Introduction
Since Zuk et al (1) extracted pluripotent stem cells from
fat in 2001, adipose-derived stem cells (ADSCs) have become a focus
of research into tissue engineering, due to their multiple
advantages, including extensive sources, convenient extraction
methods and the ability to be rapidly amplified. A number of
studies have confirmed that ADSCs can differentiate into multiple
cell types in vitro, including bone, cartilage, fat, muscle,
neural and endothelial cells (2–6),
indicating characteristics similar to bone marrow stromal stem
cells and a prospective application in tissue engineering.
Furthermore, cartilage is recognized as the most suitable tissue
for construction by tissue engineering, thus ADSCs have great
potential as seed cells for cartilage tissue engineering. Cartilage
tissue engineering requires large quantities of seed cells in the
short-term, which can maintain a chondrocyte phenotype in the
process of in vitro culture.
It is difficult to meet these requirements with
traditional static culture, whilst three-dimensional dynamic
culture is able to accelerate cell proliferation and be conducive
to chondrogenic differentiation and phenotype maintenance (7). Therefore, the present study applied
bioreactor technology combined with microcarriers to rapidly
amplify and induce chondrogenic differentiation in ADSCs. The aim
was to investigate the proliferation and differentiation of ADSCs
on microcarriers, and explore the feasibility of clinical
application in the future.
Materials and methods
Materials
Type I collagenase, dexamethasone,
2-phospho-L-ascorbic acid (vitamin C), ITS liquid media supplement
and Cytodex 3 microcarrier beads (Sigma-Aldrich, Poole, UK);
trypsin, Dulbecco’s modified Eagle’s medium (DMEM)-high glucose
(Gibco Life Technologies, Carlsbad, CA, USA); fetal bovine serum
(FBS; Beijing Yuan Heng Sheng Ma Biotechnology Institute, Beijing,
China); recombinant murine FGF-basic and TGF-β1 (PeproTech EC Ltd.,
London, UK); type II collagen mouse anti-human monoclonal antibody
(Beijing Zhongshan Co., Beijing, China); a rotating bioreactor
(Synthecon, Inc., Houston, TX, USA); and an inverted microscope
(Olympus IX70; Olympus Corporation, Tokyo, Japan).
Isolation and culture of ADSCs
The present study and the collection of human fat
tissue was under the ethical approval of the Chinese PLA General
Hospital Ethics Committee (Beijing, China). The infrapatellar fat
pad removed during knee arthroplasty was maintained in strictly
aseptic conditions, and stem cells were separated within 30 min of
harvest. The superficial fascia and small blood vessels on the
surface of the fat tissue were carefully removed with microsurgical
scissors, D-Hank’s solution was used to wash the samples three
times to remove red cells. The fat was cut into pieces, added to 5X
0.075% type I collagenase, stirred for 30 min at 37°C and
centrifuged at 671 × g for 5 min to release the fat and the
supernatant. It was then washed again with D-Hank’s solution,
filtered through a 100-mesh filter (BD Biosciences, Suzhou, China)
and centrifuged at 168 × g for 5 min. Then the precipitation was
washed twice in D-Hank’s solution, which was prepared in the Key
Laboratory of PLA (Chinese PLA General Hospital, Beijing, China)
and contained KCl (0.4 g/l), KH2PO (0.06 g/l), NaCl (8.0
g/l), NaHCO3 (0.35 g/l),
Na2HPO4.7H2O (0.06 g/l) and phenol
red (0.02 g/l). The cells were then resuspended in 10% FBS
containing high-glucose DMEM medium, with a change of medium after
48 h. When the cells were at 80% confluency, 0.25% trypsin was used
for digestion passage culture. At the second passage, the medium
was changed to cartilage-inducing liquid (5 ng/ml FGF-2; 10 ng/ml
TGF-β1; 50 μg/ml vitamin C; 10−7 M dexamethasone; and 1X
ITS) for cartilage differentiation.
Pretreatment with Cytodex 3
The dry weight of 50 mg microcarriers was placed
into 10-ml clean glass bottles, with 10 ml phosphate-buffered
saline (PBS; Sigma-Aldrich) without calcium and magnesium ions, and
incubated at room temperature for at least 3 h to expand the
microcarriers. The supernatant was discarded with a polyethylene
aluminum compound pipe straw, the microcarriers were cleaned twice
with PBS without calcium and magnesium ions, which was then
discarded prior to the addition of 10 ml fresh PBS without calcium
and magnesium ions. The samples then underwent high pressure
sterilization for 20 min, the supernatant liquid was removed and
the microcarriers were cleaned with culture liquid. They were then
stored in a refrigerator at 4°C.
Cell culture methods
The Cytodex 3 microcarriers (50 mg) and ADSCs (final
concentration, 1×105/ml) were added to a 10-ml rotary
cell culture system (RCCS; Synthecon, Inc., Houston, TX, USA)
culture container, then the cartilage inducing medium was added to
fill the container. The container with cell-microcarriers complex
was connected to the rotating base. The device was put in a 5%
CO2 incubator and was cultured at 37°C. In order to
fully mix cells, the container was rotated at a force of 5 rpm for
5 min and paused for 5 min alternately, which was repeated for
several cycles, it was then continuously rotated and gradually
adjusted to 10–12 rpm. Once the culture medium exhibited a yellow
color and a PH value <7.0, the medium was changed. Static
culture was conducted by seeding 1×105/ml ADSCs into
five wells of a 6-well plate, 2 ml in each well. During the
culture, the medium was changed every 2–3 days to maintain a
sufficient quantity of cells.
Cell morphological observation and
counting
Human ADSCs were cultured for 1, 3, 5 and 7 days,
and under the inverted microscope, the morphology of the ADSCs and
the proliferation on the surface of the microcarriers were
assessed. The chondrocytes on the surface of the microcarrier were
digested, separated and counted with a blood cell counting plate
(Yancheng Glass Instrument Company, Yancheng, China), then a cell
growth curve was constructed. Cell microcarrier samples were taken
on day 3 to be observed by scanning electron microscopy (Hitachi
S-4500; Hitachi, Tokyo, Japan), and the cell morphology and matrix
secretion was observed. The static monolayer culture of ADSCs in
the control group was observed to assess the ADSC morphology and
proliferation at days 1, 3, 5 and 7. They were then collected and
the survival rate was calculated using a trypan blue dye exclusion
test. Finally, a cell growth curve was constructed based on whole
cell counts.
Cytochemical and immunocytochemical
analysis
The ADSCs growing on the microcarriers and those in
monolayer culture were harvested, digested and smeared. They were
then subjected to safranin-O and toluidine blue staining to observe
the levels of glycosaminoglycan (GAG) synthesis and secretion from
the extracellular matrix. The type II collagen monoclonal antibody
was incubated with the cells with DAB (Beijing Hepten and Protein
Biomedical Institute, Beijing, China) as the substrate, and the
immunoperoxidase method was used for development. Brown staining
indicated positive staining, and thus the occurrence of type II
collagen secretion and synthesis.
Results
ADSC adherent growth on the surface of
the Cytodex 3 microcarriers
Three hours following inoculation, a number of cells
had adhered to the surface of microcarriers. After 24 h, the
majority of the cells had adhered to microcarriers, and only a
small number of dead cells were present in the medium. Cells
gradually changed from a spherical and hemispherical to a short
fusiform shape, and occasionally there were pseudopodium with
irregular shapes (Fig. 1A).
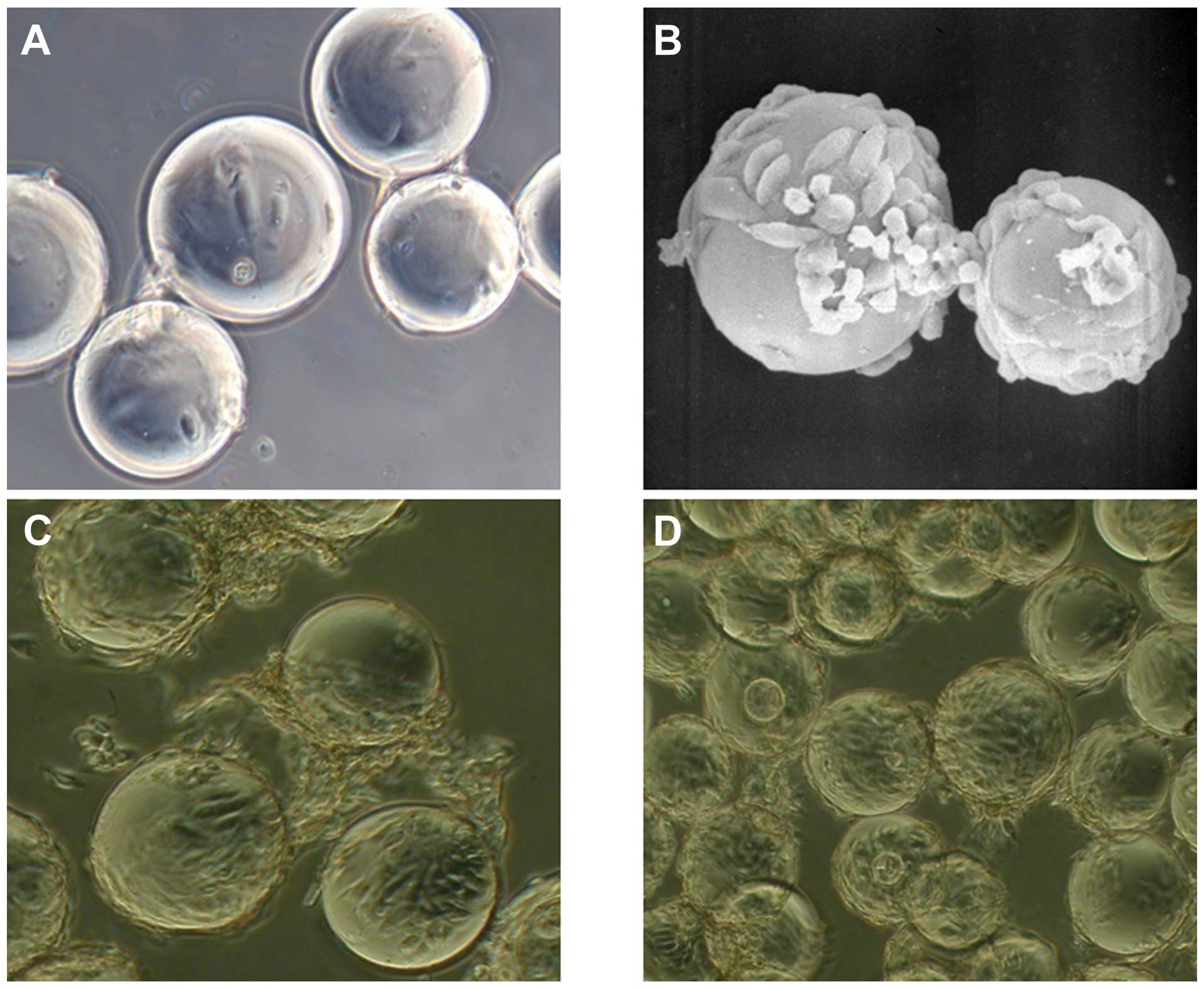 | Figure 1Morphological observation of cell
microcarriers with SEM and light microscopy. (A) ADSCs cultured on
microcarriers for 24 h and viewed with a light microscope
(magnification, ×400). Cells gradually attach to surface of the
microcarrier, becoming short and fusiform. (B) ADSCs cultured on
microcarriers for 3 days observed by SEM (magnification, ×800). The
cell number increased significantly after 3 days, and became long
and fusiform in shape. (C) ADSCs cultured on microcarriers for 5
days, observed with a light microscope (magnification, ×400). Cell
culture on microcarriers. The majority of the microcarrier surface
was covered with overlapping-growth cells and microcarriers
presented cluster-style growth. (D) ADSCs cultured on microcarriers
for 7 days, observed with a light microscope (magnification, ×400).
Microcarriers were completely covered with overlapping cells and
widespread cell connections between the microcarriers were
identified. SEM, scanning electron microscopy; ADSC,
adipose-derived stem cell. |
On day 3, the number of cells on the surface of the
microcarriers increased significantly, and the adherent cells were
apparent on a different focal plane. Cell matrix secretion around
the cells, and occasionally connecting cells between the
microcarriers, were observed. Cells were spindle-shaped and flat.
There were few dead cells in the medium, and cells adhered well to
microcarriers (Fig. 1B). On the
5th day, the majority of the microcarrier surface was covered with
cells and cells had grown in layers, with abundant extracellular
matrix secretion, indistinguishable single cell morphology, and
large cell connection bodies between microcarriers, whilst
microcarriers displayed cluster growth (Fig. 1C). On the 7th day, microcarrier
surfaces were completely covered by cells overlapping as a mass,
leading to a rough, uneven surface on the microcarriers. There were
widespread cell connections between the microcarriers, and there
were almost no dead cells in the medium (Fig. 1D).
Growth of ADSCs in static culture
Two hours after cells were treated with the
induction medium, cells began to adhere to the wall, and after 24
h, all cells were adherent. The cells were spindle-shaped or
polygonal, and were slightly larger than non-induced ADSCs. Once
fully adherent, cells began to rapidly proliferate, after 3 days
they reached a level of 50% fusion, and after 5 days they reached
80% fusion. Along with the increase in cell density, the
proliferation slowed down, and after 7 days, almost complete fusion
was reached.
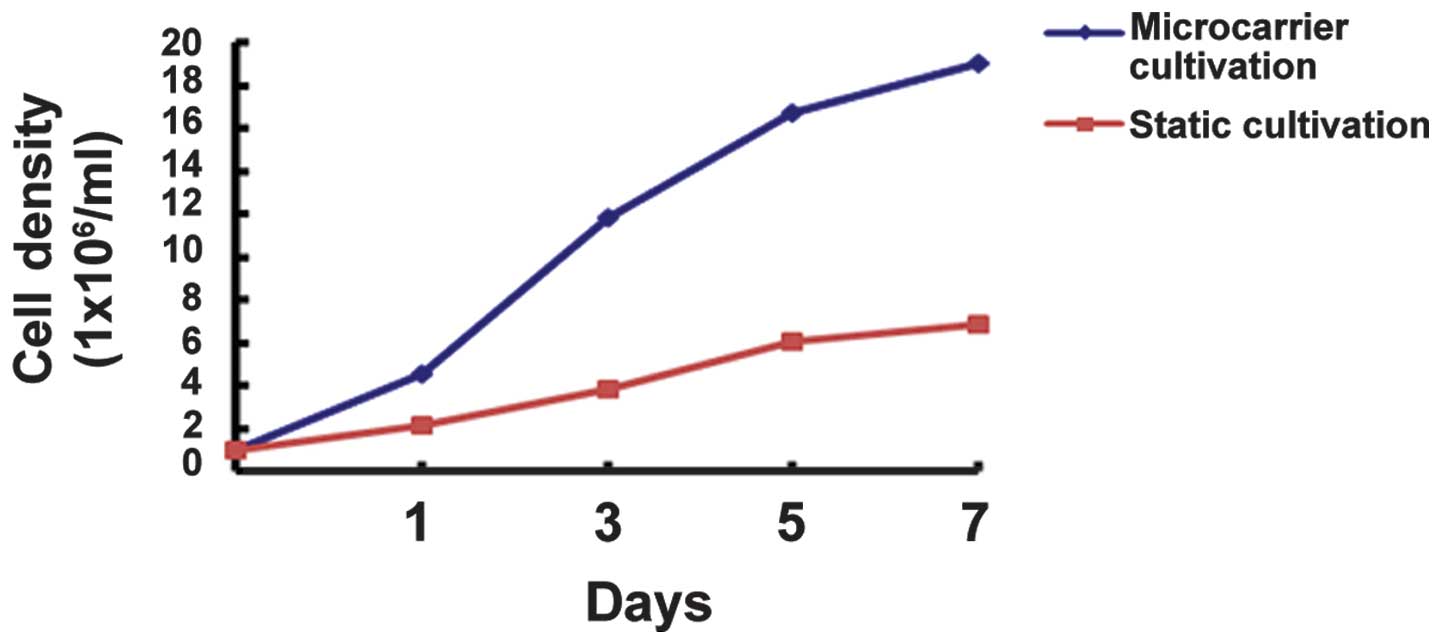
Growth curves of ADSCs under two
different culture modes
On days 1, 3, 5 and 7, ADSCs were harvested from the
RCCS container and common culture bottle and were counted, and the
growth curves were as presented in Fig. 2. The Trypan blue dye exclusion test
indicated that the survival rate of ADSCs harvested from the
microcarriers was >90%. According to the blood cell count, it
was clear that the growth of ADSCs accelerated from day 3 after
RCCS inoculation, and the cell density reached its highest value at
day 7, ~19 times the initial inoculation. On day 7, the total
number of harvested cells cultured in a static monolayer was ~6
times the original.
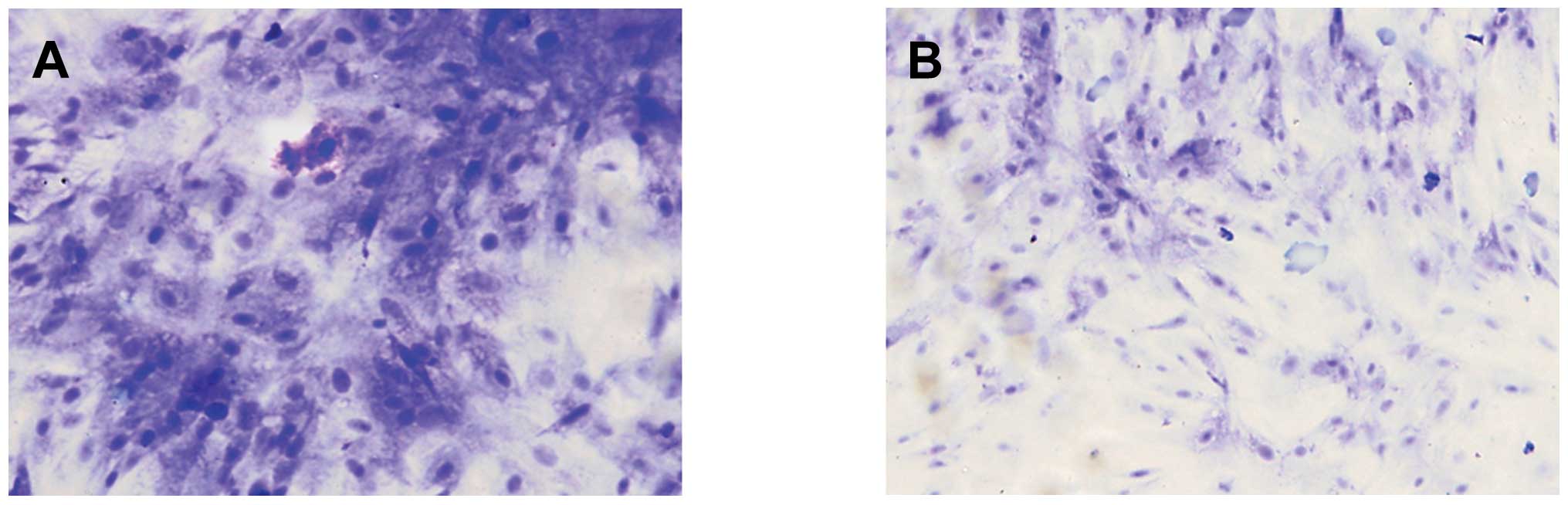
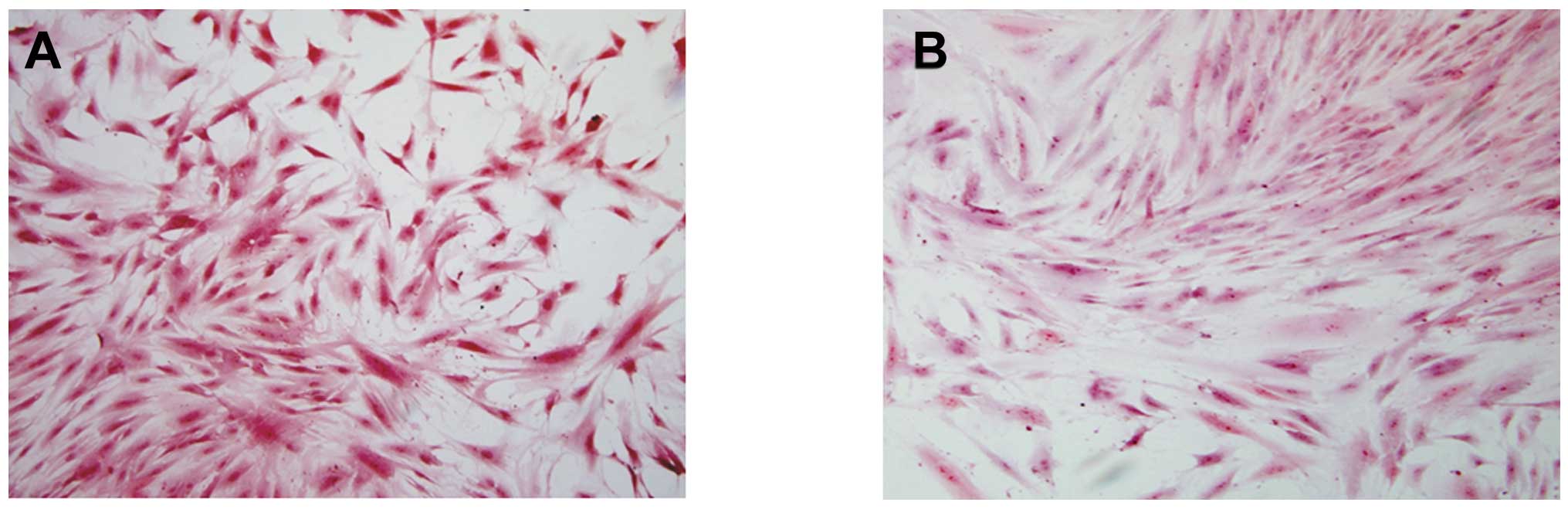
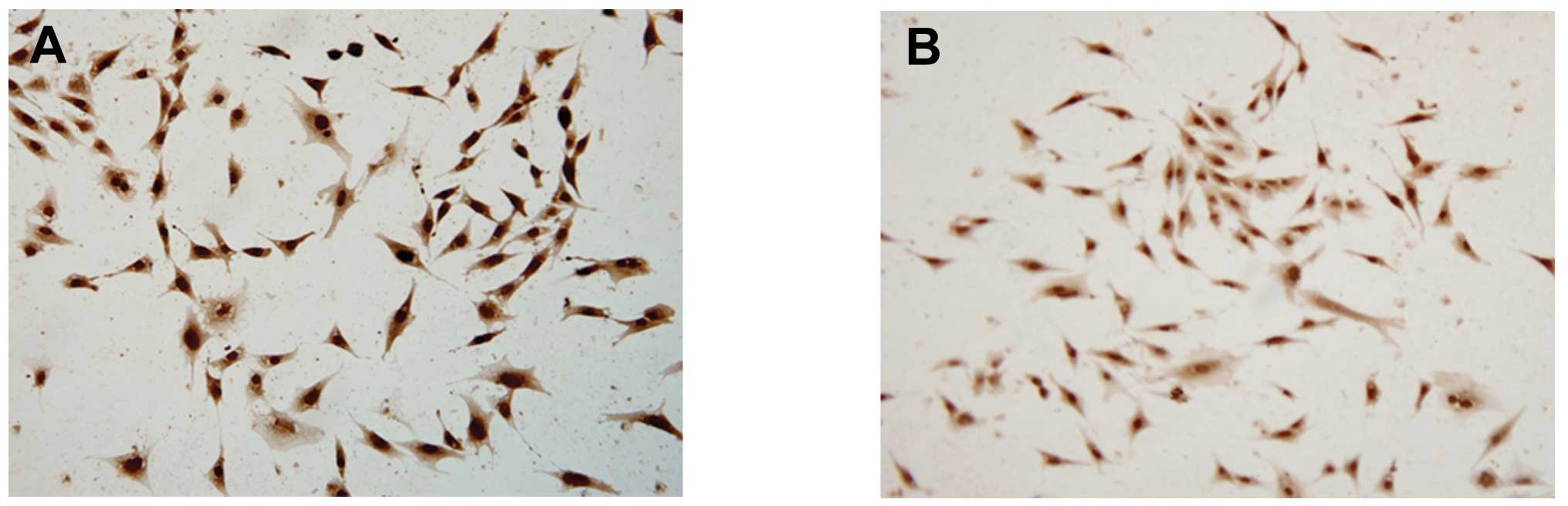
Analysis of cytochemistry and
immunocytochemical examination
It was demonstrated by ADSC smear staining in the
two groups, that toluidine blue (Fig.
3) and safranin-O (Fig. 4)
staining in the microcarrier culture was strongly positive, and was
stronger than that in the static culture group. The positive
staining indicated that the extracellular matrix contained a high
level of GAGs; and type II collagen staining was also strongly
positive (Fig. 5), indicating that
the ADSCs cultured on the microcarriers through dynamic induction
culture were able to express matrix components characteristic of
cartilage.
Discussion
ADSCs are relatively easy to obtain and have a wider
range of sources compared with bone marrow stromal stem cells. De
Ugarte et al (8) identified
no significant difference between bone marrow-derived stem cells
and ADSCs in the time taken to obtain them, the cell growth
kinetics, cell aging, gene transfection and cell adhesion
properties. Thus, ADSCs have exceptional advantages and prospects
as seed cells for cartilage tissue engineering.
The clinical application of cartilage tissue
engineering often requires a great quantity of seed cells, and
studies have demonstrated that when the cell number is
<1×107/ml, it is not able to form cartilage or can
only generate small amounts (9).
The traditional method of amplification culture is slow and does
not reliably maintain the stem cell phenotype subsequent to
induction; and studies have demonstrated that appropriate
mechanical stimulation and three-dimensional culture conditions are
conducive to the differentiation of ADSCs into chondrocytes, and
promote the synthesis of extracellular matrix (10,11);
the dynamic three-dimensional culture combines the mechanical
environment with the three-dimensional culture surface, to further
improve the biochemical and biomechanical function of cartilage
tissue engineering.
Microcarriers are widely used for the large-scale
culture of attachment-dependent cells (12,13),
as they possess an increased surface area for cell adhesion, and
thus, numerous seed cells can be harvested in a short time period
(14). The microcarrier dynamic
culture is the combination of using microcarriers with a
bioreactor, and has been widely used in the field of tissue
engineering. In the dynamic process of cultivation, external fluid
mechanical stimulation is able to significantly increase cell
proliferation and promote synthesis of DNA. Using the bioreactor
and microcarrier technique, the cell concentration can reach
1×108/ml (15,16), which is difficult to achieve under
conventional culture conditions. The microcarrier has a larger
specific surface area (110 g Cytodex 3 provides ~4600
cm2 surface area) (17)
than a common culture flask, and it can cultivate up to hundreds of
times more cells and meet the required quantities of seed cells to
construct a large volume of tissue. The rotating bioreactor (RCCS)
was also used, as the whole culture container is driven by the
motor to rotate along a horizontal axis, so the culture medium and
cell particles rotate together with the rotary container, the
microcarrier particles can form a liquid suspension track on the
horizontal axis, and the gravity, buoyancy and shear force can
achieve a balance, which constitutes a microgravity environment
conducive to cell aggregation. This balances the amplification of
growth in each direction of the microcarriers’ surface. In this
dynamic environment, cells and nutrients are prone to homogeneous
distribution, which can significantly improve the exchange of
nutrients and metabolites throughout the microenvironment,
maintaining the homogeneity and stability of the culture
environment and effectively promoting cell proliferation.
Another problem facing tissue construction is how to
maintain the induced phenotype, and not to allow cells to undergo
dedifferentiation. The correct hydrostatic pressure and shear
stress aids in maintaining the phenotype and function of cartilage
cells (18–21). The use of a biological reactor and
microcarrier with dynamic three-dimensional culture provides an
effective environment for cell proliferation, and also the periodic
mechanical stimulation required in the process of cell
differentiation. This serves an important function in the
mechanical environment, which can effectively promote
differentiation of the stem cells into chondrocytes (22–25).
In a dynamic environment, the enhanced cell activity coupled with
the inductive factors, leads to increased production and secretion
of proteins to form the specific extracellular matrix and promote
the differentiation and maturation of cells.
In conclusion, this type of dynamic culture
microenvironment provides cell aggregation, fast three-dimensional
growth and effective differentiation conditions, and provides the
most effective method for tissue engineering. The results of the
current study confirmed this. In the present study, the ADSCs
quickly attached, extended and rapidly proliferated on the
microcarriers. On day 7, cells covered the surfaces of the
microcarriers completely, and grew, projecting out from the
microcarriers. The microcarriers connected with each other and
formed masses. The number of cells in the microcarrier culture
increased by almost 20-fold in one week, while that of the static
culture increased only by 6-fold, and histochemical and
immunohistochemical analysis indicated that microcarrier culture
cells possessed improved phenotypes and extracellular matrix
production. The current study used ADSCs as seed cells to construct
cartilage tissue, providing an experimental basis, and the
foundation for future investigation of cartilage tissue
engineering.
Acknowledgements
The present study was supported by the 863 National
Program (grant no. 2002AA205021) and the Beijing Municipal Science
and Technology Commission (grant no. H060920050630).
References
|
1
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Planat-Benard V, Silvestre JS, Cousin B,
et al: Plasticity of human adipose lineage cells toward endothelial
cells: physiological and therapeutic perspectives. Circulation.
109:656–663. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogawa R, Mizuno H, Watanabe A, et al:
Osteogenic and chondrogenic differentiation by adipose-derived stem
cells harvested from GFP transgenic mice. Biochem Biophys Res
Commun. 313:871–877. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ogawa R, Mizuno H, Watanabe A, et al:
Adipogenic differentiation by adipose derived stem cells harvested
from GFP transgenic mice including relationship of sex differences.
Biochem Biophys Res Commun. 319:511–517. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fujimura J, Ogawa R, Mizuno H, et al:
Neural differentiation of adipose-derived stem cells isolated from
GFP transgenic mice. Biochem Biophys Res Commun. 333:116–121. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Marlovits S, Tichy B, Truppe M, et al:
Collagen expression in tissue engineered cartilage of aged human
articular chondrocytes in a rotating bioreactor. Int J Artif
Organs. 26:319–330. 2003.PubMed/NCBI
|
|
8
|
De Ugarte DA, Morizono K, Elbarbary A, et
al: Comparison of multi-lineage cells from human adipose tissue and
bone marrow. Cells Tissues Organs. 174:101–109. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koch RJ and Gorti GK: Tissue engineering
with chondrocytes. Facial Plast Surg. 18:59–68. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Winter A, Breit S, Parsch D, et al:
Cartilage-like gene expression in differentiated human stem cell
spheroids: a comparison of bone marrow-derived and adipose
tissue-derived stromal cells. Arthritis Rheum. 48:418–429. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ogawa R, Mizuno S, Murphy GF and Orgill
DP: The effect of hydrostatic pressure on three-dimensional
chondroinduction of human adipose-derived stem cells. Tissue Eng
Part A. 15:2937–2945. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Costa AR, Withers J, Rodrigues ME, et al:
The impact of microcarrier culture optimization on the
glycosylation profile of a monoclonal antibody. Springerplus.
2:252013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chu L and Robinson DK: Industrial choices
for protein production by large scale cell culture. Cur Opin
Biotechnol. 12:180–187. 2001. View Article : Google Scholar
|
|
14
|
Rudolph G, Lindner P, Gierse A, et al:
Online monitoring of microcarrier based fibroblast cultivations
with in situ microscopy. Biotechnol Bioeng. 99:136–145. 2008.
View Article : Google Scholar
|
|
15
|
Saris DB, Sanyal A, An KN, et al:
Periosteum reponds to dynamic fluid pressure by proliferating in
vitro. J Orthop Res. 17:668–677. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Griffiths B: Scale-up of suspension and
anchorage-dependent animal cells. Mol Biotechnol. 17:225–238. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dan JL, Xu JZ, Zhou Q, et al: Culturing
human mesenchymal stem cells in vitro in large scale on
microcarriers. Orthop J Chin. 12:1158–1160. 2004.(In Chinese).
|
|
18
|
Parkkinen JJ, Ikonen J, Lammi MJ, et al:
Effects of cyclic hydrostatic pressure on proteoglycan synthesis in
cultured chondrocytes and articular cartilage explants. Arch
Biochem Biophys. 300:458–465. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lammi MJ, Inkinen R, Parkkinen JJ, et al:
Expression of reduced amounts of structurally altered aggrecan in
articular cartilage chondrocytes exposed to high hydrostatic
pressure. Biochem J. 304:723–730. 1994.PubMed/NCBI
|
|
20
|
Simon WH, Mak A and Spirt A: The effect of
shear fatigue on bovine articular cartilage. J Orthop Res. 8:86–93.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tomatsu T, Imai N, Takeuchi N, Takahashi K
and Kimura N: Experimentally produced fractures of articular
cartilage and bone. The effects of shear forces on the pig knee. J
Bone Joint Surg Br. 74:457–462. 1992.PubMed/NCBI
|
|
22
|
van der Kraan PM, Buma P, van Kuppevelt T
and van den Berg WB: Interaction of chondrocytes, extracellular
matrix and growth factors: relevance for articular cartilage tissue
engineering. Osteoarthritis Cartilage. 10:631–637. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dickinson SC, Sims TJ, Pittarello L, et
al: Quantitative outcome measures of cartilage repair in patients
treated by tissue engineering. Tissue Eng. 11:277–287. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Z, Kupcsik L, Yao SJ, Alini M and
Stoddart MJ: Mechanical load modulates chondrogenesis of human
mesenchymal stem cells through the TGF-beta pathway. J Cell Mol
Med. 14:1338–1346. 2010. View Article : Google Scholar
|
|
25
|
Li Z, Yao SJ, Alini M and Stoddart MJ:
Chondrogenesis of human bone marrow mesenchymal stem cells in
fibrin-polyurethane composites is modulated by frequency and
amplitude of dynamic compression and shear stress. Tissue Eng Part
A. 16:575–584. 2010. View Article : Google Scholar
|