 | Cytidine
monophosphate (UMP-CMP) kinase 2, mitochondrial |
| BAZ1B | 2.7 | 9031 | Bromodomain
adjacent to zinc finger domain, 1B |
| CCND2 | 2.6 | 894 | Cyclin D2 |
| MOB1A | 2.5 | 55233 | MOB kinase
activator 1A |
| MAP4K5 | 2.5 | 11183 | Mitogen-activated
protein kinase kinase kinase kinase 5 |
| Ion binding
proteins |
| DSC2 | 4.5 | 1824 | Desmocollin 2 |
| EEA1 | 4.2 | 8411 | Early endosome
antigen 1 |
| ZC3H11A | 3.3 | 9877 | Zinc finger
CCCH-type containing 11A |
| CLSTN1 | 2.8 | 22883 | Calsyntenin 1 |
| C1S | 2.7 | 716 | Complement
component 1, s subcomponent |
| THAP6 | 2.6 | 152815 | THAP domain
containing 6 |
| RSAD2 | 2.5 | 91543 | Radical S-adenosyl
methionine domain containing 2 |
| ZNFX1 | 2.3 | 57169 | Zinc finger,
NFX1-type containing 1 |
| PLCB4 | 2.2 | 5332 | Phospholipase C,
β4 |
| TIPARP | 2.2 | 25976 | TCDD-inducible
poly(ADP-ribose) polymerase |
| WDFY1 | 2.1 | 57590 | WD repeat and FYVE
domain containing 1 |
| CHURC1 | 2.1 | 91612 | Churchill domain
containing 1 |
| ITGA4 | 2.1 | 3676 | Integrin, α4 |
| XAF1 | 2.1 | 54739 | XIAP associated
factor 1 |
| DNA/RNA
proteins |
| ZFR | 4.4 | 51663 | Zinc finger RNA
binding protein |
| TOP1 | 4.2 | 7150 | Topoisomerase (DNA)
I |
| BOD1L1 | 3.4 | 259282 | Biorientation of
chromosomes in cell division 1-like 1 |
| IFIT2 | 3.3 | 3433 | Interferon-induced
protein with tetratricopeptide repeats 2 |
| CENPF | 3.1 | 1063 | Centromere protein
F, 350/400 kDa (mitosin) |
| MBNL1 | 3.0 | 4154 | Muscleblind-like
splicing regulator 1 |
| DHX9 | 2.4 | 1660 | DEAH
(Asp-Glu-Ala-His) box polypeptide 9 |
| FMR1 | 2.4 | 2332 | Fragile X mental
retardation 1 |
| DDX58 | 2.3 | 23586 | DEAD
(Asp-Glu-Ala-Asp) box polypeptide 58 |
| BCLAF1 | 2.3 | 9774 | BCL2-associated
transcription factor 1 |
| DNMT1 | 2.2 | 1786 | DNA
(cytosine-5-)-methyltransferase 1 |
| NOL8 | 2.2 | 55035 | Nucleolar protein
8 |
| RNF213 | 2.2 | 57674 | Ring finger protein
213 |
| BDP1 | 2.2 | 55814 | B double prime
1 |
| RAD21 | 2.2 | 5885 | RAD21 homolog |
| HP1BP3 | 2.1 | 50809 | Heterochromatin
protein 1, binding protein 3 |
| SF3B1 | 2.0 | 23451 | Splicing factor 3b,
subunit 1 |
| Other |
| SAMD9L | 5.7 | 219285 | Sterile α motif
domain containing 9-like |
| C10orf118 | 5.5 | 55088 | Chromosome 10 open
reading frame 118 |
| MTUS1 | 2.7 | 57509 | Microtubule
associated tumor suppressor 1 |
| EPSTI1 | 2.6 | 94240 | Epithelial stromal
interaction 1 |
| ATXN7L3B | 2.6 | 552889 | Ataxin 7-like
3B |
| ANKRD32 | 2.5 | 84250 | Ankyrin repeat
domain 32 |
| EHBP1 | 2.4 | 23301 | EH domain binding
protein 1 |
| PPFIBP1 | 2.4 | 8496 | PTPRF interacting
protein, binding protein 1 (liprin β1) |
| TMTC3 | 2.3 | 160418 | Transmembrane and
tetratricopeptide repeat containing 3 |
| CEP350 | 2.2 | 9857 | Centrosomal protein
350 kDa |
| BTBD10 | 2.2 | 84280 | BTB (POZ) domain
containing 10 |
| BTN3A3 | 2.1 | 10384 | Butyrophilin,
subfamily 3, member A3 |
| KCTD9 | 2.1 | 54793 | Potassium channel
tetramerisation domain containing 9 |
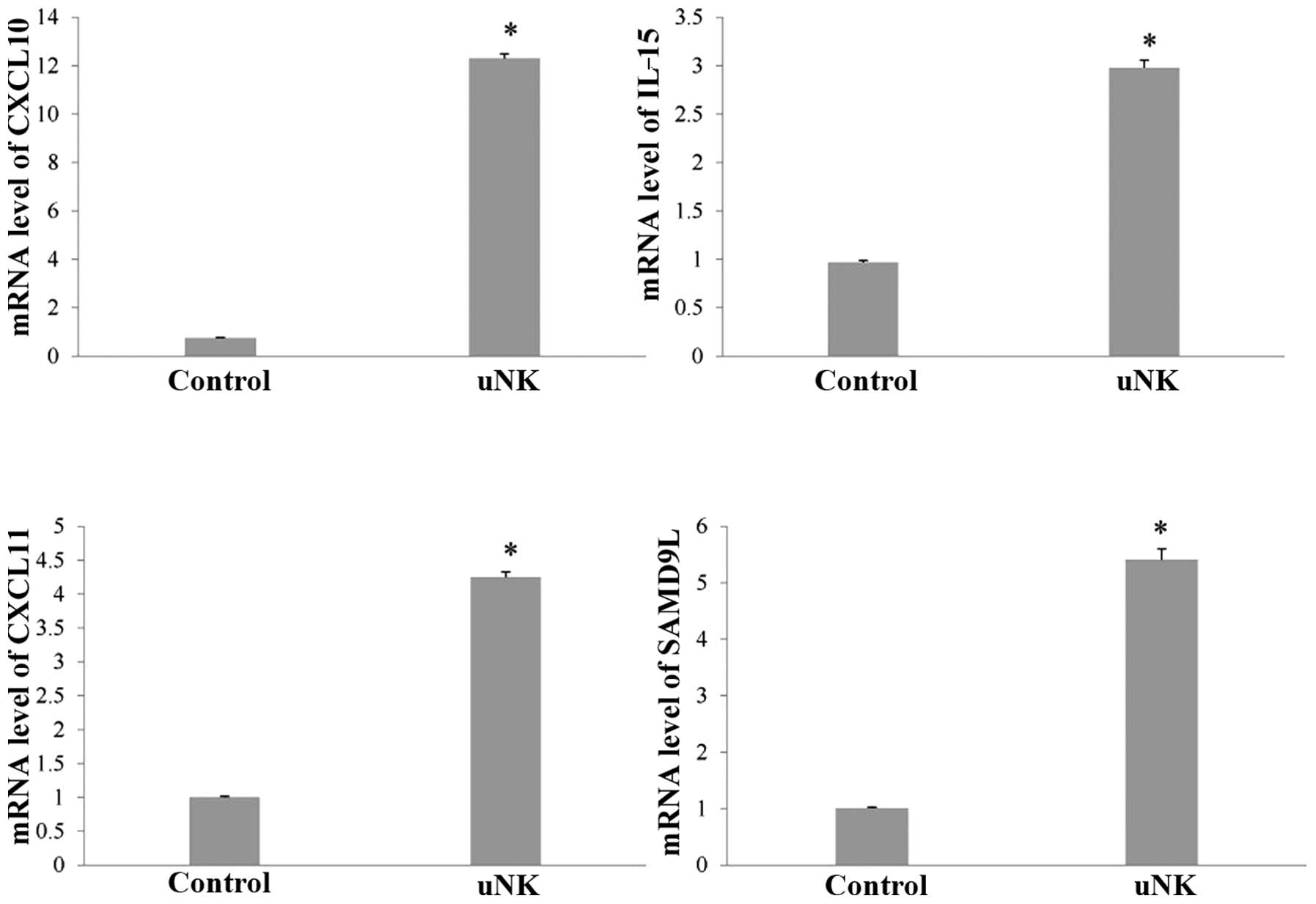
RT-qPCR analysis
In order to verify these changes, transcript levels
for certain genes were measured by RT-qPCR, including chemokine
(C-X-C) motif ligand (CXCL)10, CXCL11, IL-15 and sterile α motif
domain containing 9-like (SAMD9L; Fig.
1). The RT-qPCR results confirmed the significant changes in
expression that had been indicated by the microarray analysis. The
endometrial co-culture system treated with uNK cell-secretion
medium showed increased expression of CXCL10 (16.4-fold), CXCL11
(4.3-fold), IL-15 (3.1-fold) and SAMD9L (5.4-fold; P<0.01).
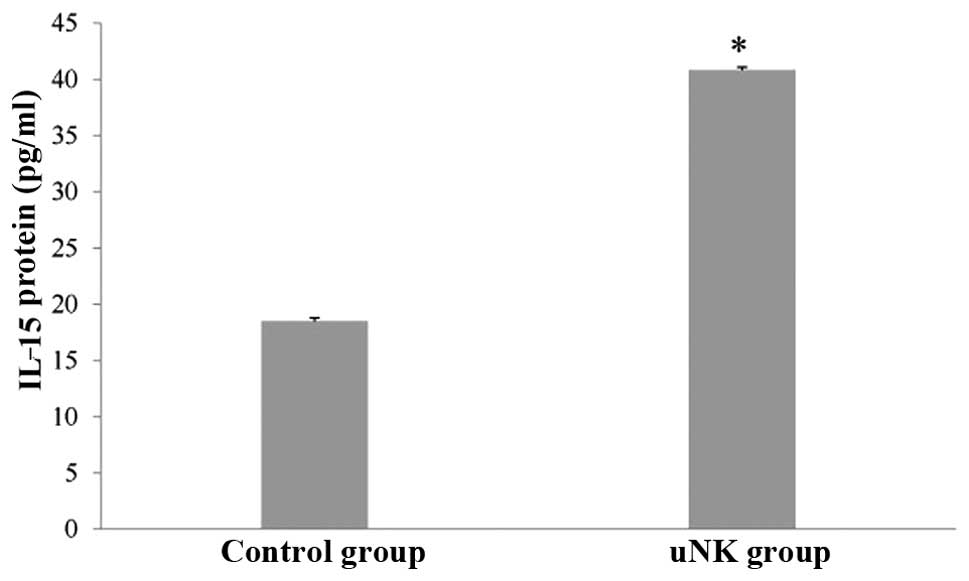
ELISA experiments
To confirm the observed changes in the IL-15 protein
level, the quantity of IL-15 was analyzed by ELISA in the
supernatant of the control and uNK cell-secretion medium-stimulated
endometrial co-culture systems. There was a significant increase in
IL-15 protein levels in the experimental groups compared with the
control group (Fig. 2;
P<0.01).
Discussion
Several observations suggest that uNK cells are
involved in reproduction. They increase in number during the luteal
period of the menstrual cycle when implantation occurs (1). They are present in the early phases
of gestation, when placental cells invade into the maternal
arteries (18). In addition, they
are particularly abundant in the area surrounding the infiltrating
fetally derived extravillous cells (19). During the progesterone-dominated
phase of the menstrual cycle, uNK cells show changes in the levels
of transcripts for VEGF-C (20).
Previous protein array studies have shown that uNK cells are the
predominant producers of angiogenic growth factors in early
pregnancy (21). In addition, in
an ex vivo chorionic plate artery model, uNK cells promoted
vessel-like assembly of extravillous cytotrophoblast cell lines
(15,22). Insufficient uNK cell activation may
reduce these processes and contribute to poor arterial remodeling
in decidua, thus increasing the risk of preeclampsia and
intrauterine fetal growth restriction (23).
In humans, CD56+ NK cells are associated
with the synthesis of immunoregulatory cytokines, particularly
IFN-γ (24). IFN-γ significantly
upregulates certain chemokines [CXCL9, CXCL10, chemokine (C-C
motif) ligand 8 and IL-15Rα], enzymes [guanylate binding protein 5,
transporter associated with antigen processing (TAP1), SOD2 and
CASP1] and transcription factors (interferon regulatory factor 1,
NFE2L3 and transcription factor AP-2 γ). It is also known to
downregulate insulin-like growth factor binding proteins (Wnt1
inducible signaling pathway 2 and insulin-like growth
factor-binding protein 3) (16).
These actions, combined with the uNK cell production of chemokines
CXCL10 and CXCR2, direct the migration and invasion of trophoblasts
(25) and promote angiogenesis in
the placental bed (26,27). The present study found similar
changes in gene expression, for example, IFNGR1 transcript levels
were significantly increased in co-culture systems stimulated by
uNK cell-secretion medium. This concordance strongly suggests that
uNK cell paracrine signaling, combined with INF-γ, regulates the
expression of genes involved in embryo and trophoblast migration,
endometrial decidualization and angiogenesis in human uterine
endometrium.
Implantation-associated decidualization in the rat
and mouse results in the accumulation of NK cells in the uterine
mesometrial decidua (28). uNK
cells are hypothesized to be involved in pregnancy-associated
uterine vascular development (20). However, it is not clear how uNK
cells communicate with the developing endometrial cells in order to
facilitate this process. Previous in vitro experimentation
has indicated that uNK cells produce factors that directly affect
the behavior of trophoblast cells (25). A number of studies have suggested
that uNK cell supernatant stimulate trophoblast invasion (29), whereas others have concluded that
it is the uNK cell supernatants that stimulate these process
(20). The results of the current
study indicate that the paracrine effects of uNK cells on
endometrial epithelial and stromal cells mediate the development of
the vasculature. Levels of ICAM-1, which is involved the migration
and network formation of the trophoblast cell line (15), increased significantly in this
study. Thus, it is likely that ICAM-1 is involved in this paracrine
network.
Although replete with cytotoxic machinery, uNK cells
remain tolerant at the maternal-fetal interface (26). A previous indicated that this is
facilitated by VEGF-C (30), and
in vitro studies have also suggested that the involvement of
IL-15 is important in promoting this tolerance (17). In addition, the non-cytotoxic
capacity of uNK cells is based on their ability to recognize
surface MHC class I molecules on target cells, which deliver
signals that suppress NK cell function. A number of studies have
suggested that a lack of engagement of MHC-specific receptors leads
to NK cell-mediated killing (17,31).
The results from this study showed that there was an increased
expression of VEGF-C, IL-15 and MHC class I polypeptide-related
sequence A/B in the co-culture systems that were treated with uNK
cell-secretion medium compared with the control group, indicating
similar non-cytotoxic mechanism to those already postulated.
Furthermore, there is evidence that endothelial cells exhibit
sensitivity to activated peripheral blood NK cells in the absence
of the expression of TAP1 (32),
as TAP-1 is a key factor essential for peptide loading for MHC
class I assembly (33). It is
proposed that VEGF-C is the predominant regulator of TAP1
expression in the uterus (30),
and the present study showed higher TAP1 expression in the uNK cell
group. These findings support a dual function of VEGF-C, in which
it acts as an angiogenic factor and also promotes immune tolerance
in the uterine microenvironment. To the best of our knowledge, this
is the first evidence that noncytotoxicity of uNK cells is directly
coupled to their vascular remodeling and angiogenesis
functions.
The origins of human uNK cells are not clear, and a
number of mechanisms have been postulated (34–36).
Several of the molecules that were altered by uNK cell-secretion
medium in this study are known to have important functions in NK
cell proliferation, including IL-15 and IL-15Rα. IL-15 has a
variety of functions, including the induction of T cell
proliferation and the activation of cytotoxic effector cells and
monocytes (37,38). The reciprocal interactions between
uNK cells and the epithelial/stromal cell co-culture system
observed in this study are similar to those in bone marrow during
NK cell development. NK cells upregulate IL-15 in the bone marrow
microenvironment, which is then bound and presented by IL-15Rα to
the stromal cell surface, promoting increased NK cell proliferation
(39). These results suggest that
this mechanism may occur in the endometrium in response to
molecules secreted by uNK cells. The results suggest that uNK cells
and non-decidualized stromal and epithelial cells may interact to
maintain immune cell homeostasis in the endometrial
microenvironment.
In conclusion, to the best of our knowledge, this is
the first detailed study of the paracrine interaction between uNK
cells and endometrial cells (epithelial/stromal cells). It
indicates that this paracrine signaling may contribute to uNK cell
proliferation and recruitment, embryo and trophoblast migration,
endometrial decidualization, angiogenesis and immune tolerance in
the uterine microenvironment.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81173292).
References
|
1
|
King A, Wellings V, Gardner L and Loke YW:
Immunocytochemical characterization of the unusual large granular
lymphocytes in human endometrium throughout the menstrual cycle.
Hum Immunol. 24:195–205. 1989. View Article : Google Scholar
|
|
2
|
Verma S, Hiby SE, Loke YW and King A:
Human decidual natural killer cells express the receptor for and
respond to the cytokine interleukin 15. Biol Reprod. 62:959–968.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moffett A and Loke C: Immunology of
placentation in eutherian mammals. Nat Rev Immunol. 6:584–594.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Parham P and Guethlein LA: Pregnancy
immunogenetics: NK cell education in the womb? J Clin Invest.
120:3801–3804. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carson DD, Wilson OF and Dutt A:
Glycoconjugate expression and interactions at the cell surface of
mouse uterineu epithelial cells and periimplantation-stage embryos.
Trophoblast Invasion and Endometrial Receptivity. Springer; New
York, NY, USA: pp. 211–241. 1990, View Article : Google Scholar
|
|
6
|
Murphy CR and Shaw TJ: Plasma membrane
transformation: a common response of uterine epithelial cells
during the peri-implantation period. Cell Biol Int. 18:1115–1128.
1994. View Article : Google Scholar
|
|
7
|
Inoue T, Kanzaki H, Iwai M, et al: Tumour
necrosis factor alpha inhibits in-vitro decidualization of human
endometrial stromal cells. Hum Reprod. 9:2411–2417. 1994.
|
|
8
|
Pawar S, Starosvetsky E, Orvis GD,
Behringer RR, Bagchi IC and Bagchi MK: STAT3 regulates uterine
epithelial remodeling and epithelial-stromal crosstalk during
implantation. Mol Endocrinol. 27:1996–2012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Germeyer A, Sharkey AM, Prasadajudio M, et
al: Paracrine effects of uterine leucocytes on gene expression of
human uterine stromal fibroblasts. Mol Hum Reprod. 15:39–48. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schlafke S and Enders AC: Cellular basis
of interaction between trophoblast and uterus at implantation. Biol
Reprod. 12:41–65. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiby SE, Walker JJ, O’shaughnessy KM, et
al: Combinations of maternal KIR and fetal HLA-C genes influence
the risk of preeclampsia and reproductive success. J Exp Med.
200:957–965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gong X, Chen Z, Liu Y, Lu Q and Jin Z:
Gene expression profiling of the paracrine effects of uterine
natural killer cells on human endometrial epithelial cells. Int J
Endocrinol. 2014:3937072014. View Article : Google Scholar
|
|
13
|
Grabstein KH, Eisenman J, Shanebeck K, et
al: Cloning of a T cell growth factor that interacts with the beta
chain of the interleukin-2 receptor. Science. 264:965–968. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou Y, Bellingard V, Feng KT, McMaster M
and Fisher SJ: Human cytotrophoblasts promote endothelial survival
and vascular remodeling through secretion of Ang2, PlGF, and
VEGF-C. Dev Biol. 263:114–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu Y, Eastabrook G, Tan R, MacCalman C,
Dutz JP and von Dadelszen P: Decidual NK cell-derived conditioned
medium enhances capillary tube and network organization in an
extravillous cytotrophoblast cell line. Placenta. 31:213–221. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kitaya K, Yasuo T, Yamaguchi T, Fushiki S
and Honjo H: Genes regulated by interferon-γ in human uterine
microvascular endothelial cells. Int J Mol Med. 20:689–697.
2007.
|
|
17
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bulmer J, Johnson P and Bulmer D:
Leukocyte populations in human decidua and endometrium.
Immunoregulation and Fetal Survival. Oxford University Press;
Oxford, UK: pp. 111–134. 1987
|
|
19
|
Loke Y: Human implantation: Cell Biology
and Immunology. Cambridge University Press; Cambridge, UK: 1995
|
|
20
|
Zhang J, Chen Z, Smith GN and Croy BA:
Natural killer cell-triggered vascular transformation: maternal
care before birth? Cell Mol Immunol. 8:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lash GE, Robson SC and Bulmer JN: Review:
Functional role of uterine natural killer (uNK) cells in human
early pregnancy decidua. Placenta. 31(Suppl): S87–S92. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Dutz JP, MacCalman CD, Yong P, Tan R
and von Dadelszen P: Decidual NK cells alter in vitro first
trimester extravillous cytotrophoblast migration: a role for
IFN-gamma. J Immunol. 177:8522–8530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wegmann TG, Lin H, Guilbert L and Mosmann
TR: Bidirectional cytokine interactions in the maternal-fetal
relationship: is successful pregnancy a TH2 phenomenon? Immunol
Today. 14:353–356. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ottaviani C, Nasorri F, Bedini C, de Pità
O, Girolomoni G and Cavani A: CD56brightCD16(−) NK cells accumulate
in psoriatic skin in response to CXCL10 and CCL5 and exacerbate
skin inflammation. Eur J Immunol. 36:118–128. 2006.
|
|
25
|
Hanna J, Goldman-Wohl D, Hamani Y, et al:
Decidual NK cells regulate key developmental processes at the human
fetal-maternal interface. Nat Med. 12:1065–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moffett-King A: Natural killer cells and
pregnancy. Nat Rev Immunol. 2:656–663. 2002. View Article : Google Scholar
|
|
27
|
Wang A, Rana S and Karumanchi SA:
Preeclampsia: the role of angiogenic factors in its pathogenesis.
Physiology (Bethesda). 24:147–158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ain R, Canham LN and Soares MJ: Gestation
stage-dependent intrauterine trophoblast cell invasion in the rat
and mouse: novel endocrine phenotype and regulation. Dev Biol.
260:176–190. 2003. View Article : Google Scholar
|
|
29
|
Smith SD, Dunk CE, Aplin JD, Harris LK and
Jones RL: Evidence for immune cell involvement in decidual spiral
arteriole remodeling in early human pregnancy. Am J Pathol.
174:1959–1971. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kalkunte SS, Mselle TF, Norris WE, Wira
CR, Sentman CL and Sharma S: Vascular endothelial growth factor C
facilitates immune tolerance and endovascular activity of human
uterine NK cells at the maternal-fetal interface. J Immunol.
182:4085–4092. 2009. View Article : Google Scholar
|
|
31
|
Riley JK and Yokoyama WM: NK cell
tolerance and the maternal-fetal interface. Am J Reprod Immunol.
59:371–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ayalon O, Hughes EA, Cresswell P, et al:
Induction of transporter associated with antigen processing by
interferon gamma confers endothelial cell cytoprotection against
natural killer-mediated lysis. Proc Natl Acad Sci USA.
95:2435–2440. 1998. View Article : Google Scholar
|
|
33
|
Cox JH, Yewdell JW, Eisenlohr LC, Johnson
PR and Bennink JR: Antigen presentation requires transport of MHC
class I molecules from the endoplasmic reticulum. Science.
247:715–718. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vosshenrich CA, García-Ojeda ME,
Samson-Villéger SI, et al: A thymic pathway of mouse natural killer
cell development characterized by expression of GATA-3 and CD127.
Nat Immunol. 7:1217–1224. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
van den Heuvel M, Peralta C, Bashar S,
Taylor S, Horrocks J and Croy BA: Trafficking of peripheral blood
CD56(bright) cells to the decidualizing uterus - new tricks for old
dogmas? J Reprod Immunol. 67:21–34. 2005.PubMed/NCBI
|
|
36
|
Gargett CE, Schwab KE, Zillwood RM, Nguyen
HP and Wu D: Isolation and culture of epithelial progenitors and
mesenchymal stem cells from human endometrium. Biol Reprod.
80:1136–1145. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carson WE, Giri JG, Lindemann MJ, et al:
Interleukin (IL) 15 is a novel cytokine that activates human
natural killer cells via components of the IL-2 receptor. J Exp
Med. 180:1395–1403. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Waldmann T and Tagaya Y: The multifaceted
regulation of interleukin-15 expression and the role of this
cytokine in NK cell differentiation and host response to
intracellular pathogens. Annu Rev Immunol. 17:19–49. 1999.
View Article : Google Scholar
|
|
39
|
Iizuka K, Chaplin DD, Wang Y, et al:
Requirement for membrane lymphotoxin in natural killer cell
development. Proc Natl Acad Sci USA. 96:6336–6340. 1999. View Article : Google Scholar : PubMed/NCBI
|