Introduction
Paclitaxel is used as a first-line chemotherapeutic
agent for a broad spectrum of solid malignancies. It exhibits
significant anticancer activity. Since the original approval for
its clinical application, paclitaxel is now routinely used in the
inductive, adjuvant, neoadjuvant and metastatic environment in a
number of human cancers including nasopharyngeal carcinoma (NPC)
(1–3). Despite its extensive range of
applications, the clinical efficiency of paclitaxel is limited by
the emergence of resistant cancer cells, which ultimately leads to
tumour recurrence and a poor prognosis (4). NPC is a prevalent type of human
cancer in Southern China and Southeast Asia, with a clear racial
and geographic preponderance (5).
Chemotherapy is currently an important therapeutic option for NPC
(3,6). However, resistance to
chemotherapeutic agents, including paclitaxel is a significant
obstacle in the treatment of NPC. Therefore, it is essential to
identify molecules associated with paclitaxel resistance and to
clarify mechanisms by which they confer resistance in cancer cells,
in order to develop novel therapeutic alternatives for use in
patients with NPC who have developed paclitaxel resistance.
Ephrin receptors (Ephs) are the largest subfamily of
transmembrane receptor tyrosine kinases in the human genome. Eph
receptors are classified into Eph type A and B (EphA and EphB)
based on sequence homology and binding capacity for two different
species of membrane-anchored ephrin ligands (7). EphA2 is a member of EphA receptor
tyrosine kinase family. It has a low level of expression in certain
noncancerous epithelial cells (8).
However, previous studies have demonstrated that the expression of
EphA2 is elevated in a large number of human epithelial
malignancies, and that elevated EphA2 expression is associated with
malignant transformation and a poor prognosis (9–14).
Gain- and loss-of-function experiments have shown that abnormal
activation of the EphA2 signalling pathway promotes carcinogenesis,
indicating that it may act as an oncogene (13,15).
EphA2 has been shown to be involved in a number of behaviours
associated with malignant cells, including malignant cell
transformation, proliferation, angiogenesis, invasion and
metastasis (13,16–18).
There is evidence that EphA2 modulates the
sensitivity of cancer cells to chemotherapeutic agents. A small
number of studies in ovarian and prostate cancer have indicated
that EphA2 silencing leads to increased sensitivity to the
anticancer drug, paclitaxel (19–21).
In addition, given the widespread expression of EphA2 in epithelial
carcinoma, novel therapeutic compounds conjugated with the EphA2
receptor may be an effective way to target paclitaxel to cancer
cells (21,22). A previous study indicated that
EphA2 protein expression is increased in specimens from patients
with NPC, and that increased expression of EphA2 is associated with
clinical progression of this disease. Furthermore, EphA2 silencing
significantly inhibited behaviours associated with malignant
transformation and enhanced the sensitivity of NPC cells to
paclitaxel in vitro (23).
Therefore, EphA2 may be a promising molecular target with which to
attempt to reverse paclitaxel resistance. However, the molecular
mechanisms underlying EphA2-mediated paclitaxel resistance in NPC
remain unclear.
Materials and methods
Antibodies and reagents
Rabbit EphA2 polyclonal antibodies and mouse β-actin
monoclonal antibodies were obtained from Santa Cruz Biotechnology,
Inc., (Dallas, TX, USA). Rabbit Akt, phosphor-Akt (p-Akt), p21,
cyclin-dependent kinase 2 (CDK2) and Cyclin E monoclonal
antibodies, and mouse p27, retinoblastoma protein (Rb),
phosphor-Rb, glycogen synthase kinase-3β (GSK-3β) and
phosphor-GSK-3β monoclonal antibodies, and the PI3K/Akt signalling
pathway small molecule inhibitor, LY294002, were obtained from Cell
Signaling Technology, Inc., (Danvers, MA, USA). Paclitaxel was
obtained from Bristol-Myers Squibb (New York, NY, USA). The EphA2
cDNA-pEGFP-N1 expression plasmid and pEGFP-N1 vector plasmid were
obtained from GeneChem Co., Ltd. (Shanghai, China).
Lipofectamine® 2000 Transfection Reagent and
Opti-MEM® I Reduced-Serum Medium were obtained from
Invitrogen Life Technologies (Carlsbad, CA, USA). 10% foetal bovine
serum (FBS) and RPMI-1640 medium were obtained from Hyclone
Laboratories, Inc. (Logan, UT, USA). Cell Counting kit-8 (CCK-8),
100 IU/ml penicillin, 100 IU/ml streptomycin, Annexin V-fluorescein
isothiocyanate, propidium iodide and BeyoECL Plus Detection system
were obtained from Beyotime Institute of Biotechnology (Jiangsu,
China). Polyvinylidene fluoride membranes (PVDF) were obtained from
EMD Millipore (Billerica, MA, USA).
Cell lines and culture conditions
5-8F NPC cells were provided by the Cell Center of
Central South University, (Changsha, China). 5-8F cells were
cultured as a monolayer in RPMI-1640 media with 10% FBS, 100 IU/ml
streptomycin and 100 IU/ml penicillin at 37°C in a humidified cell
incubator with 5% CO2. 5-8F cells in the exponential
growth phase were used for subsequent experiments.
Plasmid construction, transient
transfection and efficiency validation
EphA2-specific cDNA lentiviral plasmids
(EX-A0125-Lv105, GeneCopoeia, Guangzhou, China) are pools of
concentrated, transduction-ready viral particles designed to
overexpress EphA2 gene in human NPC 5-8F cells. 5-8F NPC cells
(5×104) were seeded in triplicate in 12-well plates and
allowed to grow for 24 h. EphA2 cDNA plasmids (2 μg) or empty
vectors (2 μg) were transfected into 5-8F NPC cells using
Lipofectamine 2000 Transfection Reagent according to the
manufacturer’s instructions. At 6 h, the initial transfection
medium was changed for fresh medium. The expression of EphA2 in
5-8F cells from each group was assessed using western blotting at
72 h post-infection.
Western blotting
Western blotting was performed as described
previously (9,24). Briefly, equal quantities of total
protein samples were separated by 12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis, transferred to a PVDF
membrane and incubated with the primary and secondary antibodies.
The signalling intensity was visualized using the BeyoECL Plus
Detection system. All experiments were performed three times.
Paclitaxel cytotoxicity assays
Cells (3×103) were separately seeded into
96-well plates in triplicate. At 24 h, cells were treated with
varying concentrations of paclitaxel (0, 0.001, 0.01, 0.1, 1, 5,
10, 20 and 30 nM/l) and incubated for a further 48 h. The optical
density values of each group were determined by CCK-8 assays. Each
experiment was performed in triplicate.
Assessment of cell cycle and apoptosis by
flow cytometry (FCM)
Cells (2×105) from each group were grown
in triplicate in 6-well plates for 24 h prior to exposure to 0.1
nM/l paclitaxel for 48 h. Cells were harvested and processed as
described previously (24).
Statistical analysis
Statistical tests were conducted with SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Quantitative data are
presented as the mean ± standard deviation. Differences between
groups were compared using a paired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
EphA2 regulates the sensitivity of NPC
cells to paclitaxel in vitro
A preliminary study demonstrated that EphA2
silencing led to increased sensitivity of 5-8F NPC cells to
paclitaxel in vitro (23).
To confirm the association between EphA2 and NPC sensitivity to
paclitaxel, an EphA2 cDNA-pEGFP-N1 expression plasmid was used to
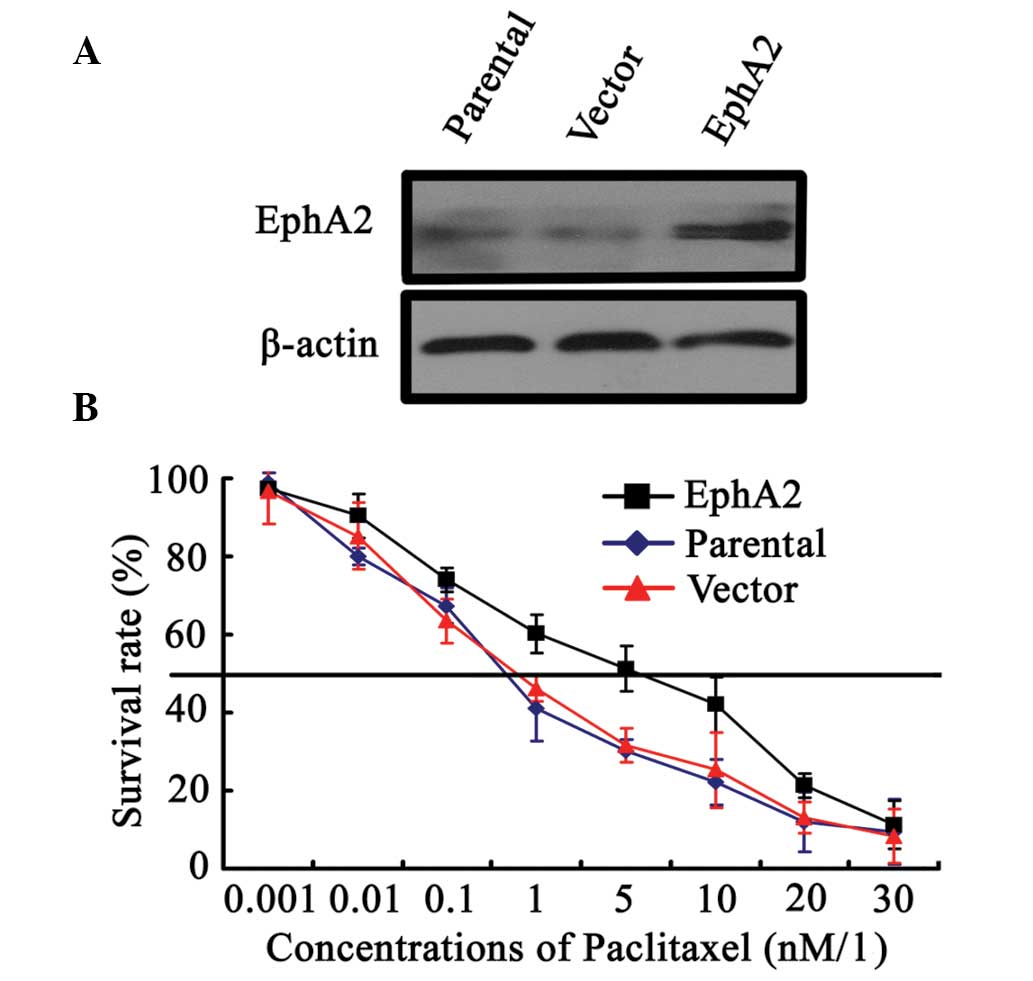
upregulate EphA2 expression in 5-8F NPC cells. As shown in Fig. 1A, EphA2 was demonstrated to be
successfully upregulated in EphA2 cDNA plasmid-transfected 5-8F
cells compared with parent and vector plasmid-transfected 5-8F
cells. Following paclitaxel stimulation with varying concentrations
for 48 h, paclitaxel IC50 values in EphA2 cDNA
plasmid-transfected, parent and vector plasmid-transfected 5-8F
cells were 3.8±0.52, 1.3±0.06 and 1.4±0.05 nM/l, respectively,
indicating that EphA2 upregulation enhanced the survival of 5-8F
NPC cells compared with control cells exposed to the same
concentrations of paclitaxel (Fig.
1B). These results confirmed the involvement of EphA2 in the
sensitivity of NPC cells to paclitaxel.
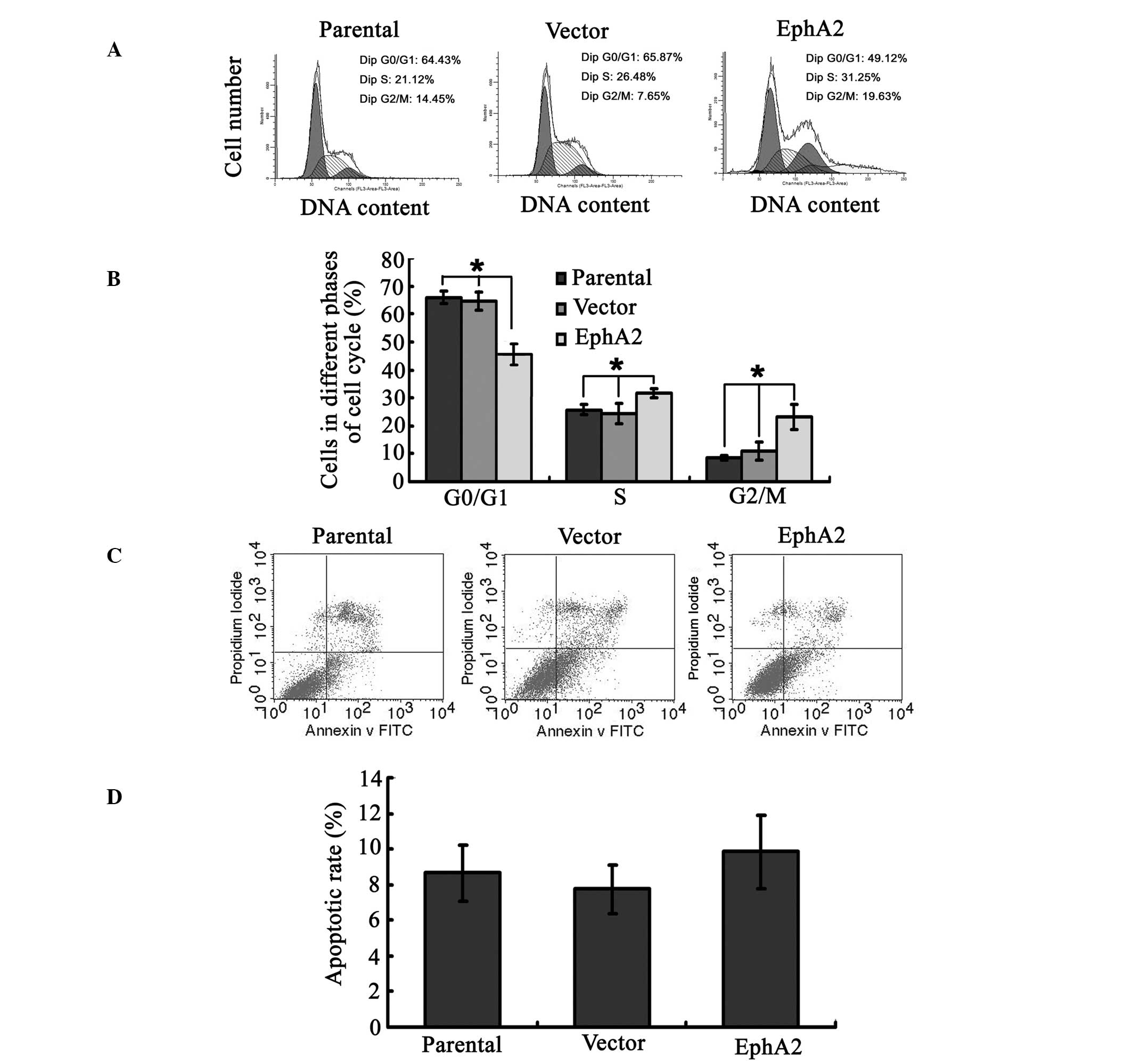
EphA2 over-expression regulates
paclitaxel-mediated cell cycle progression but not apoptosis in
NPC
To investigate the mechanisms underlying
EphA2-regulated sensitivity of NPC cells to paclitaxel, changes in
cell cycle progression and apoptosis in NPC 5-8F cells, following
over-expression of EphA2 and the administration of paclitaxel, were
assayed by FCM. The percentage of cells in G0/G1 phase in the 5-8F
cells transfected with the EphA2 cDNA plasmid was significantly
reduced compared with that in the parent and vector plasmid
transfected 5-8F cells (45.76±3.89 compared with 64.52±3.31 and
65.85±2.28%, respectively; P<0.05). By contrast, the percentage
of cells in the S phase (31.56±1.59 compared with 24.55±3.64 and
25.76±1.89%, respectively; P<0.05) and the G2/M phase
(23.10±4.55 compared with 10.94±3.27 and 8.39±0.81%, respectively;
P<0.05) were significantly increased in cells over-expressing
EphA2 compared with the other groups (Fig. 2A and B). However, the percentage of
apoptotic cells in these three groups was not significantly
different, with that of the EphA2-over-expressed group (9.84±2.08)
similar to those of the parental (8.66±1.5) and vector (7.74±1.34%)
groups (P>0.05) (Fig. 2C and
D). These results demonstrate that EphA2 over-expression
regulated paclitaxel-mediated cell-cycle progression but not
apoptosis in NPC cells.
EphA2 affects NPC cell-cycle progression
via regulation of p21, p27 and p-Rb protein, but not CDK2 and
Cyclin E
Since EphA2 expression was associated with changes
in cell cycle progression following administration of paclitaxel,
the regulation of cell cycle factors by EphA2 was investigated.
Western blot analyses indicated that ectopic expression of EphA2
did not influence the expression of the cell cycle promoters, CDK2
and Cyclin E, whereas the expression of cyclin-dependent kinase
inhibitors, p21 and p27, were significantly downregulated. In
addition, the expression of inactive p-Rb was increased without a
change in the total expression of Rb, which is also an inhibitory
factor in cell cycle progression (Fig.
3).
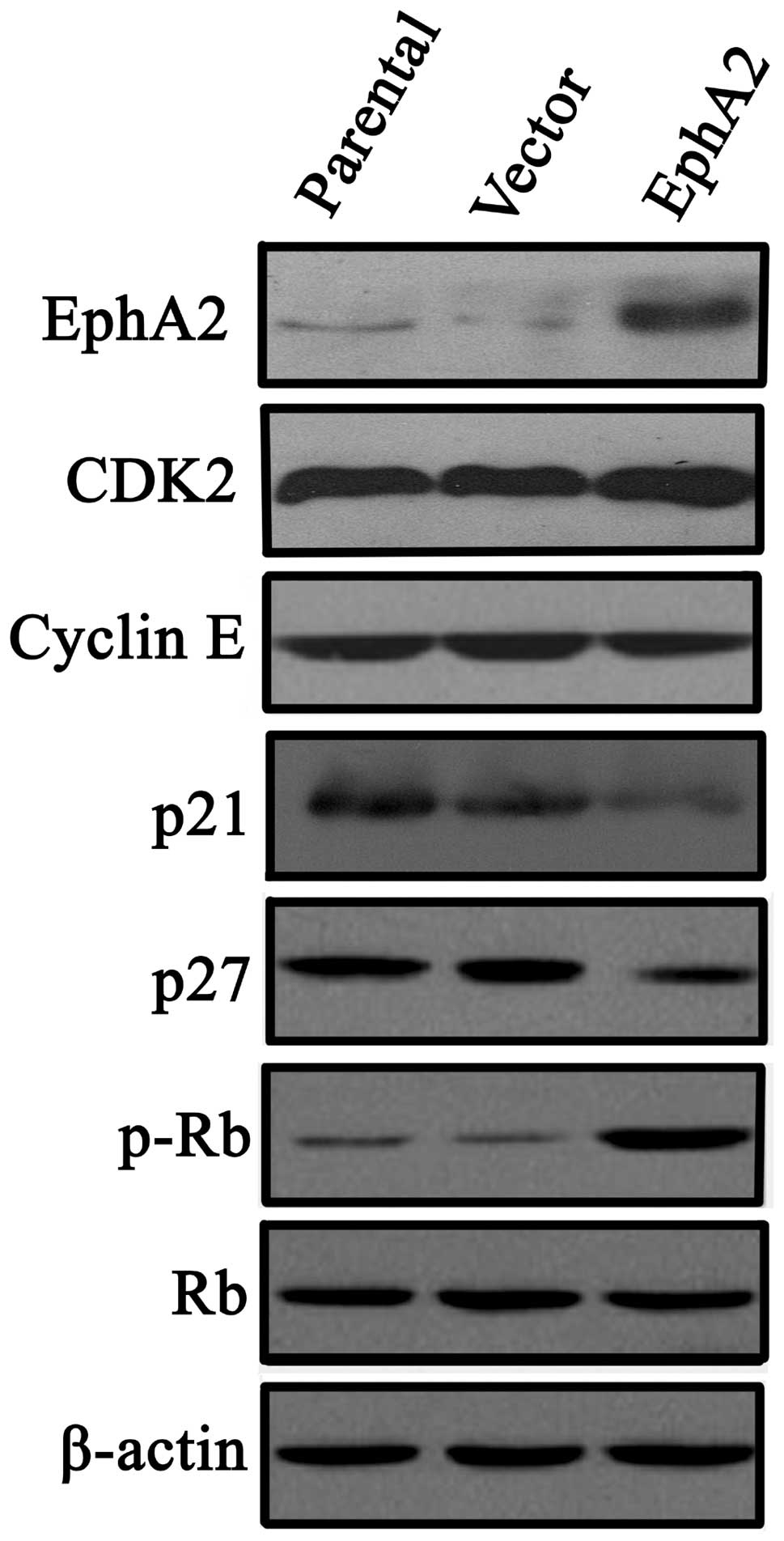 | Figure 3Effect of EphA2 over-expression on
cyclin-dependent kinase inhibitors, p21Cip1 and p27Kip1, in NPC
5-8F cells. Western blot analysis was used to detect the expression
of p21Cip1, p27Kip1 CDK2, Cyclin E and p-Rb in NPC 5-8F and CNE-2
nasopharyngeal carcinoma cells. All data were obtained by three
independent experiments, which produced similar results. EphA2,
ephrin type-A receptor 2; CDK, cyclin-dependent kinase; Rb,
retinoblastoma protein; p-Rb, phosphorylated Rb; NPC,
nasopharyngeal carcinoma. |
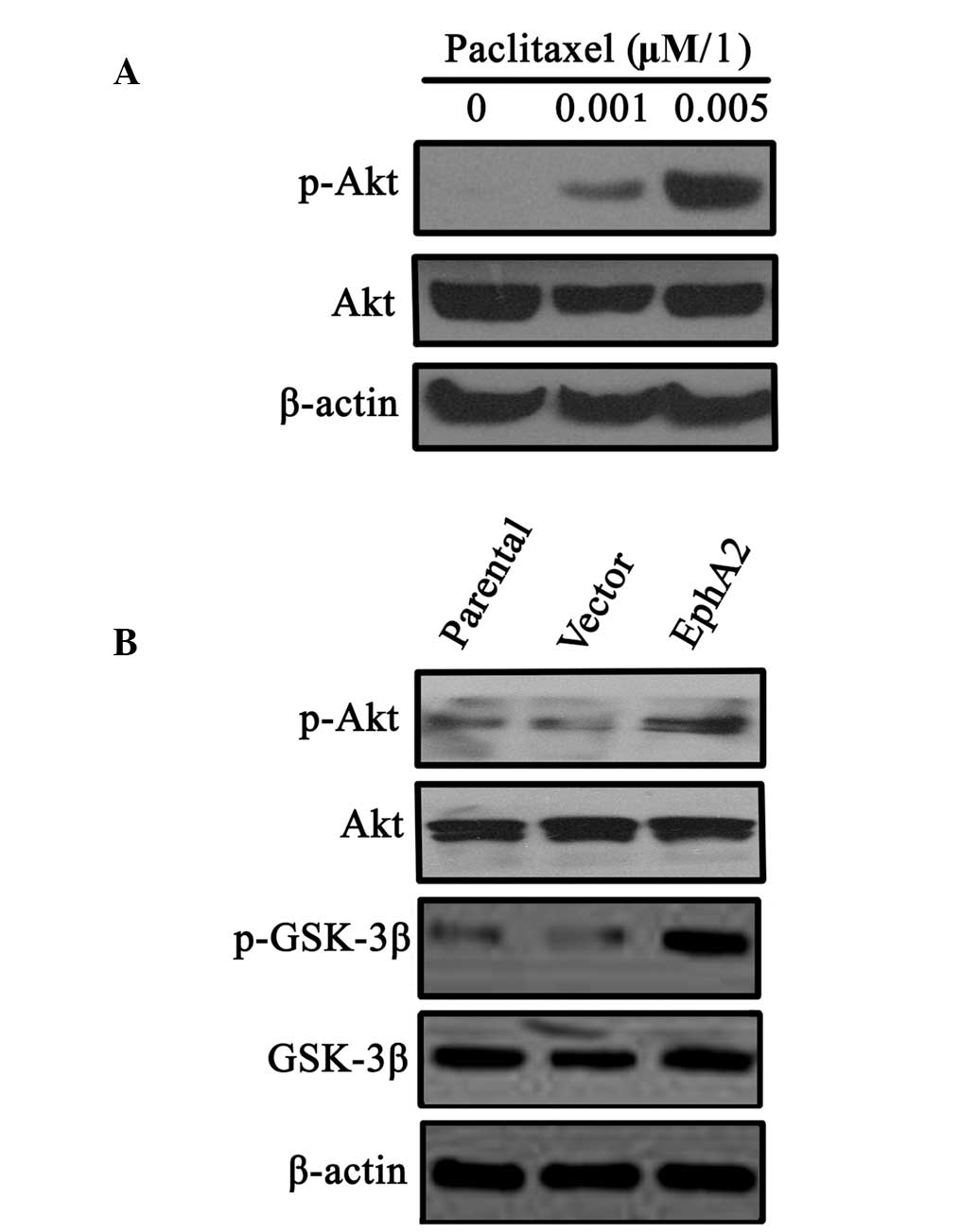
Paclitaxel stimulation and EphA2
over-expression results in activation of the PI3K/Akt signalling
pathway in NPC cells
It has been reported that abnormal activation of the
PI3K/Akt signalling pathway is involved in sensitivity to
paclitaxel in a number of human malignancies. Therefore, the
involvement of the PI3K/Akt signalling pathway in EphA2-mediated
NPC cell sensitivity to paclitaxel was investigated. The results
demonstrated that continuous paclitaxel stimulation induced an
increase in p-Akt expression in 5-8F NPC cells (Fig. 4A). Furthermore, over-expression of
EphA2 in 5-8F cells also increased p-Akt expression and the
expression of its downstream signalling molecule, p-GSK-3β,
indicating that there was aberrant activation of the PI3K/Akt
signalling pathway (Fig. 4B).
These results suggest that the PI3K/Akt pathway may be involved in
the regulation of EphA2-mediated NPC sensitivity to paclitaxel.
PI3K/Akt signalling pathway is involved
in EphA-mediated sensitivity to paclitaxel
To further investigate the role of the PI3K/Akt
signalling pathway in EphA2-mediated sensitivity to paclitaxel, a
small molecule inhibitor of the PI3K/Akt signalling pathway,
LY294002, was used to block this pathway in 5-8F cells
over-expressing EphA2, and to observe whether EphA2-mediated
changes in sensitivity to paclitaxel are reversed in
EphA2-over-expressing 5-8F cells. As shown in Fig. 5A, LY294002 significantly restored
sensitivity to paclitaxel, which had been reduced by EphA2
over-expression, in a dose-dependent manner. This was accompanied
by corresponding changes in the cell-cycle phase distribution
(Fig. 5B) but was not associated
with changes in the percentage of apoptotic rate (Fig. 5C). Furthermore, the changes in
expression of cell-cycle regulatory factors p21, p27 and p-Rb
resulting from EphA2 over-expression were also reversed by the
addition of LY294002 (Fig. 5D).
These results provide further evidence that the PI3K/Akt pathway is
involved in EphA2-mediated sensitivity to paclitaxel.
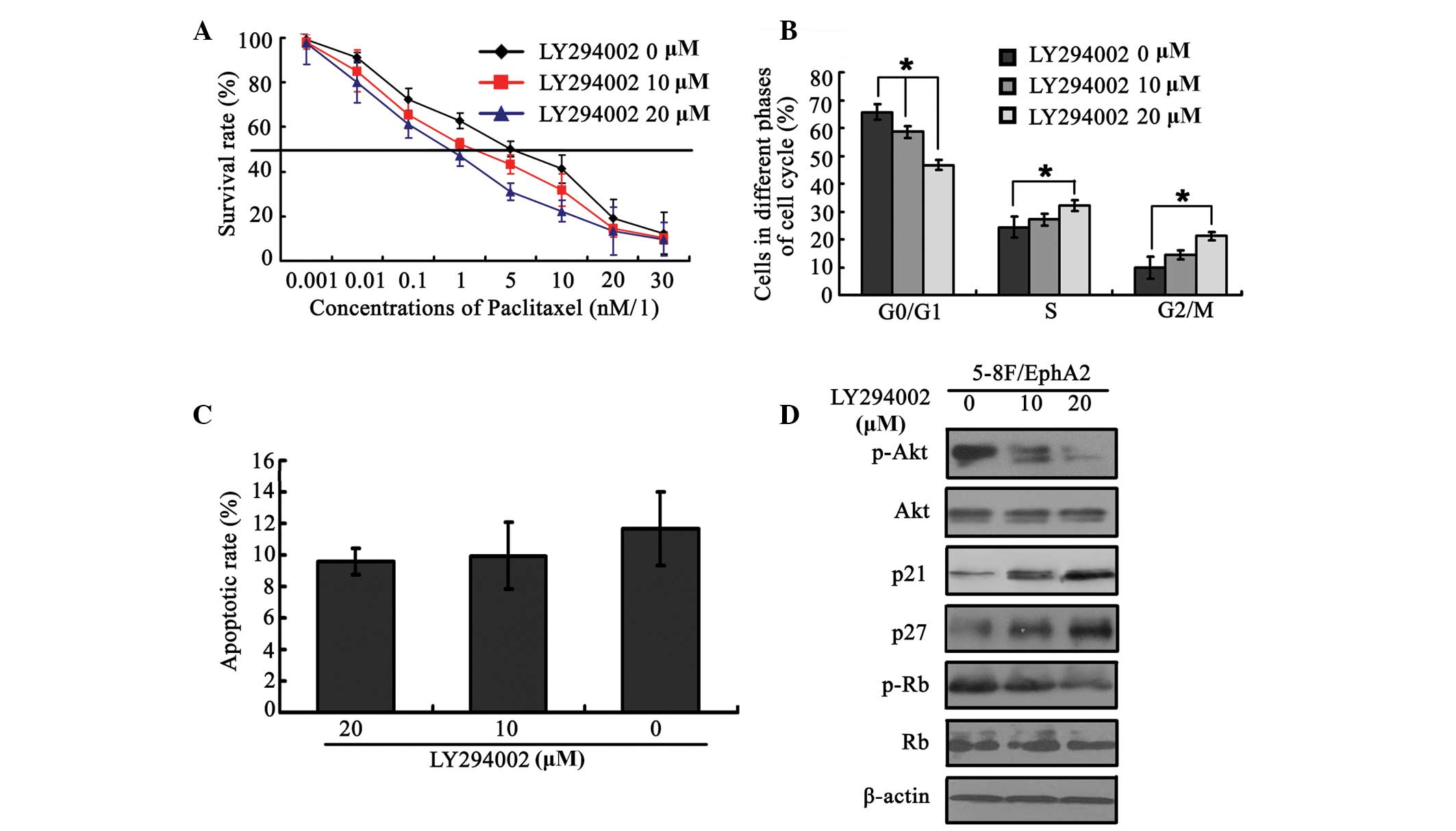 | Figure 5EphA2 mediated paclitaxel sensitivity
in NPC 5–8 cells via modulation of the PI3K/Akt signalling pathway.
(A) PI3K/Akt signalling pathway inhibitor, LY294002, reverses
paclitaxel resistance caused by EphA2 over-expression. (B) Effect
of LY294002 on the cell-cycle distribution in EphA2 over-expressing
NPC cells pre-treated with paclitaxel. (C) Effect of LY294002 on
the apoptotic rate in NPC cells over-expressing EphA2, pretreated
with paclitaxel. (D) LY294002 restores the changes in expression of
cyclin-dependent kinase inhibitors, p21Cip1 and p27Kip1, caused by
EphA2 over-expression. *P<0.05. EphA2, ephrin type-A
receptor 2; NPC, nasopharyngeal carcinoma; PI3K, phosphoinositide
3-kinase; p-Akt, phsophorylated Akt; Rb, retinoblastoma protein;
p-Rb, phosphorylated Rb. |
Discussion
The development of paclitaxel resistance is a
characteristic feature in NPC progression and is associated with a
poor prognosis and increased mortality in patients with advanced
NPC. Therefore, it is important to identify molecules and their
downstream signalling pathways that permit cancer cells to evade
the cytotoxic effects of paclitaxel and to maintain unregulated
growth.
Existing evidence has demonstrated that EphA2
expression is associated with cancer cell metastasis. Numerous
reports have focused on the impact of EphA2 on this process
(25), and a previous study also
showed that EphA2 knockdown inhibited metastasis in NPC in
vitro (23). In the present
study, EphA2 was found to modulate the sensitivity of NPC cells to
paclitaxel, which was consistent with previous studies that have
reported that EphA2 inhibition leads to increased paclitaxel
sensitivity in ovarian cancer (19,26).
Recently, a novel treatment, in which an agent targeted against
EphA2 was conjugated with paclitaxel (22). It demonstrated significantly
enhanced antitumour efficacy compared with paclitaxel alone in a
xenograft animal model of prostate cancer (21). However, a literature review
demonstrated that there has been little investigation into the
association between abnormal expression of EphA2 and paclitaxel
resistance. Paclitaxel exerts its effect partly by inducing cell
cycle arrest and activating proapoptotic signalling pathways
(27). The results from the
current study showed that EphA2 over-expression led to
downregulation of the cyclin-dependent kinase inhibitors, p21 and
p27, and an increase in the expression of the inactive p-Rb. These
changes expedited NPC cell cycle progression but did not affect
apoptosis. Thus, EphA2 renders NPC cells resistant to paclitaxel by
affecting NPC cell-cycle progression. These results indicate that
EphA2 is involved in the modulation of sensitivity to paclitaxel in
human malignancies, including NPC.
Investigation of the mechanisms underlying this
effect showed that the PI3K/Akt signalling pathway is involved in
EphA2-mediated sensitivity to paclitaxel of NPC. A number of groups
have reported that activation of PI3K/Akt may protect cancer cells
against the cytotoxic effects of anticancer drugs, including
paclitaxel (28–31). Abnormal activation of PI3K/Akt
during chemotherapy is one of the primary causes for the
development of chemotherapeutic resistance. Therefore, PI3K/Akt
activity following paclitaxel stimulation and EphA2 upregulation
was investigated. It was shown that PI3K/Akt was activated by
paclitaxel stimulation and ectopic expression of EphA2 in 5-8F NPC
cells. PI3K/Akt is known to regulate the expression of certain
mediators of cell cycle progression, and their expression in
numerous human cancers has been shown to be associated with cancer
cell survival, chemoresistance and radioresistance (32). In the present study, it was
demonstrated that ectopic expression of EphA2 in combination with
administration of paclitaxel enhanced NPC cell cycle progression
via downregulation of the cell cycle regulators, p21 and p27, and
upregulation of the inactive p-Rb. This conferred survival
advantages to 5-8F NPC cells, leading to paclitaxel resistance.
However, the PI3K/Akt inhibitor, LY294002, reversed the paclitaxel
resistance, along with changes in mediators of cell cycle
progression, which were caused by ectopic expression of EphA2.
Therefore, EphA2 inhibition is a candidate for combination
treatment with the anticancer agent, paclitaxel, which induces
activation of the PI3K/Akt signalling pathway. A previous study
demonstrated that EphA2 regulates the growth of cancer cells via
numerous signalling pathways, reflecting its complicated regulatory
network (33). The present results
suggest that abnormal activation of the PI3K/Akt pathway caused by
EphA2 over-expression is part of the mechanism underlying the
EphA2-mediated paclitaxel resistance in NPC cells. Another study
into the molecular mechanisms underlying paclitaxel resistance
showed that dysfunction of multidrug resistance (MDR)-1 gene and
its encoding protein P-glycoprotein, leads to the efflux of
paclitaxel, thus disrupting paclitaxel retention (34). A study by our group (Yunyun Wang
et al, unpublished data) showed that EphA2 regulates the
chemoresistance of paclitaxel by mediating the expression of MDR-1.
Thus, combination therapy consisting of EphA2 targeted knockdown
and paclitaxel has synergistic effects and may represent a
promising therapeutic strategy for patients with advanced NPC.
In conclusion, the present study demonstrated the
efficacy of EphA2 inhibition on enhancement of chemosensitivity to
paclitaxel in NPC in vitro. Over-expression of EphA2 led to
increased PI3K/Akt activity, resulting in promotion of cell cycle
progression in NPC cells, whilst inhibition of PI3K/Akt reversed
the EphA2-mediated reduction in paclitaxel sensitivity. Although
additional in vivo studies and clinical trials are required
to explore the efficacy and safety, the cytotoxic effect of EphA2
inhibition in combination with paclitaxel may provide a novel
treatment strategy for patients with advanced NPC.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81202128, 81272974
and 81372426), the Research Fund for the Doctoral Program of Higher
Education of China (grant nos. 20120162120049 and 20100162110036)
and the Freedom Explore Program of Central South University (grant
no. 2012QNZT099). This abstract has previously been published in
the 5th World Congress of IFHNOS and the 2014 annual meeting of the
ANHS (JAMA otolaryngology - head and neck surgery).
References
|
1
|
Huober J, Fasching PA, Hanusch C, Rezai M,
Eidtmann H, et al: Neoadjuvant chemotherapy with paclitaxel and
everolimus in breast cancer patients with non-responsive tumours to
epirubicin/cyclophosphamide (EC) ± bevacizumab - results of the
randomised GeparQuinto study (GBG 44). Eur J Cancer. 49:2284–2293.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Burg ME, Vergote I, Onstenk W,
Boere IA, Leunen K, et al: Long-term results of weekly paclitaxel
carboplatin induction therapy: an effective and well-tolerated
treatment in patients with platinum-resistant ovarian cancer. Eur J
Cancer. 49:1254–1263. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leong SS, Wee J, Rajan S, Toh CK, Lim WT,
et al: Triplet combination of gemcitabine, paclitaxel, and
carboplatin followed by maintenance 5-fluorouracil and folinic acid
in patients with metastatic nasopharyngeal carcinoma. Cancer.
113:1332–1337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yusuf RZ, Duan Z, Lamendola DE, Penson RT
and Seiden MV: Paclitaxel resistance: molecular mechanisms and
pharmacologic manipulation. Curr Cancer Drug Targets. 3:1–19. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fåhraeus R, Fu HL, Ernberg I, Finke J,
Rowe M, et al: Expression of Epstein-Barr virus-encoded proteins in
nasopharyngeal carcinoma. Int J Cancer. 42:329–338. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen C, Wang FH, An X, Luo HY, Wang ZQ, et
al: Triplet combination with paclitaxel, cisplatin and 5-FU is
effective in metastatic and/or recurrent nasopharyngeal carcinoma.
Cancer Chemother Pharmacol. 71:371–378. 2013. View Article : Google Scholar
|
|
7
|
Pasquale EB: Eph receptor signalling casts
a wide net on cell behaviour. Nat Rev Mol Cell Biol. 6:462–475.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sulman EP, Tang XX, Allen C, Biegel JA,
Pleasure DE, et al: ECK, a human EPH-related gene, maps to 1p36.1,
a common region of alteration in human cancers. Genomics.
40:371–374. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Y, Zhang X, Qiu Y, Huang D, Zhang S,
et al: Clinical significance of EphA2 expression in squamous-cell
carcinoma of the head and neck. J Cancer Res Clin Oncol.
137:761–769. 2011. View Article : Google Scholar
|
|
10
|
Kinch MS, Moore MB and Harpole DH Jr:
Predictive value of the EphA2 receptor tyrosine kinase in lung
cancer recurrence and survival. Clin Cancer Res. 9:613–618.
2003.PubMed/NCBI
|
|
11
|
Mudali SV, Fu B, Lakkur SS, Luo M,
Embuscado EE and Iacobuzio-Donahue CA: Patterns of EphA2 protein
expression in primary and metastatic pancreatic carcinoma and
correlation with genetic status. Clin Exp Metastasis. 23:357–365.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abraham S, Knapp DW, Cheng L, Snyder PW,
Mittal SK, et al: Expression of EphA2 and Ephrin A-1 in carcinoma
of the urinary bladder. Clin Cancer Res. 12:353–360. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zelinski DP, Zantek ND, Stewart JC,
Irizarry AR and Kinch MS: EphA2 overexpression causes tumorigenesis
of mammary epithelial cells. Cancer Res. 61:2301–2306.
2001.PubMed/NCBI
|
|
14
|
Herrem CJ, Tatsumi T, Olson KS, Shirai K,
Finke JH, et al: Expression of EphA2 is prognostic of disease-free
interval and overall survival in surgically treated patients with
renal cell carcinoma. Clin Cancer Res. 11:226–231. 2005.PubMed/NCBI
|
|
15
|
Brantley-Sieders DM, Zhuang G, Hicks D,
Fang WB, Hwang Y, et al: The receptor tyrosine kinase EphA2
promotes mammary adenocarcinoma tumorigenesis and metastatic
progression in mice by amplifying ErbB2 signaling. J Clin Invest.
118:64–78. 2008. View
Article : Google Scholar
|
|
16
|
Lin YG, Han LY, Kamat AA, Merritt WM,
Landen CN, et al: EphA2 overexpression is associated with
angiogenesis in ovarian cancer. Cancer. 109:332–340. 2007.
View Article : Google Scholar
|
|
17
|
Lu C, Shahzad MM, Wang H, Landen CN, Kim
SW, et al: EphA2 overexpression promotes ovarian cancer growth.
Cancer Biol Ther. 7:1098–1103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duxbury MS, Ito H, Zinner MJ, Ashley SW
and Whang EE: EphA2: a determinant of malignant cellular behavior
and a potential therapeutic target in pancreatic adenocarcinoma.
Oncogene. 23:1448–1456. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Landen CN Jr, Chavez-Reyes A, Bucana C,
Schmandt R, Deavers MT, et al: Therapeutic EphA2 gene targeting in
vivo using neutral liposomal small interfering RNA delivery. Cancer
Res. 65:6910–6918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen H, Rodriguez-Aguayo C, Xu R,
Gonzalez-Villasana V, Mai J, et al: Enhancing chemotherapy response
with sustained EphA2 silencing using multistage vector delivery.
Clin Cancer Res. 19:1806–1815. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang S, Placzek WJ, Stebbins JL, Mitra S,
Noberini R, et al: Novel targeted system to deliver
chemotherapeutic drugs to EphA2-expressing cancer cells. J Med
Chem. 55:2427–2436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang S, Noberini R, Stebbins JL, Das S,
Zhang Z, et al: Targeted delivery of paclitaxel to EphA2-expressing
cancer cells. Clin Cancer Res. 19:128–137. 2013. View Article : Google Scholar :
|
|
23
|
Tan P, Liu Y, Yu C, Su Z, Li G, et al:
EphA2 silencing in nasopharyngeal carcinoma leads to decreased
proliferation, invasion and increased sensitization to paclitaxel.
Oncol Lett. 4:429–434. 2012.
|
|
24
|
Liu Y, Yu C, Qiu Y, Huang D, Zhou X, et
al: Downregulation of EphA2 expression suppresses the growth and
metastasis in squamous-cell carcinoma of the head and neck in vitro
and in vivo. J Cancer Res Clin Oncol. 138:195–202. 2012. View Article : Google Scholar
|
|
25
|
Parri M, Taddei ML, Bianchini F, Calorini
L and Chiarugi P: EphA2 reexpression prompts invasion of melanoma
cells shifting from mesenchymal to amoeboid-like motility style.
Cancer Res. 69:2072–2081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Landen CN Jr, Lu C, Han LY, Coffman KT,
Bruckheimer E, et al: Efficacy and antivascular effects of EphA2
reduction with an agonistic antibody in ovarian cancer. J Natl
Cancer Inst. 98:1558–1570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yared JA and Tkaczuk KH: Update on taxane
development: new analogs and new formulations. Drug Des Devel Ther.
6:371–384. 2012.PubMed/NCBI
|
|
28
|
He K, Xu T, Xu Y, Ring A, Kahn M and
Goldkorn A: Cancer cells acquire a drug resistant, highly
tumorigenic, cancer stem-like phenotype through modulation of the
PI3K/Akt/β-catenin/CBP pathway. Int J Cancer. 134:43–54. 2014.
View Article : Google Scholar
|
|
29
|
Wang Y, Chen L, Huang G, He D, He J, et
al: Klotho sensitizes human lung cancer cell line to cisplatin via
PI3k/Akt pathway. PLoS One. 8:e573912013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiu P, Dong X, Dong X, Xu Z, Zhu H, Liu F,
et al: Secretory clusterin contributes to oxaliplatin resistance by
activating Akt pathway in hepatocellular carcinoma. Cancer Sci.
104:375–382. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yang YI, Lee KT, Park HJ, Kim TJ, Choi YS,
et al: Tectorigenin sensitizes paclitaxel-resistant human ovarian
cancer cells through downregulation of the Akt and NFκB pathway.
Carcinogenesis. 33:2488–2498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim TR, Cho EW, Paik SG and Kim IG:
Hypoxia-induced SM22α in A549 cells activates the IGF1R/PI3K/Akt
pathway, conferring cellular resistance against chemo- and
radiation therapy. FEBS Lett. 586:303–309. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wykosky J and Debinski W: The EphA2
receptor and ephrinA1 ligand in solid tumors: function and
therapeutic targeting. Mol Cancer Res. 6:1795–1806. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gréen H, Söderkvist P, Rosenberg P,
Horvath G and Peterson C: mdr-1 single nucleotide polymorphisms in
ovarian cancer tissue: G2677T/A correlates with response to
paclitaxel chemotherapy. Clin Cancer Res. 12:854–859. 2006.
View Article : Google Scholar : PubMed/NCBI
|